Background
Azotemia is an elevation of blood urea nitrogen (BUN) and serum creatinine levels. The reference range for BUN is 8-20 mg/dL. Reference ranges for serum creatinine vary slightly by age and sex: in adults, the normal range is 0.5-1.1 mg/dL (44-97 μmol/L) in women and 0.6-1.2 mg/dL (53-106 μmol/L) in men.
Each human kidney contains approximately 1 million functional units, nephrons, which are primarily involved in urine formation. Urine formation ensures that the body eliminates the final waste products of metabolism and excess water in an attempt to maintain a constant internal environment (homeostasis). Urine formation by each nephron involves 3 main processes, as follows:
-
Filtration at the glomerular level
-
Selective reabsorption from the filtrate passing along the renal tubules
-
Secretion by the cells of the tubules into this filtrate
Perturbation of any of these processes impairs the kidney’s excretory function, resulting in azotemia.
The quantity of glomerular filtrate produced each minute by all nephrons in both kidneys is referred to as the glomerular filtration rate (GFR). On average, the GFR is about 125 mL/min (10% less for women), or 180 L/day. About 99% of the filtrate (178 L/day) is reabsorbed, and the rest (2 L/day) is excreted.
Measurement of kidney function
Radionuclide assessment of the GFR is the best available test for measuring kidney function. However, this test is expensive and not widely available, and as a result, serum creatinine concentration, creatinine clearance (CrCl) and estimating equations for GFR (eGFR) more commonly are used to estimate GFR.
An inverse relation between serum creatinine and the GFR exists; however, serum creatinine and CrCl are not sensitive measures of kidney damage, for 2 reasons. First, substantial kidney damage can take place before any decrease in the GFR occurs. Second, a substantial decline in the GFR may lead to only a slight elevation in serum creatinine (see the image below). Because of compensatory hypertrophy and hyperfiltration of the remaining healthy nephrons, an elevation in serum creatinine is apparent only when the GFR falls to about 60-70 mL/min.
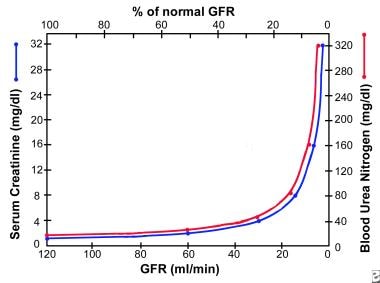 Graph shows relation of glomerular filtration rate (GFR) to steady-state serum creatinine and blood urea nitrogen (BUN) levels. In early renal disease, substantial decline in GFR may lead to only slight elevation in serum creatinine. Elevation in serum creatinine is apparent only when GFR falls to about 70 mL/min.
Graph shows relation of glomerular filtration rate (GFR) to steady-state serum creatinine and blood urea nitrogen (BUN) levels. In early renal disease, substantial decline in GFR may lead to only slight elevation in serum creatinine. Elevation in serum creatinine is apparent only when GFR falls to about 70 mL/min.
Because creatinine normally is filtered as well as secreted into the renal tubules, CrCl may cause the GFR to be substantially overestimated, especially as kidney failure progresses because of maximal tubular excretion. More accurate determinations of GFR require the use of inulin clearance or a radiolabeled compound (eg, iothalamate). In practice, precise knowledge of the GFR is not required, and the disease process usually can be adequately monitored by using the estimated GFR (eGFR), which may be obtained with a number of different methods.
CrCl is best calculated by obtaining a 24-hour urine collection for creatinine and volume and then using the following formula:
CrCl (mL/min) = U/P × V
where U is the urine creatinine in mg/dL, P is the serum creatinine in mg/dL, and V is the 24-hour volume divided by 1440 (the number of minutes in 24 hours). See also the CrCl from 24h Urine calculator. An adequate 24-hour collection usually reflects a creatinine generation of 15-20 mg/kg in women and 20-25 mg/kg in men. When 24-hour creatinine is measured, the adequacy of the collection must be established prior to calculation of the creatinine clearance. 24-hr creatinine clearance measurements have largely been replaced by estimating equations described below.
Alternatively, several formulas are available for estimating GFR. The Cockcroft-Gault formula, a bedside formula that uses the patient’s serum creatinine (mg/dL), age (y), and lean weight (kg), is as follows:
CrCl (mL/min) = [(140 – age) × weight]/(72 × serum creatinine)
For women, the result of the equation is multiplied by 0.85.
An online calculator for the Cockcroft-Gault formula is available. Although the Cockcroft-Gault formula is rarely used in clinical practice, it is still the preferred formula used in clinical trials that require kidney function assessment, due to its longevity and its use in many drug trials.
Another formula was derived from data collected in the Modification of Diet in Renal Disease (MDRD) study. The MDRD formula, also called the Levey formula, became widely accepted as more accurate than the Cockcroft and Gault formula; see the MDRD eGFR and MDRD eGFR (6 Variable) calculators.
Both formulas have limitations: the Cockcroft-Gault formula is simple to use but overestimates the GFR by 10-15% because creatinine is both filtered and secreted. The MDRD formula is much more complex and has been found to underestimate GFR by 6.2% in patients with chronic kidney disease (CKD) and by 29% in healthy persons. [1] Furthermore the MDRD formula overstimated eGFR by a factor of 21% in Black CKD patients resulting in delayed referrals of Black patients for needed renal care.
A third formula, the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation, is based on the same four variables as the MDRD Study formula but uses a 2-slope “spline” to model the relationship between estimated GFR and serum creatinine, and a different relationship for age, sex, and race. See the eGFR using CKD-EPI calculator. The National Kidney Foundation (NKF) recommended using the CKD-EPI equation to estimate GFR [2] until recently, when researchers noted that although the CKD-EPI equation performed much better than MDRD, it still overestimated the eGFR in Black patients, although to a lesser extent (by 16%), which would also lead to delayed referral of Black patients for renal care.
As a result, a new formula was developed in 2023, the CKD-EPI Refit equation; see the eGFR calculator without race eGFR calculator. The CKD EPI-Refit equation does not include race, thereby eliminating the health disparity issue. [3] Inputs for the eGFR calculator without race are the patient's serum creatinine level, age, and gender. Users have the option to input cystatin C level rather than creatinine level, to get a cystatin C-based eGFR. Cystatin C–based eGFR estimates perform better than creatinine-based eGFR estimates because cystatin C is produced by all nucleated cells and thereby has a better steady state than creatinine, which is produced by muscles alone, so levels can fluctuate based on muscle mass. However, cystatin C assays are expensive and not for routine use. For clinical use, the creatinine-based eGFR estimate without race is the current standard.
Pathophysiology
There are three pathophysiologic states in azotemia, as follows:
-
Prerenal azotemia
-
Intrarenal azotemia
-
Postrenal azotemia
Prerenal azotemia
Prerenal azotemia refers to elevations in BUN and creatinine levels resulting from problems in the systemic circulation that decrease blood flow to the kidneys. The decreased renal flow stimulates salt and water retention to restore volume and pressure.
Decreases in blood volume or pressure activate the baroreceptor reflexes located in the aortic arch and carotid sinuses. This leads to sympathetic nerve activation, resulting in renal afferent arteriolar vasoconstriction and renin secretion through β1 receptors. Constriction of the afferent arterioles causes a decrease in intraglomerular pressure, which reduces the GFR proportionally. Reduction in renal blood flow results in the generation of renin, which converts angiotensinogen to angiotensin I. Angiotensin-converting enzyme then converts angiotensin I to angiotensin II, which, in turn, stimulates aldosterone release. The increase in aldosterone levels results in salt and water absorption in the distal collecting tubule.
A decrease in volume or pressure is a nonosmotic stimulus for hypothalamic production of antidiuretic hormone, which exerts its effect in the medullary collecting duct for water reabsorption. Through unknown mechanisms, activation of the sympathetic nervous system leads to enhanced proximal tubular reabsorption of salt and water, as well as BUN, creatinine, calcium, uric acid, and bicarbonate. The net result of those 4 mechanisms of salt and water retention is decreased urine output and decreased urinary excretion of sodium (< 20 mEq/L).
Intrarenal azotemia
Intrarenal azotemia, also known as acute kidney injury (AKI), renal-renal azotemia, and (in the past) acute renal failure (ARF), refers to elevations in BUN and creatinine resulting from problems in the kidney itself. Definitions of AKI include a rise in serum creatinine levels of about 30% from baseline or a sudden decline in output below 500 mL/day. If output is preserved, AKI is nonoliguric; if output falls below 500 mL/day, AKI is oliguric. Any form of AKI may be so severe that it virtually stops urine formation; this condition is called anuria (< 100 mL/day).
The most common causes of nonoliguric AKI are acute tubular necrosis (ATN), aminoglycoside nephrotoxicity, lithium toxicity, and cisplatin nephrotoxicity. Tubular damage is less severe in nonoliguric AKI than it is in oliguric AKI. However, normal output in nonoliguric AKI does not reflect a normal GFR: Patients may still make 1440 mL/day of urine even when the GFR falls to about 1 mL/min because of decreased tubular reabsorption.
Some studies indicate that nonoliguric forms of AKI are associated with less morbidity and mortality than is oliguric AKI. Uncontrolled studies also suggest that volume expansion, potent diuretic agents, and renal vasodilators can convert oliguric AKI to nonoliguric AKI if administered early.
The pathophysiology of acute oliguric or nonoliguric AKI depends on the anatomic location of the injury. In ATN, epithelial damage leads to functional decline in the ability of the tubules to reabsorb salt, water, and other electrolytes. Excretion of acid and potassium is also impaired. In more severe ATN, the tubular lumen is filled with epithelial casts, causing intraluminal obstruction and resulting in a declining GFR.
Acute interstitial nephritis is characterized by inflammation and edema, which result in azotemia, hematuria, sterile pyuria, white blood cell (WBC) casts with variable eosinophiluria, proteinuria, and hyaline casts. The net effect is a loss of urinary concentrating ability, with low osmolality (< 500 mOsm/L), low specific gravity (< 1.015), high urinary sodium (> 40 mEq/L), and, occasionally, hyperkalemia and renal tubular acidosis. However, if there is superimposed prerenal azotemia, the specific gravity, osmolality, and sodium may be misleading.
Glomerulonephritis or vasculitis is suggested by the presence of hematuria, red blood cells (RBCs), WBCs, granular and cellular casts, and a variable degree of proteinuria. Nephrotic syndrome usually is not associated with active inflammation and often presents as proteinuria greater than 3.5 g/24 h.
Glomerular diseases may reduce GFR by changing basement membrane permeability and stimulating the renin-aldosterone axis. Such diseases often manifest as nephrotic or nephritic syndrome.
In nephrotic syndrome, the urinary sediment is inactive, and there is gross proteinuria (> 3.5 g/day), hypoalbuminemia, hyperlipidemia, and edema. Azotemia and hypertension are uncommon initially, but their presence may indicate advanced disease.
Some patients with nephrotic syndrome may present with AKI. Impairment of capillary circulation in the kidney due to edema (nephrosarca) and tubular obstruction from protein casts, as well as decreased effective circulating volume, have been proposed as potential mechanisms for the development of AKI in patients with nephrotic syndrome.
In nephritic syndrome the urinary sediment is active, with WBC or RBC casts, and granular casts, and azotemia is present. Proteinuria is less obvious, but increased salt and water retention in glomerulonephritis can lead to hypertension, edema formation, decreased output, low urinary excretion of sodium, and increased specific gravity.
Acute vascular diseases include vasculitis syndromes, malignant hypertension, scleroderma renal crisis, and thromboembolic disease, all of which cause renal hypoperfusion and ischemia leading to azotemia. Chronic vascular diseases include hypertensive benign nephrosclerosis, which has not been conclusively associated with end-stage renal disease (ESRD), and ischemic renal disease from bilateral renal artery stenosis. [4]
In bilateral renal artery stenosis, maintenance of adequate intraglomerular pressure for filtration greatly depends on efferent arteriolar vasoconstriction. Azotemia sets in when angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) cause efferent arteriolar dilatation, thereby decreasing intraglomerular pressure and filtration. Therefore, ACE inhibitors and ARBs are contraindicated in bilateral renal artery stenosis.
In addition to accumulation of urea creatinine and other waste products, a substantial reduction in GFR in CKD results in the following:
-
Decreased production of erythropoietin (causing anemia) and vitamin D-3 (causing hypocalcemia, secondary hyperparathyroidism, hyperphosphatemia, and renal osteodystrophy)
-
Reduction in acid, potassium, salt, and water excretion (causing acidosis, hyperkalemia, hypertension, and edema)
-
Platelet dysfunction (leading to increased bleeding tendencies)
The syndrome associated with the signs and symptoms of accumulation of toxic waste products (uremic toxins) is termed uremia and often occurs at a GFR of about 10 mL/min. Some of the uremic toxins (eg, urea, creatinine, phenols, and guanidines) have been identified, but none have been found responsible for all the manifestations of uremia.
Postrenal azotemia
Postrenal azotemia refers to elevations in BUN and creatinine levels resulting from obstruction in the collecting system. Obstruction to flow leads to reversal of the Starling forces responsible for glomerular filtration. Progressive bilateral obstruction causes hydronephrosis with an increase in the Bowman capsular hydrostatic pressure and tubular blockage that leads to progressive decline in and ultimate cessation of glomerular filtration, azotemia, acidosis, fluid overload, and hyperkalemia.
Unilateral obstruction rarely causes azotemia. There is evidence that if complete ureteral obstruction is relieved within 48 hours of onset, relatively complete recovery of GFR can be achieved within a week; little or no further recovery occurs after 12 weeks. Complete or prolonged partial obstruction can lead to tubular atrophy and irreversible renal fibrosis. Hydronephrosis may be absent if obstruction is mild or acute or if the collecting system is encased by retroperitoneal tumor or fibrosis.
Etiology
Prerenal azotemia occurs as a consequence of impaired renal blood flow or decreased perfusion resulting from decreased blood volume, decreased cardiac output (congestive heart failure), decreased systemic vascular resistance, decreased effective arterial volume from sepsis or hepatorenal syndrome, [5] or renal artery abnormalities. It may be superimposed on a background of chronic kidney disease. Iatrogenic factors, such as excessive diuresis and treatment with ACE inhibitors, should be ruled out.
Intrarenal azotemia occurs as a result of injury to the glomeruli, tubules, interstitium, or small vessels. It may be acute oliguric, acute nonoliguric, or chronic. Systemic disease, nocturia, proteinuria, loss of urinary concentrating ability (low urine specific gravity), anemia, and hypocalcemia are suggestive of chronic intrarenal azotemia.
Postrenal azotemia occurs when an obstruction to urine flow is present. It is observed in bilateral ureteral obstruction from tumors or stones, retroperitoneal fibrosis, neurogenic bladder, and bladder neck obstruction from prostatic hypertrophy or carcinoma and posterior urethral valves. It may be superimposed on a background of chronic kidney disease.
Epidemiology
United States statistics
The reported incidence of hospital or community-acquired AKI varies considerably. In one report, community-acquired AKI occurred in about 1% of all hospital admissions. Overall, AKI occurs in about 5% of all hospital admissions. However, differences exist between the incidence of AKI occurring in the intensive care unit (ICU; about 15%) and that in the coronary care unit (CCU; about 4%). [6]
In CKD, progressive azotemia leading to ESRD necessitating dialysis or kidney transplantation occurs in a number of chronic diseases, including the following:
-
Diabetes (36%)
-
Hypertension (24%)
-
Glomerulonephritis (15%)
-
Cystic kidney disease (4%)
-
Other known miscellaneous kidney disorders (15%)
International statistics
In a report from Madrid that evaluated 748 cases of AKI at 13 tertiary hospital centers, conditions included the following [7] :
-
ATN (45%)
-
Prerenal (21%)
-
AKI or CKD, mostly due to ATN and prerenal disease (13%)
-
Urinary tract obstruction (10%)
-
Glomerulonephritis or vasculitis (4%)
-
Acute interstitial nephritis (2%)
-
Atheroemboli (1%)
A study of the epidemiology of community-acquired AKI in eastern India in 1983–95 versus 1996–2008 found that the incidence rate rose from 1.95 to 4.14 per 1000 hospital admissions. In addition, the etiology of AKI shifted: the incidences of obstetric, surgical, and diarrheal AKI decreased significantly, whereas those of AKI associated with malaria, sepsis, nephrotoxic drugs, and liver disease increased. [8]
Etiologies of CKD differ around the world. Diabetic nephropathy as a cause of CKD is on the rise in developed and developing countries.
Age-, sex-, and race-related demographics
According to the 2022 annual report of the United States Renal Data System (USRDS), rates of hospitalization with AKI in older adults (events per 1000 person-years) were highest in patients age 85 years and older (132.8, versus 73.2 in those age 75-84), in men (75.5, 52.6 in women), and in Blacks (114.5, vs 58.9 in Whites). The USRDS reported that in 2017-March 2020, 14.0% of the US adult population had CKD, and that at the end of 2020 there were 807,920 prevalent cases of ESRD in the US. [9] Prevalence by race/ethnicity was as follows:
-
Black: 6306 cases per million population (pmp)
-
Native American: 3478 cases pmp
-
Hispanic: 3378 cases pmp
-
Asian: 2350 cases pmp
-
White: 1475 cases pmp
Prognosis
The prognosis for patients with AKI generally is poor and depends on the severity of the underlying disease and the number of failed organs. Whereas mortality in patients with simple AKI without other underlying disease is 7-23%, that in ICU patients on mechanical ventilation is as high as 80%.
The prognosis of CKD depends on the etiology. Patients with diabetic kidney disease, hypertensive nephrosclerosis, and ischemic nephropathy (ie, large-vessel arterial occlusive disease) tend to have progressive azotemia resulting in ESRD. Different types of glomerulonephritis have major differences in prognosis: some are quite benign and rarely progress to ESRD, whereas others progress to ESRD within months. About 50% of patients with polycystic kidney disease progress to ESRD by the fifth or sixth decade of life.
-
Graph shows relation of glomerular filtration rate (GFR) to steady-state serum creatinine and blood urea nitrogen (BUN) levels. In early renal disease, substantial decline in GFR may lead to only slight elevation in serum creatinine. Elevation in serum creatinine is apparent only when GFR falls to about 70 mL/min.
-
Diagnostic indices in azotemia. Although such indices are helpful, it is not necessary to perform all these tests on every patient. Comparison should always be made with patients' baseline values to identify trends consistent with increase or decrease in effective circulating volume. Use of some of these indices may be limited in certain clinical conditions, such as anemia (hematocrit), hypocalcemia (serum calcium), decreased muscle mass (serum creatinine), liver disease (blood urea nitrogen [BUN], total protein, and albumin), poor nutritional state (BUN, total protein, and albumin), and use of diuretics (urine sodium). Fractional excretion of urea and fractional excretion of trace lithium appear to be superior for assessing prerenal status in patients on diuretics.







