Background
Paroxysmal supraventricular tachycardia (paroxysmal SVT) is an episodic condition with an abrupt onset and termination. (See Etiology and Presentation.)
SVT in general is any tachyarrhythmia that requires atrial and/or atrioventricular (AV) nodal tissue for its initiation and maintenance. It is usually a narrow-complex tachycardia that has a regular, rapid rhythm; exceptions include atrial fibrillation (AF) and multifocal atrial tachycardia (MAT). Aberrant conduction during SVT results in a wide-complex tachycardia. (See Etiology and Workup.)
SVT is a common clinical condition that occurs in persons of all age groups, and treatment can be challenging. Electrophysiologic studies are often needed to determine the source of the conduction abnormalities. (See Epidemiology, Prognosis, Workup, Treatment, and Medication.)
Manifestations of SVT are quite variable; patients may be asymptomatic or they may present with minor palpitations or more severe symptoms. Results from electrophysiologic studies have helped to determine that the pathophysiology of SVT involves abnormalities in impulse formation and conduction pathways. The most common mechanism identified is reentry. (See Etiology, Prognosis, Presentation, and Workup.) [1, 2, 3, 4]
Rare complications of paroxysmal SVT include myocardial infarction, congestive heart failure, syncope, and sudden death. (See Prognosis and Presentation.)
Classification
The development of intracardiac electrophysiologic studies has dramatically changed the classification of SVT, with intracardiac recordings having identified the various mechanisms involved in the condition. Depending on the site of origin of the dysrhythmia, SVT may be classified as an atrial or AV tachyarrhythmia. Another way to separate the arrhythmias is to classify them into conditions having either regular or irregular rhythms. [5, 6]
Atrial tachyarrhythmias include the following:
-
Sinus tachycardia
-
Inappropriate sinus tachycardia (IST)
-
Sinus nodal reentrant tachycardia (SNRT)
-
Atrial tachycardia
-
Multifocal atrial tachycardia
-
Atrial flutter
-
Atrial fibrillation
AV tachyarrhythmias include the following:
-
AV nodal reentrant tachycardia (AVNRT)
-
AV reentrant tachycardia (AVRT)
-
Junctional ectopic tachycardia (JET)
-
Nonparoxysmal junctional tachycardia (NPJT)
Patient education
For patient education information, see the Heart Health Center, as well as Supraventricular Tachycardia (SVT, PSVT), Atrial Fibrillation (AFib), Atrial Flutter, and Arrhythmias (Heart Rhythm Disorders).
Etiology
SVT and paroxysmal SVT are triggered by a reentry mechanism. This may be induced by premature atrial or ventricular ectopic beats. Other triggers include hyperthyroidism and stimulants, including caffeine, drugs, and alcohol.
Paroxysmal SVT is observed not only in healthy individuals; it is also common in patients with previous myocardial infarction, mitral valve prolapse, rheumatic heart disease, pericarditis, pneumonia, chronic lung disease, and current alcohol intoxication. [7, 8, 9, 10] Digoxin toxicity also may be associated with paroxysmal SVT. [9, 10, 11]
Atrial tachyarrhythmias
Sinus tachycardia
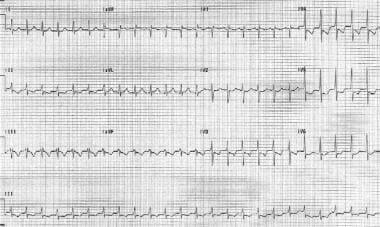
Sinus tachycardia is the most common regular SVT. It has an accelerated sinus rate that is a physiologic response to a stressor. It is characterized by a heart rate faster than 100 beats per minute (bpm) and generally involves a regular rhythm with p waves before all QRS complexes. (See the image below.)
 Sinus tachycardia. Note that the QRS complexes are narrow and regular. The patient's heart rate is approximately 135 bpm. P waves are normal in morphology.
Sinus tachycardia. Note that the QRS complexes are narrow and regular. The patient's heart rate is approximately 135 bpm. P waves are normal in morphology.
Underlying physiologic stresses such as hypoxia, hypovolemia, fever, anxiety, pain, hyperthyroidism, and exercise usually induce sinus tachycardia. [12, 13] Certain drugs, such as stimulants (eg, nicotine, caffeine), medications (eg, atropine, salbutamol), recreational drugs (eg, cocaine, amphetamines, ecstasy), and hydralazine, can also induce the condition. Treatment involves addressing the basic underlying stressor.
Inappropriate sinus tachycardia
IST is an accelerated baseline sinus rate in the absence of a physiologic stressor. In this situation, healthy adults may have an elevated resting heart rate and an exaggerated heart rate response to even minimal exercise. This tachyarrhythmia is observed most commonly in young women without structural heart disease. [10, 14, 15]
The underlying mechanism of IST may be hypersensitivity of the sinus node to autonomic input or an abnormality within the sinus node and/or its autonomic input. P wave morphology is normal on ECG and it is a diagnosis of exclusion. [10, 14, 15]
Sinus nodal reentrant tachycardia
SNRT is frequently confused with IST. SNRT is due to a reentry circuit, either in or near the sinus node. Therefore, it has an abrupt onset and offset. The heart rate is usually 100-150 bpm, and electrocardiographic tracings usually demonstrate a normal sinus P wave morphology. [14, 15, 10]
Atrial tachycardia
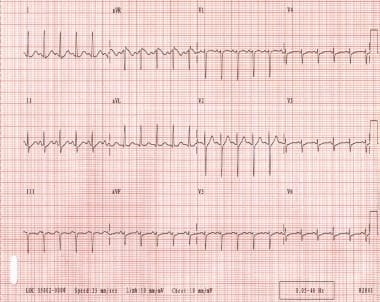
Atrial tachycardia is an arrhythmia originating in the atrial myocardium. Enhanced automaticity, triggered activity, or reentry may result in this rare tachycardia. [16, 17, 18, 19, 10] The heart rate is regular and is usually 120-250 bpm. The P-wave morphology is different from the sinus P waves and is dependent on the site of origin of the tachycardia. (See the image below.)
Because the arrhythmia does not involve the AV node, nodal blocking agents, such as adenosine and verapamil, are usually unsuccessful in terminating this arrhythmia. Atrial tachycardia has also been associated with digoxin toxicity via the triggered mechanism. [16, 17, 18, 19, 10]
Multifocal atrial tachycardia
Multifocal atrial tachycardia is a tachyarrhythmia that arises within the atrial tissue; it is composed of 3 or more P-wave morphologies and heart rates. This arrhythmia is fairly uncommon; it is typically observed in elderly patients with pulmonary disease. The heart rate is greater than 100 bpm, and electrocardiographic findings typically include an irregular rhythm, which may be misinterpreted as atrial fibrillation (see the image below). Treatment involves correcting the underlying disease process. [20, 21, 22] Magnesium and verapamil may sometimes be effective.
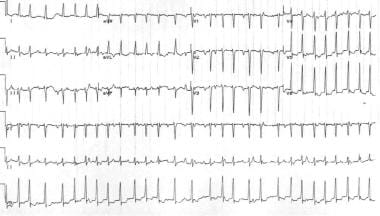 Multifocal atrial tachycardia. Note the different P-wave morphologies and irregularly irregular ventricular response.
Multifocal atrial tachycardia. Note the different P-wave morphologies and irregularly irregular ventricular response.
Atrial flutter
Atrial flutter is a tachyarrhythmia arising above the AV node with an atrial rate of 250-350 bpm. The mechanism behind atrial flutter is generally reentrant in nature. Typically, counterclockwise atrial flutter is due to a macroreentrant right atrial circuit. It is commonly observed in patients with any of the following conditions:
-
Ischemic heart disease
-
Myocardial infarction
-
Cardiomyopathy
-
Myocarditis
-
Pulmonary embolus
-
Toxic ingestion (eg, alcohol)
-
Chest trauma
Atrial flutter may be a transitional rhythm and can progress to atrial fibrillation. Electrocardiographic findings of typical atrial flutter include negative sawtooth flutter waves in leads II, III, and aVF. AV conduction is most commonly 2:1, which yields a ventricular rate of approximately 150 bpm. (See the image below.) [12, 23, 11]
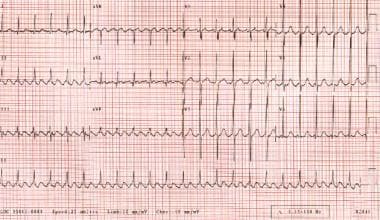 Atrial flutter. The patient's heart rate is approximately 135 bpm with 2:1 conduction. Note the sawtooth pattern formed by the flutter waves.
Atrial flutter. The patient's heart rate is approximately 135 bpm with 2:1 conduction. Note the sawtooth pattern formed by the flutter waves.
Atrial fibrillation
Atrial fibrillation is an extremely common arrhythmia arising from chaotic atrial depolarization. The atrial rate is usually 300-600 bpm, while the ventricular rate may be 170 bpm or more. Electrocardiographic findings characteristically include an irregular rhythm with fibrillatory atrial activity. (See the image below.)
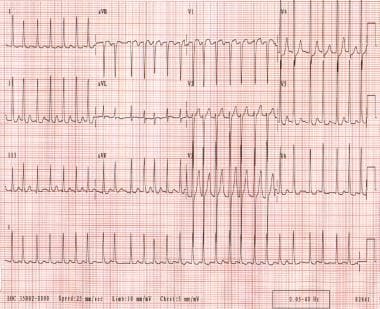 Atrial fibrillation. The patient's ventricular rate varies from 130-168 bpm. The rhythm is irregularly irregular. P waves are not discernible.
Atrial fibrillation. The patient's ventricular rate varies from 130-168 bpm. The rhythm is irregularly irregular. P waves are not discernible.
This arrhythmia is associated with the following conditions [12, 23, 11] :
-
Rheumatic heart disease
-
Hypertension
-
Ischemic heart disease
-
Pericarditis
-
Thyrotoxicosis
-
Alcohol intoxication
-
Mitral valve prolapse and other disorders of the mitral valve
-
Digitalis toxicity
When atrial fibrillation occurs in young or middle-aged patients in the absence of structural heart disease or any other apparent cause, it is called lone or idiopathic atrial fibrillation.
AV tachyarrhythmias
AV nodal reentrant tachycardia
One of the common causes of paroxysmal SVT is AVNRT. AVNRT is diagnosed in 50-60% of patients who present with regular narrow QRS tachyarrhythmia and is often in people older than 20 years. [3, 23, 24, 25] The heart rate is 120-250 bpm and is typically quite regular. (See the images below.)
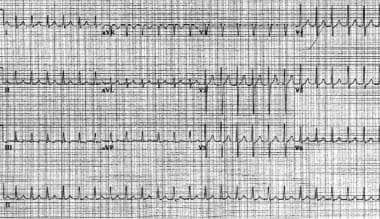 Atrioventricular nodal reentrant tachycardia. The patient's heart rate is approximately 146 bpm with a normal axis. Note the pseudo S waves in leads II, III, and aVF. Also note the pseudo R' waves in V1 and aVR. These deflections represent retrograde atrial activation.
Atrioventricular nodal reentrant tachycardia. The patient's heart rate is approximately 146 bpm with a normal axis. Note the pseudo S waves in leads II, III, and aVF. Also note the pseudo R' waves in V1 and aVR. These deflections represent retrograde atrial activation.
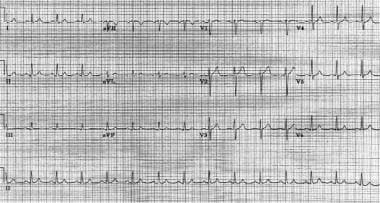 Same patient as in the previous image. The patient is in sinus rhythm following atrioventricular nodal reentrant tachycardia.
Same patient as in the previous image. The patient is in sinus rhythm following atrioventricular nodal reentrant tachycardia.
AVNRT may occur in healthy, young individuals, and it occurs most commonly in women. [25] Most patients do not have structural heart disease. However, occasionally these individuals may have an underlying heart condition such as rheumatic heart disease, pericarditis, myocardial infarction, mitral valve prolapse, or preexcitation syndrome. [3, 24, 25]
An understanding of the electrophysiology of AV nodal tissue is very important in comprehending the mechanism of AVNRT. In most people, the AV node has a single conducting pathway that conducts impulses in an anterograde manner to depolarize the bundle of His. In certain cases, AV nodal tissue may have 2 conducting pathways with different electrophysiologic properties. One pathway (alpha) is a relatively slow conducting pathway with a short refractory period, while the second pathway (beta) is a rapid conducting pathway with a long refractory period.
The coexistence of these functionally different pathways serves as the substrate for reentrant tachycardia. [3, 23, 24, 9] Electrophysiologic studies have demonstrated dual AV nodal pathways in 40% of patients.
The onset of AVNRT is triggered by a premature atrial impulse. A premature atrial impulse may reach the AV node when the fast pathway (beta) is still refractory from the previous impulse but the slow pathway (alpha) may be able to conduct. The premature impulse then conducts through the slow pathway (alpha) in an anterograde manner; the fast pathway (beta) continues to recover because of its longer refractory period.
After the impulse conducts in an anterograde manner through the slow pathway (alpha), it may find the fast pathway (beta) recovered. The impulse then conducts in a retrograde manner via the fast (beta) pathway. If the slow pathway (alpha) has repolarized by the time the impulse completes the retrograde conduction, the impulse can reenter the slow pathway (alpha) and initiate AVNRT. (See the image below.)
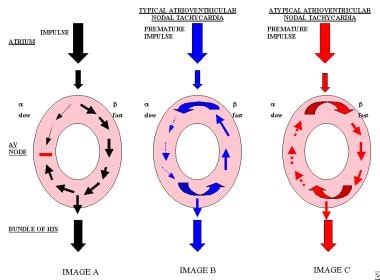 Image A displays the slow pathway and the fast pathway, with a regular impulse being conducted through the atrioventricular node. Image B displays a premature impulse that is conducted in an anterograde manner through the slow pathway and in a retrograde manner through the fast pathway, as is seen in typical atrioventricular nodal tachycardia. Image C displays the premature impulse conducting in a retrograde manner through the pathway and the impulse reentering the pathway with anterograde conduction, which is seen commonly in patients with atypical atrioventricular nodal tachycardia.
Image A displays the slow pathway and the fast pathway, with a regular impulse being conducted through the atrioventricular node. Image B displays a premature impulse that is conducted in an anterograde manner through the slow pathway and in a retrograde manner through the fast pathway, as is seen in typical atrioventricular nodal tachycardia. Image C displays the premature impulse conducting in a retrograde manner through the pathway and the impulse reentering the pathway with anterograde conduction, which is seen commonly in patients with atypical atrioventricular nodal tachycardia.
Importantly, note that AVNRT does not involve the ventricles as part of the reentry circuit; the necessity of perinodal atrial tissue to the circuit is controversial. Because the impulse typically conducts in an anterograde manner through the slow pathway and in a retrograde manner through the fast pathway, the PR interval is longer than the RP interval. Thus, in patients with typical AVNRT, the P wave is usually located at the terminal portion of the QRS complex. [3, 23, 11, 24, 9]
In patients with atypical AVNRT, anterograde conduction is via the fast pathway, while retrograde conduction is via the slow pathway. For these atypical patients, the RP interval is longer than the PR interval. [24, 26, 23, 25, 3, 9, 27, 11]
AV reentrant tachycardia
AVRT is another common form of paroxysmal SVT. The incidence rate of AVRT in the general population is 0.1-0.3%. AVRT is more common in males than in females (male-to-female ratio of 2:1), and patients with AVRT commonly present at a younger age than do patients with AVNRT. AVRT is associated with the Ebstein anomaly, although most patients with AVRT do not have evidence of structural heart disease.
AVRT results from the presence of 2 or more conducting pathways; specifically, the AV node and 1 or more bypass tracts. In a normal heart, only a single route of conduction is present. Conduction begins at the sinus node, progresses to the AV node, and then to the bundle of His and the bundle branches. However, in AVRT, 1 or more accessory pathways connect the atria and the ventricles. The accessory pathways may conduct impulses in an anterograde manner, a retrograde manner, or both. [28, 29, 24, 30, 31, 32, 9, 10]
When impulses travel down the accessory pathway in an anterograde manner, ventricular preexcitation results. This produces a short PR interval and a delta wave, as is observed in persons with Wolff-Parkinson-White (WPW) syndrome. A delta wave is the initial deflection of the QRS complex, owing to depolarization of the ventricles. (See the image below.) [28]
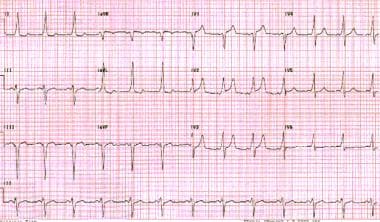 Wolff-Parkinson-White pattern. Note the short PR interval and slurred upstroke (delta wave) to the QRS complexes.
Wolff-Parkinson-White pattern. Note the short PR interval and slurred upstroke (delta wave) to the QRS complexes.
Importantly, note that not all accessory pathways conduct in an anterograde manner. Concealed accessory pathways are not evident during sinus rhythm, and they are only capable of retrograde conduction.
A reentry circuit is most commonly established by impulses traveling in an anterograde manner through the AV node and in a retrograde manner through the accessory pathway; this is called orthodromic AVRT. (See the image below.)
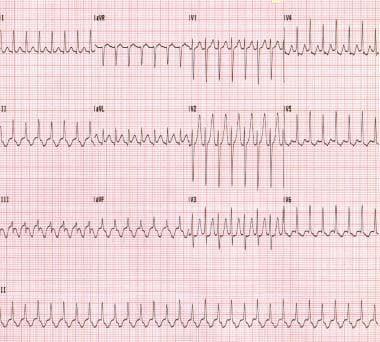 Orthodromic atrioventricular reentrant tachycardia. This patient has Wolff-Parkinson-White syndrome.
Orthodromic atrioventricular reentrant tachycardia. This patient has Wolff-Parkinson-White syndrome.
A reentry circuit may also be established by a premature impulse traveling in an anterograde manner through a manifest accessory pathway and in a retrograde manner through the AV node; this is called antidromic AVRT. While the orthodromic AVRT is typically a narrow-complex tachycardia, antidromic AVRT inscribes a bizarre, wide-complex tachycardia. (See the images below.) [33, 34, 35]
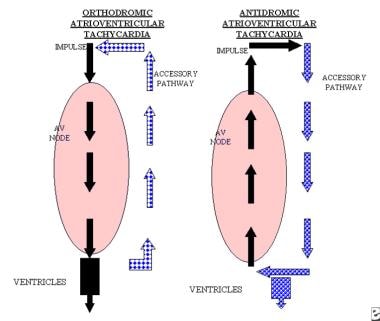 The left image displays the atrioventricular node with the accessory pathway. The impulse is conducted in an anterograde manner in the atrioventricular node and in a retrograde manner in the accessory pathway. This circuit is known as orthodromic atrioventricular reentrant tachycardia and can occur in patients with concealed accessory tracts or Wolff-Parkinson-White syndrome. The right image displays the impulse being conducted in an anterograde manner through the accessory pathway and in a retrograde manner via the atrioventricular node. This type of circuit is known as antidromic atrioventricular reentrant tachycardia and occurs only in patients with Wolff-Parkinson-White syndrome. Both patterns may display retrograde P waves after the QRS complexes.
The left image displays the atrioventricular node with the accessory pathway. The impulse is conducted in an anterograde manner in the atrioventricular node and in a retrograde manner in the accessory pathway. This circuit is known as orthodromic atrioventricular reentrant tachycardia and can occur in patients with concealed accessory tracts or Wolff-Parkinson-White syndrome. The right image displays the impulse being conducted in an anterograde manner through the accessory pathway and in a retrograde manner via the atrioventricular node. This type of circuit is known as antidromic atrioventricular reentrant tachycardia and occurs only in patients with Wolff-Parkinson-White syndrome. Both patterns may display retrograde P waves after the QRS complexes.
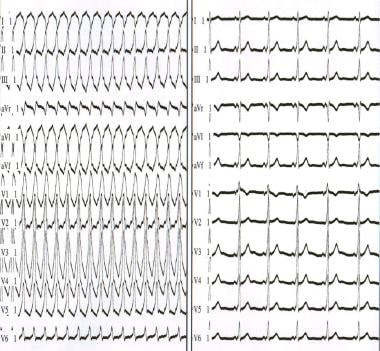 The left panel depicts antidromic atrioventricular reentrant tachycardia. The right panel depicts sinus rhythm in a patient with antidromic atrioventricular reentrant tachycardia. Note that the QRS complex is an exaggeration of the delta wave during sinus rhythm.
The left panel depicts antidromic atrioventricular reentrant tachycardia. The right panel depicts sinus rhythm in a patient with antidromic atrioventricular reentrant tachycardia. Note that the QRS complex is an exaggeration of the delta wave during sinus rhythm.
Patients with WPW syndrome can develop atrial fibrillation and atrial flutter (see the image below). The rapid conduction via the accessory pathways can result in extremely rapid rates, which can degenerate to ventricular fibrillation and cause sudden death. Patients with preexcitation syndromes with atrial fibrillation must not be administered an AV nodal blocking agent; these agents can further increase conduction via the accessory pathway, which increases the risk of ventricular fibrillation and death. [36, 37, 7, 33, 38, 8, 39]
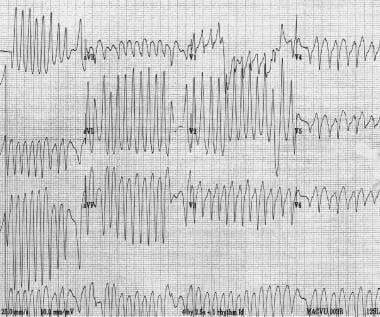 Atrial fibrillation in a patient with Wolff-Parkinson-White syndrome. Note the extremely rapid ventricular rate and variability in QRS morphology. Several minutes later, the patient developed ventricular fibrillation.
Atrial fibrillation in a patient with Wolff-Parkinson-White syndrome. Note the extremely rapid ventricular rate and variability in QRS morphology. Several minutes later, the patient developed ventricular fibrillation.
Junctional ectopic tachycardia and nonparoxysmal junctional tachycardia
JET and NPJT are rare; they presumably arise because of increased automaticity, triggered activity, or both. They are usually observed following valvular surgery, after myocardial infarction, during active rheumatic carditis, or with digoxin toxicity. These tachycardias are also observed in children following congenital heart surgery. Electrocardiographic findings include a regular narrow QRS complex, although P waves may not be visible. Patients with AV dissociation have also been described. [9, 40, 41]
Epidemiology
Occurrence in the United States
The incidence of paroxysmal SVT is approximately 1-3 cases per 1000 persons, with a prevalence of 0.2%. Atrial fibrillation is the most common, affecting 3 million people in the United States alone, with prevalence of 0.4-1% in the population. It is estimated that atrial fibrillation will affect more than 7.5 million people by 2050. [7, 8, 9, 10, 42, 43, 44]
AVNRT and AVRT
In a population-based study, the incidence of paroxysmal SVT was 35 cases per 100,000 person-years and peak incidence was in the middle age people. [45] AVNRT is more common in patients who are middle-aged or older, while adolescents are more likely to have SVT mediated by an accessory pathway like AVRT. [43, 46] The incidence rate of the WPW pattern on ECG tracings is 0.1-0.3% in the general population, although not all patients develop SVT. [7, 8, 9, 43]
Paroxysmal SVT is observed not only in healthy individuals; it is also common in patients with previous myocardial infarction, mitral valve prolapse, rheumatic heart disease, pericarditis, pneumonia, chronic lung disease, and current alcohol intoxication. [7, 8, 9, 10] Digoxin toxicity also may be associated with paroxysmal supraventricular tachycardia. [9, 10, 11]
Sex- and age-related demographics
Most series of catheter ablation reflect a higher proportion of female patients with AVNRT than male patients. This may reflect a true higher incidence in women, or it may reflect the sample of patients who are referred (or choose) to undergo extensive evaluation and/or catheter ablation. In a population-based study, the risk of developing paroxysmal SVT was twice as high in women as it was in men. [45] However, the prevalence of atrial fibrillation is the same in men and women. [47]
The prevalence of paroxysmal SVT increases with age. AVNRT is seen more commonly in persons who are middle aged or older, while adolescents usually have SVT from an accessory pathway. [45] The relative frequency of tachycardia mediated by an accessory pathway decreases with age.
Prognosis
Patients with symptomatic Wolff-Parkinson-White Syndrome (WPW) syndrome have a small risk of sudden death. Otherwise, prognosis in paroxysmal SVT is dependent on any underlying structural heart disease; patients with a structurally normal heart have an excellent prognosis.
Morbidity and mortality
Paroxysmal SVT may start suddenly and last anywhere from seconds to days. Patients may or may not be symptomatic, depending on their hemodynamic reserve, heart rate, the duration of the paroxysmal SVT, and coexisting diseases.
Paroxysmal SVT can result in heart failure, pulmonary edema, myocardial ischemia, and/or myocardial infarction secondary to an increased heart rate in patients with poor left ventricular function. [9, 10, 11] In fact, one study found that one third of patients with SVT experienced syncope or required cardioversion. [48] Incessant SVT can cause tachycardia-induced cardiomyopathy.
Patients with WPW syndrome may be at risk for cardiac arrest if they develop atrial fibrillation or atrial flutter in the presence of a rapidly conducting accessory pathway (ie, a pathway with a short anterograde refractory period).
Extremely rapid ventricular rates during atrial fibrillation or atrial flutter can cause deterioration to ventricular fibrillation. This complication is unusual and occurs primarily in patients who have had prior symptoms due to WPW syndrome. Sudden death may be the initial presentation of WPW syndrome, but how often this occurs is unclear.
In the absence of manifest preexcitation (ie, WPW syndrome), the risk of sudden death with paroxysmal SVT is extremely small.
A study by Bánhidy et al of 252 pregnant women indicated that the presence of paroxysmal SVT in the second and/or third gestational month of pregnancy increases the risk that the resulting child will have a secundum atrial septal defect. [49]
-
Sinus tachycardia. Note that the QRS complexes are narrow and regular. The patient's heart rate is approximately 135 bpm. P waves are normal in morphology.
-
Atrial tachycardia. The patient's heart rate is 151 bpm. P waves are upright in lead V1.
-
Multifocal atrial tachycardia. Note the different P-wave morphologies and irregularly irregular ventricular response.
-
Atrial flutter. The patient's heart rate is approximately 135 bpm with 2:1 conduction. Note the sawtooth pattern formed by the flutter waves.
-
Atrial fibrillation. The patient's ventricular rate varies from 130-168 bpm. The rhythm is irregularly irregular. P waves are not discernible.
-
Atrioventricular nodal reentrant tachycardia. The patient's heart rate is approximately 146 bpm with a normal axis. Note the pseudo S waves in leads II, III, and aVF. Also note the pseudo R' waves in V1 and aVR. These deflections represent retrograde atrial activation.
-
Same patient as in the previous image. The patient is in sinus rhythm following atrioventricular nodal reentrant tachycardia.
-
Image A displays the slow pathway and the fast pathway, with a regular impulse being conducted through the atrioventricular node. Image B displays a premature impulse that is conducted in an anterograde manner through the slow pathway and in a retrograde manner through the fast pathway, as is seen in typical atrioventricular nodal tachycardia. Image C displays the premature impulse conducting in a retrograde manner through the pathway and the impulse reentering the pathway with anterograde conduction, which is seen commonly in patients with atypical atrioventricular nodal tachycardia.
-
Wolff-Parkinson-White pattern. Note the short PR interval and slurred upstroke (delta wave) to the QRS complexes.
-
The left image displays the atrioventricular node with the accessory pathway. The impulse is conducted in an anterograde manner in the atrioventricular node and in a retrograde manner in the accessory pathway. This circuit is known as orthodromic atrioventricular reentrant tachycardia and can occur in patients with concealed accessory tracts or Wolff-Parkinson-White syndrome. The right image displays the impulse being conducted in an anterograde manner through the accessory pathway and in a retrograde manner via the atrioventricular node. This type of circuit is known as antidromic atrioventricular reentrant tachycardia and occurs only in patients with Wolff-Parkinson-White syndrome. Both patterns may display retrograde P waves after the QRS complexes.
-
Orthodromic atrioventricular reentrant tachycardia. This patient has Wolff-Parkinson-White syndrome.
-
The left panel depicts antidromic atrioventricular reentrant tachycardia. The right panel depicts sinus rhythm in a patient with antidromic atrioventricular reentrant tachycardia. Note that the QRS complex is an exaggeration of the delta wave during sinus rhythm.
-
Atrial fibrillation in a patient with Wolff-Parkinson-White syndrome. Note the extremely rapid ventricular rate and variability in QRS morphology. Several minutes later, the patient developed ventricular fibrillation.