Practice Essentials
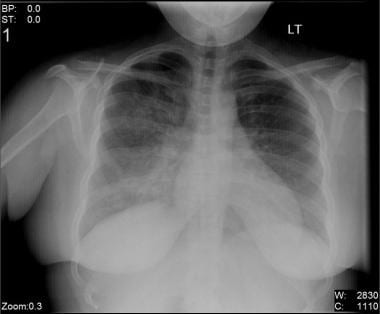
The reported incidence of viral pneumonia (see the image below) has increased during the past decade. In part, this apparent increase simply reflects improved diagnostic techniques, but an actual increase appears to have also occurred. Depending on the virulence of the organism, as well as the age and comorbidities of the patient, viral pneumonia can vary from a mild, self-limited illness to a life-threatening disease.
Signs and symptoms
The common constitutional symptoms of viral pneumonia are as follows:
-
Fever
-
Chills
-
Nonproductive cough
-
Rhinitis
-
Myalgias
-
Headaches
-
Fatigue
During physical examination, the patient may also display the following:
-
Tachypnea and/or dyspnea
-
Tachycardia or bradycardia
-
Wheezing
-
Rhonchi
-
Rales
-
Sternal or intercostal retractions
-
Dullness to percussion
-
Decreased breath sounds
-
Pleurisy
-
Pleural friction rub
-
Cyanosis
-
Rash
-
Acute respiratory distress
Influenza pneumonia
The influenza viruses are the most common viral cause of pneumonia. Primary influenza pneumonia manifests with persistent symptoms of cough, sore throat, headache, myalgia, and malaise for more than three to five days. The symptoms may worsen with time, and new respiratory signs and symptoms, such as dyspnea and cyanosis, appear.
Respiratory syncytial virus pneumonia
Respiratory syncytial virus (RSV) is the most frequent cause of lower respiratory tract infection in infants and children and the second most common viral cause of pneumonia in adults.
Patients with RSV pneumonia typically present with fever, nonproductive cough, otalgia, anorexia, and dyspnea. Wheezes, rales, and rhonchi are common physical findings.
Parainfluenza virus pneumonia
Parainfluenza virus (PIV) is second in importance only to RSV as a cause of lower respiratory tract disease in children and pneumonia and bronchiolitis in infants younger than 6 months. PIV pneumonia and bronchiolitis are caused primarily by the PIV-3 strain. The signs and symptoms include fever, cough, coryza, dyspnea with rales, and wheezing.
See Clinical Presentation for more detail.
Diagnosis
Laboratory studies
-
Cytologic evaluation: Intranuclear inclusions often exist in cells infected with a deoxyribonucleic acid (DNA) virus. Cytoplasmic inclusions usually are present in cells infected with a ribonucleic acid (RNA) virus.
-
Viral culture
-
Rapid antigen detection
-
Polymerase chain reaction (PCR) assay
-
Serologies: Particularly useful for definitively confirming the diagnosis
Radiography
Chest radiography usually demonstrates bilateral lung involvement, but none of the viral etiologies of pneumonia result in pathognomonic findings with this modality
Lung biopsy and histologic studies
Infrequently, lung biopsy is required to establish a diagnosis in very ill patients, who often are immunocompromised.
See Workup for more detail.
Management
All patients with viral pneumonia must receive supportive care with the following:
-
Oxygen
-
Rest
-
Antipyretics
-
Analgesics
-
Nutrition
-
Close observation
-
Intravenous fluids
-
Mechanical ventilation
Specific treatments for the various types of viral pneumonia include the following:
-
Influenza pneumonia: Amantadine hydrochloride and rimantadine hydrochloride are approved for the prevention and treatment of influenza A virus infection. Their efficacy in patients with influenza viral pneumonia or severe influenza is unknown.
-
RSV pneumonia: Ribavirin is the only effective antiviral agent available for the treatment of RSV pneumonia, [1] but there are conflicting data regarding its efficacy.
-
PIV pneumonia: Treatment is mainly supportive, but aerosolized and oral ribavirin have been associated with reduction in PIV shedding and clinical improvement in immunocompromised patients.
See Treatment and Medication for more detail.
Background
Viruses account for the largest proportion of childhood pneumonia. Viral pneumonia decreases in frequency in healthy young and middle-aged adults, but it then increases substantially among the elderly. Studies on community-acquired pneumonias consistently demonstrate viruses to be the second most common etiologic cause (behind Streptococcus pneumoniae), ranging from 13-50% of diagnosed cases. [2, 3, 4, 5, 6, 7]
The reported incidence of viral pneumonia has increased during the past decade. In part, this apparent increase simply reflects improved diagnostic techniques, but an actual increase has also appeared to occur. This observation is explained by the growing population of patients who are immunocompromised. [8] (See Epidemiology.)
Depending on the virulence of the organism as well as the age and comorbidities of the patient, viral pneumonia can vary from a mild and self-limited illness to a life-threatening disease. Especially in immunocompromised patients, viral pneumonia may result in respiratory failure, severe hypoxemia, and other pulmonary pathology. (See Prognosis.)
The four most frequent etiologies of viral pneumonia in children and immunocompetent adults are influenza virus, respiratory syncytial virus (RSV), adenovirus, and parainfluenza virus (PIV). Influenza virus types A and B are responsible for more than half of all community-acquired viral pneumonia cases, particularly during influenza outbreaks. (See Etiology.)
The image below depicts right-middle-lobe infiltrate in a two-month-old boy with pneumonia due to RSV
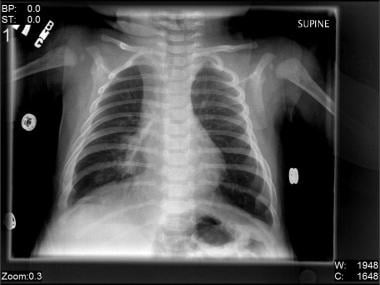 Right-middle-lobe infiltrate in a 2-month-old boy with pneumonia due to respiratory syncytial virus (RSV).
Right-middle-lobe infiltrate in a 2-month-old boy with pneumonia due to respiratory syncytial virus (RSV).
The relative importance of additional viruses (eg, parainfluenza, rhinoviruses, coronaviruses, human metapneumovirus) will likely increase as diagnostic tests such as reverse-transcription polymerase chain reaction (PCR), become more widely available. [9] (See Workup.)
Outbreaks of adenovirus of various serotypes frequently occur in military recruits. Adenovirus type 14 (Ad 14), a new variant in the United States, has been documented to cause severe and sometimes fatal acute respiratory illness in patients of all ages but especially the young, the old, patients with underlying comorbid conditions, and those who are immunocompromised.
Viral pneumonia is a subset of the pneumonitides, which were at one time called atypical pneumonias. In the past, all pneumonias were labeled atypical if a bacterial pathogen could not be identified with Gram staining and if the pneumonia did not respond to antibiotics.
Many viral pneumonias have overlapping clinical presentations with each other and with bacterial pneumonia—and may occur together with bacterial pneumonia—making diagnosis on purely clinical grounds difficult or impossible. [2] A number of rapid tests to determine viral etiologies have now been developed, and their use in the emergency department (ED) has allowed bedside diagnosis of the etiology of viral pneumonia.
An accurate and early etiologic diagnosis is important because specific therapies are used against certain viruses (see Treatment and Management). Even with currently available tests, however, in some series a causative microorganism could not be identified in 50-80% of symptomatic patients.
Agents used to treat cases of viral pneumonia include acyclovir, ganciclovir, and immunoglobulin. (See Medication.)
For more information, see Medscape’s Pneumonia Resource Center and Influenza Resource Center.
Pathophysiology
A full understanding of the pathophysiology and pathogenesis of viral diseases does not presently exist.
After contamination, most respiratory viruses tend to multiply in the epithelium of the upper airway and secondarily infect the lung by means of airway secretions or hematogenous spread. Severe pneumonias may result in extensive consolidation of the lungs with varying degrees of hemorrhage, with some patients developing bloody pleural effusions and diffuse alveolar damage. [10]
The mechanism of damage to tissues depends on the virus involved. Some viruses are mainly cytopathic, directly affecting the pneumocytes or the bronchial cells. With others, overexuberant inflammation from the immune response is the mainstay of the pathogenic process.
Immune responses can be categorized according to patterns of cytokine production. Type 1 cytokines promote cell-mediated immunity, while type 2 cytokines mediate allergic responses. Children infected with respiratory syncytial virus (RSV) who develop acute bronchiolitis, rather than mild upper respiratory infection symptoms, have impaired type 1 immunity or augmented type 2 immunity. [11]
In addition to humoral responses, cell-mediated immunity appears to be important for recovery from certain respiratory viral infections. [12] Impaired type 1 response may explain why immunocompromised patients have more severe viral pneumonias.
Respiratory viruses damage the respiratory tract and stimulate the host to release multiple humoral factors, including histamine, leukotriene C4, and virus-specific IgE in RSV infection and bradykinin, interleukin (IL)–1, IL-6, and IL-8 in rhinovirus infections. RSV infections can also alter bacterial colonization patterns, increase bacterial adherence to respiratory epithelium, reduce mucociliary clearance, and alter bacterial phagocytosis by host cells.
Influenza virus
Infection by influenza virus leads to cell death, especially in the upper airway. When direct viral infection of lung parenchyma occurs, hemorrhage is seen along with a relative lack of inflammatory cells. Mucociliary clearance is impaired, and bacterial adherence to respiratory epithelium occurs.
Infection with the influenza virus impairs T lymphocytes, neutrophils, and macrophage function, which leads to impairment of host defenses and may foster bacterial infection of normally sterile areas, including the lower respiratory tract. This impairment of host defenses may explain why as many as 53% of outpatients with bacterial pneumonia have a concurrent viral infection.
Adenoviruses
Little is known regarding mechanisms of pathogenicity of adenoviruses. Studies in children have identified increased production of cytokines, particularly tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and interleukin 8 (IL-8). Age, health of the patient, and other unknown host factors are believed to play key roles.
Viral pneumonia in elderly persons
Elderly persons are at increased risk of infection and complications in viral pneumonia because of comorbidities. Waning cellular, humoral, and innate immune functioning may impair viral clearance, which allows spread of the virus to the lower respiratory tract resulting in increased inflammation. Elderly persons also have decreased respiratory muscle strength and impaired protection of the respiratory tract from mucus. [13]
Viral transmission
The mechanism of viral transmission varies with the type of virus. Routes include large-droplet spread over short distances (< 1 m), hand contact with contaminated skin and fomites and subsequent inoculation onto the nasal mucosa or conjunctiva (eg, rhinovirus, RSV), and small-particle aerosol spread (eg, influenza, adenovirus). Some viruses are extremely fastidious, whereas others have the capability of surviving on environmental surfaces for as long as 7 hours, on gloves for 2 hours, and on hands for 30 minutes.
Transmission routes for selected viral pneumonias are as follows:
-
Environmental factors (adenovirus, enterovirus, rhinovirus)
-
Direct contact with contaminated objects (VZV)
-
Transplantation of contaminated organs (cytomegalovirus [CMV]) or blood products (CMV)
-
Lower-respiratory aspiration of virus asymptomatically shed in the saliva (CMV, herpes simplex virus [HSV])
-
Reactivation of a latent infection (HSV, CMV)
-
Hematogenous spread (CMV)
-
Spread by healthcare personnel (SARS, measles, adenovirus, parainfluenza virus, RSV).
Hantavirus transmission is thought to occur primarily through inhalation of infected excreta from diseased rodents. The virus is also present in rodent saliva, so transmission can also occur from bites.
A number of viruses, including adenoviruses, influenza virus, measles virus, PIV, RSV, rhinoviruses, and VZV, are easily transmitted during hospital stays and cause nosocomial pneumonia. Adenoviruses, influenza viruses, PIV, and RSV account for 70% of nosocomial pneumonias due to viruses.
Pulmonary host defense
The pulmonary host defense is complex and includes the following components:
-
Mechanical barriers
-
Humoral immunity
-
Phagocytic cells
-
Cell-mediated immunity
Mechanical barriers are hairs from the nostrils that filter particles larger than 10 microns, mucociliary clearance, and sharp-angle branching of the central airways that helps the 5- to 10-micron particles to become impacted in the mucosa.
Humoral immunity is represented by mucosal immunoglobulin A (IgA), alveolar immunoglobulin M (IgM), and immunoglobulin G (IgG) present in transudates from the blood.
Phagocytic cells consist of polymorphonuclear (PMN) cells; alveolar, interstitial, and intravascular macrophages; and respiratory dendritic cells. Alveolar macrophages provide the first defense involved in internalizing and degrading the viral pathogens. They act as antigen-presenting and opsonin-producing cells.
Respiratory dendritic cells undergo maturation, activation, and early migration into the regional lymph nodes after the viral exposure. They act as antigen-presenting cells and are involved in the activation and differentiation of CD8+ T cells.
Cell-mediated immunity is the most important defense mechanism against the intracellular viral pathogens. This immunity is involved in antibody production, cytotoxic activity, and cytokine production. CD8+ memory or effector T cells tend to dominate the lymphocyte component of the virus-induced inflammatory component.
Experimental models demonstrated that 30-90% of CD8+ T cells recovered from bronchoalveolar lavage (BAL) are virus specific at the peak of the primary response. Studies in transgenic mice infected with influenza viruses documented that the CD8+ T cells are not recruited in the lung during the viral infection. They are resting memory cells formed after a previous encounter with the antigen, or they are acutely activated T cells after a nonrespiratory infection that undergo early migration in the lung and that are maintained there by specific ligands.
A substantial number of peripheral CD8+ memory T cells reside in the lung after a viral infection.
A secondary infection induces extensive renewal of CD8+ T cells in both lymphoid nodes and lungs. This replacement takes place in the absence of substantial inflammation or a substantial effector-cell population in the lungs. Respiratory infection allows numerous T cells to enter the airways and may permanently alter the permeability of the lung and mediastinal lymph nodes to lymphocytes.
Etiology
Both DNA and RNA viruses are involved in the etiology of viral pneumonia. Some are well-known lung pathogens that produce common clinical and radiologic manifestations. Others are rarely involved as lung pathogens.
Etiologic viruses include various families, as follows:
-
Adenoviridae ( adenoviruses)
-
Bunyaviridae (arboviruses) - Hantavirus
-
Orthomyxoviridae (orthomyxoviruses) - Influenza virus
-
Papovaviridae (polyomavirus) - JC virus, BK virus
-
Paramyxoviridae (paramyxoviruses) - Parainfluenza virus (PIV), respiratory syncytial virus (RSV), human metapneumovirus (hMPV), measles virus
-
Picornaviridae (picornaviruses) - Enteroviruses, coxsackievirus, echovirus, enterovirus 71, rhinovirus
-
Reoviridae ( rotavirus)
-
Retroviridae (retroviruses) - Human immunodeficiency virus (HIV), human lymphotropic virus type 1 (HTLV-1)
Most of the members of Herpesviridae family are documented lung pathogens in hosts with compromised cell immunity and include the following:
-
Herpes simplex virus 1 (HSV-1) and herpes simplex virus 2 (HSV-2), also called human herpesvirus 1 (HHV-1) and human herpesvirus 2 (HHV-2), respectively
-
Herpesvirus 6, herpesvirus 7, and herpesvirus 8
-
Varicella-zoster virus (VZV)
-
Cytomegalovirus (CMV)
-
Epstein-Barr virus (EBV)
Influenza virus, respiratory syncytial virus, adenovirus, parainfluenza virus, coronavirus, rhinovirus, and human metapneumovirus may cause community-acquired viral pneumonia.
Influenza virus
The influenza viruses are enveloped, single-stranded, RNA viruses of the family Orthomyxoviridae and are the most common viral cause of pneumonia. Three serotypes of influenza virus exist: A, B, and C.
Influenza type A can alter surface antigens and infect livestock. This characteristic may account for its ability to create a reservoir for infection and cause epidemics in humans. The virus is spread by means of small-particle aerosol and targets the columnar epithelial cells along the entire respiratory tract.
Influenza type B causes illness that usually is seen in relatively closed populations such as boarding schools. Influenza type C is less common and occurs as sporadic cases.
Influenza type A is usually the most virulent pathogen. The influenza virus has two envelope glycoproteins, hemagglutinin (H) and neuraminidase (N), which are important for a number of reasons. The hemagglutinin initiates infectivity by binding to cellular sialic acid residues, whereas the N protein cleaves newly synthesized virus from sialic acid on cell surfaces, thus allowing spread of the virus to other cells.
The influenza virus maintains its infectivity by undergoing antigenic drift (small number of amino acid substitutions) and shift (large number of amino acid substitutions) due to changes in the protein structure of the surface protein, hemagglutinin. Epidemics occur when a viral drift occurs, and pandemics are seen with viral shift (two influenza A viruses exchange H or N genes during infection of the same hosts) because most people have no prior immunity to the virus.
Two influenza types have emerged of particular importance: H5N1 avian influenza strain and the novel H1N1 swine influenza strain.
Respiratory syncytial virus
Respiratory syncytial virus (RSV) is the most frequent cause of lower respiratory tract infection among infants and children and the second most common viral cause of pneumonia in adults. It is a medium-sized virus of the Paramyxoviridae family that consists of only 1 serotype. Structurally, RSV has 10 unique viral polypeptides, 4 of which are associated with virus envelope, and 2 of these (F and G) are important for infectivity and pathogenicity. Classic RSV infection causes syncytia formation in cell culture, giving the virus its name.
RSV is highly contagious, spreading via droplet and fomite exposure. Most children are infected before age 5 years—the infection rate during an epidemic approaches 100% in certain settings such as daycare centers—but the resulting immunity is incomplete. Reinfection in older children and young adults is common but mild. However, the likelihood of more severe disease and pneumonia increases with advancing age.
Adenoviruses
Adenoviruses are enveloped DNA viruses that cause a wide spectrum of clinical illnesses depending on the serotype of the infecting agent. These include asymptomatic illness, conjunctivitis, febrile upper respiratory disease, pneumonia, gastrointestinal illness, hemorrhagic cystitis, rash, and neurologic disease. Pneumonia is less common in adults outside of military recruit camps and similar facilities, but fulminant disease has been described in infants and in the immunocompromised population and can occur in apparently healthy hosts. [15]
Although 52 serotypes exist, classified into 7 subgroups or species (A-G), pulmonary disease is predominantly caused by serotypes 1, 2, 3, 4, 5, 7, 14, and 21. Type 7 viruses can cause bronchiolitis and pneumonia in infants. Types 4 and 7 viruses are responsible for outbreaks of respiratory disease in military recruits.
Adenovirus serotype 14 (subgroup B) is a more virulent strain that has been reported to cause severe respiratory illness and pneumonia. Emergence of this strain was reported in 2005 among civilian and military populations, with outbreaks occurring subsequently at military training centers throughout the United States.
In 2007, adenovirus serotype 14 caused a large, sustained outbreak of febrile respiratory illness among military trainees in Texas and, more recently, in a residential care facility in Washington State. [16, 17, 18] In a community outbreak in Oregon, the median age was 52 years, and 76% required hospitalization, 47% required critical care, 24% required vasopressors, and 18% died. The majority of these patients were otherwise immunocompetent adults. [19]
Spread of adenovirus is by respiratory secretions, infectious aerosols, feces, and fomites. Neonates may acquire infection from exposure to cervical secretions at birth.
Contaminated environmental surfaces can harbor virus capable of causing infection for weeks. The virus is resistant to lipid disinfectants but is inactivated by heat, formaldehyde, and bleach.
Adenoviruses are extremely contagious. Studies of new military recruits have shown seroconversion rates of 34-97% over a 6-week period. [16] The majority of children have serologic evidence of prior adenovirus infection by the age of 10.
Parainfluenza virus
Parainfluenza virus (PIV) is a common virus that infects most persons during childhood. PIV is second in importance to only RSV in causing lower respiratory tract disease in children and pneumonia and bronchiolitis in infants younger than six months. Transmission is through direct person-to-person contact or large-droplet spread.
PIV is characterized by nucleocapsids, which develop in the cytoplasm of infected cells, with hemagglutinin present in the virion envelope.
There are four subtypes of PIV, based on antigenic characteristics. PIV type 3 is endemic year-round, while types 1 and 2 peak during the fall season. Immunity is short term, and recurrent upper or lower respiratory tract infections occur throughout life. The infections vary from a mild illness to life-threatening croup, bronchiolitis, or pneumonia. Infection in immunocompromised hosts can result in life-threatening pneumonia with lung injury and respiratory failure. In one study, 44% of hematopoietic stem cell transplant (HSCT) patients with PIV progressed to develop pneumonia, of which 37% died. [20]
Rhinovirus
Some authors report that rhinovirus accounts for up to 30% of cases of all virus-related pneumonia. Clinical studies show that rhinovirus is the second most frequently recognized agent associated with pneumonia and bronchiolitis in infants and young children. Rhinovirus infection is linked to asthma hospitalizations in both adults and children.
A study of 211 French children with rhinovirus infection revealed bronchiolitis or bronchitis in 25.6% and pneumonia in 6.2%, after cases of dual bacterial or viral infections were eliminated.
A study from the Netherlands demonstrated that rhinoviruses cause 32% of all lower respiratory tract infections with an identified pathogen in the elderly (> 60 y) symptomatic population. Rhinoviruses were identified more frequently than coronaviruses (17%) or influenza viruses (7%).
Human metapneumovirus
Human metapneumovirus (hMPV) is a relatively newly discovered respiratory pathogen, initially described in the Netherlands in 2001. [21] hMPV is in the Paramyxoviridae family (like RSV and PIV) and is a pleomorphic-shaped virus surrounded by surface protein projections. This virus is a ubiquitous organism, and most surveys indicate that by age five years, almost all children have been exposed to it. However, reinfection occurs throughout life, including in adults. This virus is spread via droplet and fomite exposure.
As a human pathogen, hMPV may have been underestimated. In children and infants, hMPV was reported to be a notable cause of lower respiratory tract infections such as bronchiolitis (59%), croup (18%), asthma (14%), and pneumonia (8%).
As with other viruses, the severity of infection increases with older age and with comorbid (cardiopulmonary disease) or immunosuppressive conditions. The most common diagnoses associated with adult hospitalizations with hMPV infection are chronic obstructive pulmonary disease (COPD) exacerbations, bronchitis, and pneumonia. [22] In immunocompromised hosts (eg, hematologic malignancies), severe pneumonitis requiring intensive care or resulting in death has been reported. [23, 24]
Coronavirus
Coronaviruses are from the family Coronaviridae and are single-stranded RNA viruses, the surface of which is covered by crownlike projections, giving the virus its name. This virus is spread via droplet and fomite exposure. Long known to cause upper respiratory infections, coronaviruses were not felt to significantly cause pneumonia until relatively recently. However, the severe acute respiratory syndrome (SARS) pandemic in 2003 brought the ability of this virus to cause life-threatening pneumonia to worldwide attention (see Zoonotic Viral Pneumonia, below).
Seven human coronaviruses (HCoVs) have now been identified: HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, SARS-COV (which causes severe acute respiratory syndrome), MERS-COV (Middle East respiratory syndrome), and 2019-nCoV. All but 2019-nCoV appear to be established human pathogens with worldwide distribution, causing upper and lower respiratory tract infections, especially in children. Typically, HCoV infection follows a seasonal pattern similar to that of influenza, although Hong Kong researchers found that HCoV-NL63 infections mainly occurred in early summer and autumn. [25]
A novel coronavirus (2019-nCoV) was first reported in late 2019 and early 2020 in China. [14]
Varicella-zoster virus
Varicella-zoster virus (VZV) is a highly contagious herpes virus that is spread by the respiratory route or direct contact with skin lesions. Primary infection manifests as chickenpox. The reactivation results in zoster (shingles).
Pneumonia is a significant and life-threatening complication in otherwise healthy adults (including pregnant women) and immunocompromised hosts. This pneumonia is rare in otherwise healthy children but does occur in immunocompromised children.
Complications include secondary bacterial infections, encephalitis, hepatitis, and, with concomitant aspirin use, Reye syndrome. VZV pneumonia also tends be more severe in individuals who smoke.
Measles virus
Measles virus is a member of the Paramyxoviridae family and the genus Morbillivirus. It is a single-stranded RNA virus contained within a nucleocapsid and surrounded by an envelope. Measles is a respiratory tract virus that causes a febrile illness with rash in children. Mild pneumonia often occurs but is usually of no consequence in healthy adults.
Measles may result in severe lower respiratory tract infection and high morbidity in hosts who are immunocompromised and malnourished. This virus is highly contagious and is transmitted from person to person by droplets. The incubation period is 10-14 days and peaks in late winter and early spring.
Cytomegalovirus
Cytomegalovirus (CMV) is a herpesvirus that is a common cause of infections, usually asymptomatic, in the general population. In hosts who are immunocompetent, acute CMV infection causes a mononucleosis-like syndrome. Transmission is primarily through body fluid contact. The virus has been found in the cervix and in human milk, semen, and blood products. The prevalence of antibodies to CMV in adults ranges from 40-100%, with higher rates in lower socioeconomic areas.
Reactivation of latent infection is almost universal in transplant recipients and individuals infected with the human immunodeficiency virus. CMV pneumonia may occur and is often fatal in immunocompromised individuals, primarily hematopoietic stem cell transplant (HSCT) and solid organ transplant (SOT) recipients. The severity of pneumonia is related to the intensity of immunosuppression. Additionally, CMV infection is itself immunosuppressive, causing further immunocompromise in these patients.
In cancer patients receiving allogeneic bone marrow transplants, CMV pneumonia has a prevalence of 15% and a mortality rate of 85%, making it the most common cause of death in this population. Acute graft-versus-host disease is the major risk factor for CMV pneumonia in these patients.
Interestingly, although CMV is a well-recognized pathogen in patients with AIDS (manifesting as retinitis, colitis, encephalitis, polyradiculitis, and/or cholangiopathy), clinically relevant pneumonia is very uncommon in this group, even if CMV is cultured from alveolar fluid and/or seen on lung histology.
Herpes simplex virus
Herpes simplex virus (HSV) is a rare cause of lower respiratory tract infections and is seen primarily in severely immunocompromised patients, primarily HSCT and SOT recipients, patients who are undergoing chemotherapy or are neutropenic, or those who have congenital immunodeficiency. [26] HSV pneumonia develops either secondary to upper airway infection (because of direct extension of the virus from the upper to the lower respiratory tract) or following viremia secondary to dissemination of HSV from genital or oral lesions.
Herpes simplex virus is spread by contact with active lesions or viral shed by asymptomatic excreters. While not a classic respiratory virus, herpes simplex virus can cause pneumonia in compromised hosts, with a mortality rate of 80%. Pneumonia may develop from primary infection or reactivation.
Zoonotic viral pneumonias
Zoonotic viral pneumonias include those caused by the hantavirus, avian influenza, severe acute respiratory syndrome (SARS), and H1N1 (swine) influenza.
Hantavirus
Hantavirus is a genus of enveloped RNA viruses in the family Bunyaviridae. The majority are transmitted by arthropod vectors. Hantaviruses, however, are harbored by rodents, with each viral species having one major rodent host species. Rodents, which are chronically infected, excrete hantaviruses from urine, saliva, and feces. Infection occurs after aerosols of infectious excreta are inhaled.
The hantavirus pulmonary syndrome (HPS) is seen in the Americas and is an acute pneumonitis caused by the North American hantavirus, most notably the Sin Nombre Virus. [27, 28] Two other agents, isolated in other parts of North America, can also cause HPS.
Hantaviruses originally were recognized in the four-corners region of the southwestern United States (New Mexico, Arizona, Utah, and Colorado) in May 1993. The deer mouse (Peromyscus maniculatus) was identified to be the reservoir.
Cases of HPS have continued to be reported in the United States. As of July 2010, 545 cases of HPS had been reported in the United States from 32 states. [29]
Avian influenza
In Hong Kong in 1997, an influenza virus (H5N1 virus) previously known to infect only birds was found to infect humans. Manifestations included pneumonia, which in some cases led to fatal acute respiratory distress syndrome (ARDS) or multisystem organ failure.
Prior to the human outbreak, the H5N1 virus caused widespread deaths in chickens on three farms in Hong Kong. Epidemiologic investigations of this outbreak demonstrated that individuals in close contact with the index case or with exposure to poultry were at risk of being infected.
Concern is growing that avian influenza, which is a subtype of influenza A, may result in a worldwide pandemic in the near future. The avian influenza virus A/H5N1 has several ominous characteristics, including increased virulence and human-to-human transmission in several cases, rather than bird-to-human transmission, as is usually necessary. The disease causes high mortality as a result of pneumonia and respiratory failure.
The 1997 outbreak in Hong Kong was thought to be controlled by depopulating 1.5 million chickens in local farms and markets. However, human infections occurred in 2001 through 2003 in other parts of Asia, and the virus has been found in poultry and birds in Europe.
The rising incidence and widespread reporting of disease from H5N1 influenza viruses can probably be attributed to the increasing spread of the virus from existing reservoirs in domestic waterfowl and live bird markets, leading to greater environmental contamination. As of January 2014, 650 cases of H5N1 human infections have been reported from 16 countries since 2003, with 386 deaths (59% mortality). [30]
Severe acute respiratory syndrome
Severe acute respiratory syndrome (SARS) was due to a novel coronavirus (CoV) that crossed the species barrier through close contact between humans and infected animals. Viral isolation and genomic sequencing have revealed that the SARS virus originated in the masked palm civet cat (Paguma larvata), raccoon dog (Nyctereutes procyonoides), and possibly the Chinese ferret-badger (Melogale moschata), with subsequent interspecies jumping, during which a partial loss of genome probably led to more efficient human-to-human transmission.
Horseshoe bats (Rhinolophus sinicus) have also been found to harbor SARS-like coronaviruses (more distantly related to SARS-CoV than that of the palm civets), raising the possibility of bats being a reservoir for future SARS infections.
SARS was a particularly challenging disease because its long incubation period allowed seemingly healthy travelers who were infected with the virus to spread it. The SARS coronavirus (SARS-CoV) quickly spread from China to the rest of the world over a period of 1 year, affecting more than 8000 patients in 29 countries and resulting in 774 deaths.
Global transmission of SARS was halted in June 2003 after the World Health Organization instituted traditional public health measures, including finding and isolating case-patients, quarantining contacts, and using enhanced infection control. [31] No cases of SARS have been reported since 2004.
H1N1 (swine) influenza
Initially reported as an outbreak in Mexico and subsequently the United States, infection from a novel swine-origin influenza A (H1N1) virus rapidly spread to become a worldwide pandemic in 2009. The World Health Organization declared an end to the pandemic in August 2010.
Virus-associated hemophagocytic syndrome may play an important role in development of multiorgan failure and ensuing death in H1N1 infection. [32]
For more information on H1N1 influenza, see H1N1 Influenza (Swine Flu). Rare causes of viral pneumonia include Epstein-Barr virus and rotavirus.
Epstein-Barr virus
Epstein-Barr virus (EBV) is transmitted through infected saliva. Pneumonia as a complication of mononucleosis is very uncommon. The virus can cause pneumonia in the absence of mononucleosis.
Lung involvement secondary to EBV infections is more often reported in immunocompromised people than in others. In 25% of pediatric patients with HIV infection, EBV can cause lesions related to lymphocytic interstitial pneumonia or pulmonary lymphoid hyperplasia. [33]
Rotavirus
Although upper respiratory tract infection secondary to rotavirus is common, rotavirus pneumonia is rare. Just a few cases have been reported.
Epidemiology
Traditionally, viruses were felt to cause approximately 8% of cases of community-acquired pneumonia for which patients are hospitalized. More recent investigations have demonstrated viruses to play a larger role, causing 13-50% of pathogen-diagnosed community-acquired pneumonia cases as sole pathogens and 8-27% of cases as mixed bacteria-virus infections. [3, 4, 5, 34]
Influenza virus types A and B account for more than 50% of all community-acquired viral pneumonias in adults. Various studies have reported differing frequencies of the other viruses causing community-acquired pneumonias, with RSV ranging from 1-4%, adenovirus 1-4%, PIV 2-3 %, hMPV 0-4%, and coronavirus 1-14% of pathogen-diagnosed pneumonia cases. [3, 4, 5, 34]
The impact of influenza is high in elderly persons and greatest for those with chronic illnesses. It has been estimated that at least 63% of the 300,000 influenza-related hospitalizations and 85% of 36,000 influenza-related deaths occur in patients aged 65 years or older, despite the fact that this group accounts for only 10% of the population. [35]
RSV is the most common etiology of viral pneumonia in infants and children. [36] In addition, RSV has become an increasingly important pathogen in the elderly population and is now the second most commonly identified cause of pneumonia in elderly persons. According to the CDC each year in the U.S., RSV leads to approximately 60,000-160,000 hospitalizations and 6,000-10,000 deaths among adults 65 years of age and older.
In the United States from 1999 to 2018, the highest mean mortality rate per 100,000 population for RSV and influenza was among adults aged 65 years or older at 14.7 (95% CI, 13.8-15.5) for RSV and 20.5 (95% CI, 19.4-21.5) for influenza. [13]
Some studies have suggested that RSV-related disease is as frequent as influenza in elderly persons. Approximately 10% of nursing home patients develop RSV infection annually, while 10% of these patients will develop pneumonia.
An international study found adults with congestive heart failure (CHF) had 8 times the rate of RSV-associated hospitalization compared with adults without CHF. The adjusted RSV hospitalization rate was 26.7 (95% CI: 22.2, 31.8) per 10,000 population in adults with CHF versus 3.3 (95% CI: 3.3, 3.3) per 10,000 in adults without CHF. [37]
For information regarding RSV hospitalizations, see the CDC RSV-NET interactive Dashboard.
Parainfluenza infection is the second most common viral illness, after RSV, in infants.
Adenovirus accounts for 10% of pneumonias in children. Disease from adenovirus can occur at any time of the year. Various adenovirus serotypes are responsible for essentially continuous epidemics of acute respiratory disease at military recruit training facilities in the United States and worldwide. During the prevaccination era, up to 20% of recruits had to be removed from duty due to illness. [38] Unfortunately, the vaccine against adenovirus is no longer available for administration to military personnel.
In late 2019, a novel coronavirus (2019-nCoV) was identified as the cause of viral pneumonia cases in Wuhan City, Hubei Province, China. See the Medscape Drugs & Diseases article 2019-nCoV Coronavirus for updated information on this outbreak.
Viral pneumonia in immunocompromised hosts
Although immunocompromised patients are at higher risk for viral pneumonia from CMV, VZV, HSV, measles, and adenoviruses, seasonal viruses (influenza, RSV, PIV) remain a major cause of pneumonia. HSCT and SOT recipients are particularly at risk for acquiring lower respiratory tract infection due to CMV and RSV. [39, 40]
CMV pneumonia has been observed in 10-30% of patients with HSCT and 15-55% of heart-lung transplant recipients, making this virus the most common cause of viral pneumonia in the former patient group. [41] After CMV, the frequency of viruses isolated from HSCT patients vary, with influenza virus ranging from 14-52%, RSV 14-48%, adenovirus 2-21%, and PIV 11-49% of viral isolates. [42]
Although HSV has been shown to cause pneumonia in this patient population, it is relatively rare when compared with the other viral pathogens, with one study showing HSV to cause 5% of nonbacterial pneumonias in HSCT recipients, compared with 46% for CMV. [43]
Viral pneumonia in pregnancy
Acute viral pneumonia is common and often underdiagnosed in pregnancy. Although the severity of bacterial pneumonia does not seem to be increased in pregnancy, viral pneumonia can have a serious clinical evolution.
Among the viral pathogens, influenza virus, VZV, and measles virus are reported as etiologic agents in severe lower respiratory tract infection. The infection may result in acute respiratory decompensation, respiratory failure, and/or ARDS, which can lead to maternofetal hypoxia, preterm labor, multisystem organ failure, and even death.
Pregnant women seem to be at increased risk for influenza pneumonia. VZV pneumonia is rare but potentially lethal, with mortality rates of 35-40% in pregnant women, compared with 10% in the general population.
Measles virus can be a considerable cause of pneumonia in pregnant women. Further bacterial superinfection can complicate the clinical and radiologic picture.
Despite reports of a high mortality rate during outbreaks of hantavirus pulmonary syndrome, no cases of maternal fatalities secondary to this disorder have been reported to date.
Sex differences in viral pneumonia
Men who are infected develop viral pneumonia at a slightly higher rate than women. Pregnant women with viral pneumonia have a higher risk for severe disease than other females. Pregnant patients have a disproportionate risk of severe disease with 2009 H1N1 infection. Treatment should be initiated as soon as the diagnosis is suspected. [44]
Age-related differences in viral pneumonia
Most viruses that can cause pneumonia generally infect children and cause a mild illness. Healthy adults also develop mild disease. In contrast, elderly persons and persons who are immunosuppressed develop severe viral pneumonia, resulting in high morbidity and mortality rates. [13]
The main exception to this was seen in the 2009-2010 H1N1 influenza pandemic, in which severe infection was more common in the population aged 5-59 years than in the elderly. This was thought to be from lack of exposure, and thus immunity, to the 1957 (and earlier) H1N1 influenza strain(s). [45]
Mortality and Morbidity
The US census for 2000-2001 listed pneumonia/influenza as the seventh leading cause of death (down from sixth) despite a 7.2% decrease in the mortality rate for these diseases during this period. Severe influenza seasons can result in more than 40,000 excess deaths and more than 200,000 hospitalizations.
Patients aged 65 years or older are at particular risk for death from viral pneumonia as well as from influenza not complicated by pneumonia. Deaths in these patients account for 89% of all pneumonia and/or influenza deaths.
Morbidity, especially in elderly persons, is also high. Up to 10-12% of patients older than 65 years required a higher level of assistance for activities of daily living after hospitalization for acute respiratory illnesses. In one nursing home outbreak, residents with acute influenza illness showed significant functional decline. [46]
Pneumonia from adenovirus serotypes other than Ad 14 has a low fatality rate, and most serotypes have a low morbidity rate.
Influenza virus
Influenza virus represents a common cause of pneumonia in the adult population, affecting 4-8% of healthy adults. Rates have been 10-20% during outbreaks and as high as 50% during epidemics. Morbidity and mortality rates related to influenza pneumonia in both the general population and in selected groups (eg, patients with chronic diseases, the elderly) are substantial.
The highest rates of hospitalization for influenza occur in preschool-aged children and in the elderly population. During outbreaks, the hospitalization rates are 27.9 cases per 10,000 persons younger than 5 years and 55 cases per 10,000 persons older than 65 years. Between 1972 and 1992, 426,000 deaths related to influenza pneumonia were reported in United States. Individuals 85 years or older were 16 times more likely than those aged 65-69 years to die from influenza.
Also in contrast with seasonal influenza, mortality was higher in younger patients with H1N1 influenza, with 87% of deaths and 71% of severe pneumonia in the age group of 5-51 years. [45] The higher mortality in patients younger than 60 years may reflect this cohort’s lack of exposure to the 1957 (and earlier) H1N1 influenza strains. Exposure to those early strains may have conferred some immunity in the older population. Also of interest is a report that identified obesity as a possible risk factor for more severe disease/mortality. [47]
As of February 2010, the CDC had estimated that 8,330 to 17,160 H1N1-related deaths occurred between April 2009 and January 16, 2010 in the United States, [48] and the World Health Organization (WHO) has estimated that, worldwide, at least 16,226 deaths have been directly attributable to H1N1. [49]
The H5N1 avian influenza seems to be more virulent than seasonal influenza, with a 59% mortality rate in cases reported thus far. [30] The median time from disease onset to death is nine days. The majority of these patients had no underlying medical problems.
Respiratory syncytial virus
RSV pneumonia is responsible for an average of 80,000 pediatric hospitalizations and 500 deaths every year. The mortality rate depends on the patient's immunologic status. In healthy children, the reported mortality rate is 0.5-1.7% but is higher in immunosuppressed patients (< 80-100% in untreated HSCT recipients vs 22% in treated control subjects).
In adults, RSV pneumonia is associated with a mortality rate ranging from 11-78%, depending on the severity of underlying immune suppression. In long-term care facilities, 5-27% of respiratory tract infections have been estimated to be caused by RSV, 10% of which will develop into pneumonia and 1-5% of which will be fatal. [50] In immunocompromised patients, particularly HSCT recipients, the mortality rate for RSV pneumonia is high, at 41%. [42]
Adenovirus
Adenovirus infection has been associated with low mortality in healthy adults, but death from a 2009 community outbreak of serotype 14 pneumonia was 18%. [19] In immunocompromised patients, adenovirus can be acquired not only by person-to-person transmission but also from reactivation, to produce a wide variety of syndromes, including gastroenteritis, hepatitis, and hemorrhagic cystitis (in addition to pneumonia), with mortality rates ranging from 38-100% and with a cumulative mortality rate of 56% in HSCT patients. [42]
Parainfluenza virus
PIV pneumonia causes 250,000 emergency visits annually, resulting in 70,000 admissions. Fully 18% of hospitalized children with respiratory tract infections in the United States have this disease.
Rates of PIV pneumonia are increased in immunocompromised pediatric groups, such as recipients of BMT, HSCT, lung transplantation, and solid-organ transplantation. PIV has been associated with 10% of acute respiratory illnesses in healthy adults and 10-50% in transplant recipients. In the latter group, the mortality rates range from 15-73%. [42] One study documented that 56% of PIV isolates were associated with upper respiratory symptoms in HSCT recipients but that 44% developed pneumonia, of whom 37% died. [20]
Human metapneumovirus
HMPV accounts for up to 10% of unexplained respiratory infections in children. Some authors report that HMPV can account for 30% of unidentified, suspected cases of viral pneumonia in transplant patients.
The relatively recent recognition of hMPV in causing pneumonia and the difficulty in its diagnosis has precluded accurate estimates of mortality rates, but case reports of deaths exist, primarily in patients with hematologic malignancies undergoing chemotherapy or HSCT. [24, 51] Mortality rates in transplant recipients have ranged from 50% in lung transplant patients to 80% in HSCT recipients. [52] A 2009 hMPV outbreak at a psychiatric ward in Taiwan, all in immunocompetent patients, resulted in 1 (of 13 diagnosed patients) death from respiratory failure. [53]
Varicella-zoster virus
In the United States, varicella pneumonia occurs with a frequency of 0.32-1.36 cases per 100,000 persons per year. Among Americans hospitalized because of varicella, 1.0-2.3 in 400 develop pneumonia.
Varicella pneumonia complicates approximately 2-10% of the cases of VZV infection in adults. At least 25% of the fatalities from varicella in adults occur in persons who develop varicella pneumonia. The severity of varicella pneumonia is highest in immunosuppressed persons, with mortality rates of 15-18%, and in pregnant women in the second/third trimesters, with a mortality rate of 41%.
The overall mortality in the general population has decreased from 19% (range, 10-30%) in 1960-1970 to 6%. In renal transplant patients, the mortality decreased from 53% in 1981-1990 to 22% in 1991-2000. The mortality rate is around 50% in intubated patients with acute respiratory failure. In HIV-infected patients, a mortality rate of 43% is reported, and in pregnant women, the mortality rate is about 41%.
Measles virus
In the United States, pneumonia is responsible for 60% of the measles mortality in infants. Although deaths from measles in the United States decreased steadily throughout the 20th century—from approximately 12 per 100,000 population in 1912 to approximately 0.2 per 100,000 population in 1960—mortality rates declined markedly after a measles vaccine was licensed in 1963. [54]
Measles is almost eradicated in the Western Hemisphere. Although only 71 cases were confirmed in the United States in 2009, a sharp increase occurred in 2014. Through August 1, 2014, 593 confirmed measles cases were reported to CDC's National Center for Immunization and Respiratory Diseases (NCIRD). [55] This is the highest number of cases since measles elimination was documented in the US in 2000. Measles-virus pneumonia is still a notable cause of mortality and morbidity in nonvaccinated children and immunocompromised adults.
In 1990, 6.5% of Americans with measles developed pneumonia. A study of 3220 US military recruits demonstrated that 3.3% had measles-related pneumonia. Most cases of pneumonia were secondary to bacterial superinfection. No deaths were reported among these otherwise healthy adults. However, in another study using different diagnostic criteria, pneumonia was found in 50% of recruits with measles. A follow-up study of Afghani children hospitalized for measles revealed an 85.4% rate of bronchopneumonia.
The CDC reported four cases of measles pneumonia, with two fatalities, among HIV-infected children in 1986-1987. [56] In children, mortality rates due to measles bronchopneumonia are high (28%). The mortality rate due to measles pneumonia is even higher in immunocompromised groups: 70% in those with cancer and 40% in those with AIDS. Investigators reported 10 fatalities secondary to measles pneumonia in 12 children with leukemia.
Cytomegalovirus
CMV pneumonia is considered the most common life-threatening complication of bone marrow transplantation (BMT) and solid-organ transplantation.
The rate of CMV pneumonia in BMT recipients is 10-50%, as reported in different studies. In patients receiving solid-organ transplants, CMV reactivation is reported in as many 70% of patients, but only 20% develop clinically significant infections. Studies of CMV pneumonia in BMT recipients demonstrate a 31% mortality rate in treated patients and a decrease from previously reported rates of 56-100% in untreated patients. The mortality rate is reportedly 75% in untreated immunosuppressed persons. [57]
Herpes simplex virus
HSV pneumonia develops mainly in immunocompromised patients. The rate of HSV pneumonia can be as high as 70-80% in hematopoietic stem-cell transplantation (HSCT) recipients not receiving prophylaxis, and it can be decreased to 5% with acyclovir prophylaxis. As with CMV, the mortality rate is high if disease remains untreated in immunosuppressed patients (>80% mortality). [58]
Hantavirus
As of July 2010, 545 cases of HPS had been reported in the United States from 32 states, mostly New Mexico, Colorado, Arizona, California, Washington, Texas, and Utah (in decreasing order of prevalence). [29] HPS is also reported in South America and in Canada. The mortality rate for HPS is 35%. Of individuals with HPS, 61% are men and 39% are women, with a mean age of 37 years. Caucasian patients account for 77%, people of Native American descent account for about 20%, and those of Hispanic descent account for 13%.
Prognosis
The prognosis is good in the vast majority of patients with viral pneumonia. It is guarded in elderly or immunocompromised patients. Some adenovirus serotypes, especially 2, 3, 7, and 21 have been the cause of serious chronic morbidity after acute respiratory illness, including irreversible atelectasis, bronchiectasis, bronchiolitis obliterans, and unilateral hyperlucent lung. [17] An estimated 14-60% of these children will suffer some degree of permanent lung damage. Many of these patients presented with pharyngitis, tonsillitis, and bronchitis. Adenovirus 14 has a high fatality and morbidity rate in healthy patients. Serious sequelae occurred in those who survived.
Viral pneumonia may leave patients with residual disability from interstitial fibrosis. Infants hospitalized with lower lung infection due to RSV are much more likely to later develop asthma.
Patient Education
For patient education resources, see the Lung Disease & Respiratory Health Center and the Cold and Flu Center. In addition, see the patient education articles Viral Pneumonia and Flu in Adults.
-
Pneumonia, viral: A 52-year-old woman developed fever, cough, and dyspnea. She also developed a rash that was prominent over the face and the trunk. The chest radiograph showed interstitial infiltrates, with suggestion of a micronodular process. The Tzanck smear results from the skin vesicle suggest varicella-zoster virus.
-
Pneumonia, viral: A 52-year-old woman developed fever, cough, and dyspnea. She also developed a rash that was prominent over the face and the trunk. The chest radiograph showed interstitial infiltrates, with suggestion of a micronodular process. The Tzanck smear results from the skin vesicle suggest varicella-zoster virus. She was treated with acyclovir; resolution of varicella-zoster virus infection occurred after 7 days of therapy.
-
Bilateral interstitial infiltrates in a 31-year-old patient with influenza pneumonia.
-
Right-middle-lobe infiltrate in a 2-month-old boy with pneumonia due to respiratory syncytial virus (RSV).
-
High-power Papanicolaou (Pap) stain showing a virus on a bronchial wash. Viruses in general show "smudgy" nuclei, may or may not show nuclear or cytoplasmic inclusions, and may demonstrate other features such as multinucleation or margination of chromatin to the periphery of the cell nucleus. Certain features are more indicative of one virus over another. This is an example of herpes virus (cells infected are located in the center right).
-
High-power Papanicolaou (Pap) stain of cytomegalovirus (center). This cell has a very large, dark intranuclear inclusion.
-
High-power hematoxin-and-eosin stain of giant cell pneumonia following measles. A multinucleated cell (center) contains eosinophilic intranuclear inclusions.
-
High-power hematoxin-and-eosin stain of numerous cells infected with the cytomegalovirus (large cells with enlarged nuclei containing dark-purple intranuclear inclusions surrounded by a clear halo).
-
High-power hematoxin-and-eosin stained herpes simplexvirus, characterized by "smudgy" degenerating nuclei. Some cells are multinucleated with margination of the chromatin to the periphery of the nuclei and molding of the nuclei to each other.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Supportive Care
- Influenza Pneumonia
- Respiratory Syncytial Virus Pneumonia
- Adenovirus Pneumonia
- Parainfluenza Virus Pneumonia
- Human Metapneumovirus Pneumonia
- Coronavirus Pneumonia
- Varicella-Zoster Virus Pneumonia
- Measles Pneumonia
- Cytomegalovirus Pneumonia
- Herpes Simplex Virus Pneumonia
- Hantavirus Pneumonia
- Prevention
- Consultations
- Show All
- Medication
- Questions & Answers
- Media Gallery
- Tables
- References