Background
Parainfluenza viruses (PIVs) are paramyxoviruses of the order Mononegavirales, the family Paramyxoviridae, and the subfamily Paramyxovirinae. Human PIVs (HPIVs) are currently divided into 5 serotypes—HPIV-1, HPIV-2, HPIV-3, HPIV-4a, and HPIV-4b—in 2 different genera: Respirovirus (HPIV-1 and HPIV-3) and Rubulavirus (HPIV-2 and HPIV-4). HPIV is closely related to Hendra virus, Nipah virus, and metapneumoviruses. [1]
HPIVs primarily affect young children, in whom the pathogenic spectrum includes upper and lower respiratory tract infections. They are responsible for 30-40% of all acute respiratory tract infections in infants and children. These conditions include common cold with fever, laryngotracheobronchitis (croup), bronchiolitis, and pneumonia. HPIVs are an important cause of outbreaks in daycare facilities, pediatric wards nursing homes and other institutional settings. [38] HPIVs are also a cause of community-acquired respiratory tract infections of variable severity in adults.
HPIV-1 is most commonly associated with croup. HPIV-2 is also associated with croup. HPIV-3 is second only to respiratory syncytial virus (RSV) as a cause of pneumonia and bronchiolitis in infants and young children. HPIV-3 is the most commonly isolated serotype worldwide. [38] HPIV-4 has ubiquitous distribution but is detected in patients less often due to difficulty growing them in cell cultures. HPIV-4 causes less severe disease hence is not included in many of the respiratory panel. [39] Reinfection with HPIV can occur throughout life, with elderly and immunocompromised persons being at a greater risk for serious complications of infections.
The seasonal patterns of HPIV-1, HPIV-2, and HPIV-3 are curiously interactive. HPIV-1 causes the largest, most defined outbreaks, which are marked by sharp biennial rises in croup cases in the autumn of odd-numbered years. Outbreaks of infection with HPIV-2, although erratic, usually follow HPIV-1 outbreaks. Outbreaks of HPIV-3 infections occur yearly, mainly in spring and summer, and last longer than outbreaks of HPIV-1 and HPIV-2. Because HPIV-4 is infrequently isolated, infection with this pathogen is less well characterized. [2] The HPIV-3 is the most virulent of the HPIVs and is associated with significant morbidity and mortality. [3]
Patients with HPIV infection typically present with a history of coryza and low-grade fever; they then develop the classic barking cough associated with croup. On physical examination, HPIV infection is associated with a broad range of findings, which may include fever, nasal congestion, pharyngeal erythema, nonproductive to minimally productive cough, inspiratory stridor, rhonchi, rales, and wheezing. Systemic flulike symptoms are not common in HPIV-infected patients, but adult patients more frequently present with flulike symptoms compared with children. [4]
Supportive care is mandatory. Oxygen mist is often helpful. Corticosteroids and nebulizers may be used to treat respiratory symptoms and to help reduce the inflammation and airway edema of croup. Antiviral agents are of uncertain benefit; antibiotics are used only if bacterial complications (eg, otitis and sinusitis) develop.
Pathophysiology
HPIVs can infect many different animals both naturally and under experimental conditions. Asymptomatic infection can be induced in hamsters, guinea pigs, and adult ferrets by all 5 serotypes of HPIV (HPIV-1, HPIV-2, HPIV-3, HPIV-4a, and HPIV-4b). Chimpanzees, macaques, squirrels, owls, and rhesus monkeys have been asymptomatically infected with HPIV-3 or HPIV-4, and only marmosets have developed symptomatic upper respiratory tract infections (URTIs) with HPIV-3 and Sendai virus.
Newcastle disease virus is a rubulavirus that infects poultry, penguins, and other birds and has been responsible for conjunctivitis in bird handlers and laboratory workers. There have been reports of human infections by some of the other nonhuman PIVs, but these have not been well established.
HPIV transmission occurs via direct inoculation of contagious secretions from the hands or via large-particle aerosols into the eyes and nose. Prolonged survival of HPIV on skin, cloth, and other objects emphasizes the importance of fomites in nosocomial spread. Ciliated respiratory epithelium appears to be the major site of virus binding and subsequent infection.
The infection is initiated in the upper respiratory tract (nose, oropharynx) and descends to lower respiratory tract within 2-5 days.
Previous exposure to the infecting serotype, viral load in upper respiratory tract and genetic predisposition determines the progression of the infection to lower respiratory tract. [41]
The viruses attach to the host cells through hemagglutinin, which specifically combines with neuraminic acid receptors in the host cells. Subsequently, the viruses enter the cell via fusion with the cell membrane mediated by F1 and F2 receptors. Absence of extensive cytopathic changes in infected cells in in-vitro studies is strongly suggestive of significant contribution of host immune response in the pathogenesis. [41]
When HPIV infects a cell, the first observable morphologic changes may include focal rounding and growing of the cytoplasm and nucleus and decreased host-cell mitotic activity. Other observable changes include single or multilocular cytoplasmic vacuoles, basophilic or eosinophilic inclusions, and formation of multinucleated giant cells. These giant cells (fusion cells) usually develop late in the infection, and each giant cell contains between 2 and 7 nuclei.
Viral replication starts with the fusion of the virus and the host cell lipid membranes, followed by the expulsion of the HPIV nucleocapsid into the cytoplasm of the cell. In the cytoplasm, transcription takes place by the help of virus-specific RNA-dependent RNA polymerase. Viral mRNAs are then translated into viral proteins by the cellular ribosomes.
There is full-length replication of the virus genome, first into a positive-sense RNA strand and then into the appropriate negative strand. Once produced, these single negative-sense strands of RNA then are encapsidated with nucleoprotein and may be used in further rounds of transcription and replication or may be packaged for export as a new virion.
The pathogenicity also depends on the accessory proteins being present with the anti-interferon (IFN) properties. [5] The dynamics of primary infection depend on the mode of transmission. In a study, it was observed that following contact transmission of HPIV, the virus grew in large numbers in the upper respiratory tract and later spread minimally to the lungs, while following airborne transmission, the virus predominated in the trachea with dissemination throughout the respiratory tract and extensive involvement of the lungs. [6]
For cytoplasmic RNA viruses such as HPIVs, viral RNA synthesis is the most potent stimulus for innate immune response. C ORF of HPIVs encodes certain proteins that prevent IFN induction, IFN signaling, and apoptosis. In a similar manner but in varying intensities among the different PIVs, these mechanisms inhibit the innate immune response. [7]
Young age and lack of prior exposure to the infecting HPIV type are two major factors associated with HPIV lower respiratory tract infection (LRTI). Young infants are at greater risk in part because of their smaller airways, which are more susceptible to obstruction, and in part because their immune response is muted owing to immunologic immaturity and pre-existing maternal antibodies, which may suppress the immune response. [7]
HPIV is affected by the external environmental conditions (eg, temperature, humidity, and pH). Above 37°C, viral survival decreases significantly, and above 50°C, HPIV is inactivated within 15 minutes. The survival of myxoviruses at room temperature has shown considerable survival variability by decreasing titers by more than 50% in as little as 2 hours or as long as 1 week. HPIV have their greatest stability at 4°C or when frozen (eg, –70°C).
Mechanisms of airway inflammation
HPIV infection in the respiratory tract leads to secretion of high levels of inflammatory cytokines such as interferon alfa, interleukin (IL)–2, IL-6, and tumor necrosis factor (TNF)-α. The peak duration of secretion is 7 to 10 days after initial exposure.
Increasing levels of certain chemokines such as RANTES (regulated upon activation, normal T-cell expressed and secreted) and macrophage inflammatory protein (MIP)–K are detected in the nasal secretions of pediatric patients. These are responsible for pathologic changes in the respiratory tract and clinical manifestations of this condition.
Studies have shown a possible role of virus-specific immunoglobulin E (IgE) antibodies earlier and in larger amounts in patients with HPIV URTI than in age-matched controls. Faster and increased production of this virus-specific IgE mediates histamine release in the trachea and the subglottic region, in turn producing croup. [8]
The chief pathologic features include airway inflammation, necrosis and sloughing of respiratory epithelium, edema, excessive mucus production, and interstitial infiltration of the lung. Edema of the mucus layer causes swelling in the vocal cords, larynx, trachea, and bronchi. These changes lead to obstruction of the airway inflow and subsequent stridor, which is characteristic of croup.
Microarray analysis of epithelial cells infected with HPIVs has indicated a role for NF-Kb, IRF3, and type 1 IFN pathways in the regulation of cellular antiviral and anti-inflammatory response. HPIV C protein plays an important role in the suppression of these immune responses. Elevated nasal wash concentrations of inflammatory cytokines IL-8/CXCL8, MIP1α+1β/CCL3+4, RANTES/CCL5, and CXCL9, have been described in children with parainfluenza virus disease. CXCR3 ligands such as IP-10 and I-TAC, which attract activated Th1 cells, are dominant chemokines observed during HPIV infection. High concentrations of IL-8 and IP-10 have been correlated with severe HPIV infection. [7]
In animal models, increased levels of histamine and eosinophils are detected in bronchoalveolar lavage (BAL) samples after infection with HPIV, suggesting a state of hyperresponsiveness of the respiratory tract.
HPIV-2 and HPIV-3 infection in humans is known to induce expression of intercellular adhesion molecule-1 (ICAM-1) in tracheal and other cells of the respiratory tract. These molecules serve as receptors for rhinoviruses, thus paving the way for rhinoviral superinfection.
The virus continues to be excreted in respiratory exudates for 3 to 16 days after primary infection and 1 to 4 days after infection.
Immune response
Host defense against HPIVs is mediated largely by humoral immunity to both surface glycol proteins of the virus, which are most immunogenic: hemagglutinin-neuraminidase (HN) and fusion (F). Most children are born with neutralizing antibodies to all 5 of the HPIV serotypes due to transfer of maternal IgG, [41] but these titers quickly fall during the first 6 months of life.
Most antibody response appears to involve serum immunoglobulin G1 (IgG1), but levels of serum immunoglobulin G3 (IgG3), immunoglobulin G4 (IgG4), serum immunoglobulin A (IgA), and immunoglobulin M (IgM) rise significantly in 30% of adults. Long-term protection is provided by neutralizing antibodies. Secretory IgA plays an important but not fully defined role in the protection against natural HPIV infections. Secretory IgA provides short-term protection against reinfection however duration of protection increases with two or more infections. Cellular immunity contributes to the inhibition of viral replication and helps to prevent primary HPIV infection. Cellular immunity provides short term protection against reinfection. Frequent upper respiratory infections are common as long-term resistance is higher in the upper respiratory tract than the lower respiratory tract. [41]
A cell-mediated immune response to HPIV antigens, in addition to an HPIV-specific IgE response, has been documented to be greater among infants with HPIV bronchiolitis than among infants who developed only upper respiratory tract illness.
After natural infection with HPIV, most children and adults develop measurable levels of these antibodies in the serum; these antibodies have been shown to be correlated with disease prevention and amelioration in adults. Local interferon production has been noted in about 30% of children with HPIV infection.
Although immunity to HPIV infection is long-lasting, reinfection may occur many times throughout life and at variable intervals, even in the presence of neutralizing antibodies. This cannot be explained solely by the relatively stable antigenic determinants of HPIVs; thus, more research is needed. Reinfections usually are milder and do not progress to acute lower respiratory infection. [41]
In a recent study involving mice models, the level of reinfection was noted to show an inverse correlation with the level of primary infection in the same tissue. In this study, it was observed that primary airborne transmission of the HPIV rendered protection from reinfection throughout the respiratory tract, whereas contact transmission of the HPIV resulted in protection from reinfection in the upper respiratory tract, with only partial protection in the lungs. [6]
HPIV infections tend to be more severe in individuals with defective cell-mediated immunity, indicating that T cells may have a greater role in containing the disease.
Etiology
HPIVs are pleomorphic viruses whose envelope is derived from the host cell they last infected. These viruses are 150-300 nm in diameter and possess a single-stranded, nonsegmented, negative-sense RNA genome with nucleoprotein P and L proteins. Noninfectious virions with positive RNA polarity have been reported. [9] The HPIV genome contains approximately 15,000 to 16,000 nucleotides, which are organized to encode at least 6 common structural proteins: the nucleocapsid protein (NP), the phosphoprotein (P), the matrix protein (M), the fusion glycoprotein (F), the hemagglutinin neuraminidase glycoprotein (HN), and RNA polymerase (L). [38] A “rule of six” has been coined for HPIV, with the advent of reverse genetics. This means that the most efficient replication and transcription of HPIV takes place when the genome is divisible by six, although exceptions have been found. [9]
A lipid bilayer covered with glycoprotein spikes surrounds a helical nucleocapsid that measures 12-17 nm in diameter (see the image below), and matrix protein resides between the core and the envelope. These glycoproteins are the HN and F proteins, which play a major role in the pathogenesis of the disease caused by HPIVs.
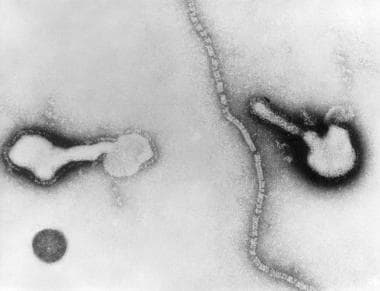 Transmission electron micrograph of parainfluenza virus. Two intact particles and free filamentous nucleocapsid.
Transmission electron micrograph of parainfluenza virus. Two intact particles and free filamentous nucleocapsid.
HPIVs belong to the order Mononegavirales, the family Paramyxoviridae, and the subfamily Paramyxovirinae. They currently comprise 5 serotypes—HPIV-1, HPIV-2, HPIV-3, HPIV-4a, and HPIV-4b—which display substantial serologic cross-reactivity. (The two serotypes of HPIV-4 are differentiated on the basis of hemadsorption inhibition pattern and monoclonal antibody reactivity. [10] ) Serologic and antigenic analysis of all of the species in the Paramyxovirinae subfamily demonstrates the following four basic genera, two of which include HPIVs:
-
Respirovirus – HPIV-1, HPIV-3, Sendai virus, and bovine PIV-3
-
Rubulavirus – HPIV-2, HPIV-4, mumps virus, and simian viruses 5 and 41
-
Morbillivirus – Measles virus and distemper virus
-
Megamyxovirus – Hendra virus and Nipah virus
The most common primary and secondary cell lines that support the growth of HPIV are LLC-MK2, Vero, HMV-II, HEp-2, MDCK, BHK, HeLa, primary human embryo, and HEF. Organ cultures from mice, guinea pigs, ferrets, and human fetal respiratory epithelium can also be used. When isolated for the first time from children with croup in 1935, these viruses were known as croup-associated viruses. [38]
The following clinical conditions are caused by the various HPIV types:
-
Croup – HPIV-1, HPIV-2, HPIV-3
-
Bronchitis – HPIV-1, HPIV-3
-
Bronchopneumonia – HPIV-1, HPIV-3
-
Minor URTI – HPIV-1, HPIV-3, HPIV-4
-
Pneumonia and bronchiolitis – HPIV-1, HPIV-3
Respiratory secretions from infected humans are the source of infection. Transmission is via respiratory droplets or via direct person-to-person contact with infected secretions or fomites; the virus can survive in aerosols for over an hour. The inoculating dose is very small. The incubation period for HPIVs ranges from 1 to 7 days.
HPIVs are common community-acquired respiratory pathogens without ethnic, socioeconomic, gender, age, or geographic boundaries. Many factors have been found that predispose individuals to these infections, including the following:
-
Malnutrition
-
Overcrowding
-
Vitamin A deficiency
-
Lack of breastfeeding
-
Environmental smoke or toxins
-
Infants without pneumococcal vaccination [38]
Epidemiology
United States statistics
Infections with HPIV-1 and HPIV-2 occur during autumn months. Infections with HPIV-3 occur throughout the year but appear to peak in the spring. HPIV-3 is the second most common cause of lower respiratory tract infections (LRTIs) treated in the United States, second only to RSV. HPIV-4 infection patterns are not well defined.
HPIV-3 infections occur earliest and most frequently. According to seroepidemiologic studies, 50% of US children aged 1 year and almost all US children aged 6 years have been infected by HPIV-3. Antibodies against HPIV-1 and HPIV-2 develop less rapidly, but 80% of children have antibodies against these types by age 10 years. Although HPIV-4 induces few clinical illnesses, infections with this serotype are apparently common: 70-80% of children aged 10 years have antibodies against HPIV-4.
A 2016 study of surveillance data collected over 10 years concerning estimates of parainfluenza virus–associated hospitalizations in children younger than 5 years revealed the cyclic pattern of HPIV circulation in the United States. HPIV-1 circulation was observed every other year, whereas HPIV-2 and HPIV-3 circulated annually. The study also analyzed the percentage of HPIV-associated manifestations in hospitalized children younger than 5 years. Of the bronchiolitis-associated admissions, 3.2% were associated with HPIV, with the highest percentage seen in children aged 1-2 years. Most hospitalizations were associated with HPIV-3 infection. Among croup-associated hospitalizations, HPIV was isolated in 46.6% of cases; HPIV-1 accounted for most cases (26.3%). HPIV was associated with 5.5% of hospitalizations due to pneumonia, with HPIV-3 being the most common HPIV isolate. HPIV-1 followed a biennial pattern, with circulation beginning to rise between May and July, peaking in September or October, and decreasing between December and January of odd-numbered years. HPIV-3 circulated every year, with proportions of detections rising in March and peaking between April and July. Circulation of HPIV-2 was less defined than that of HPIV-1 or HPIV-3, but highest activity was seen between August and January. [11]
International statistics
Internationally, HPIV-1, HPIV-2, HPIV-3, and HPIV-4 have a worldwide distribution, and epidemics are known to occur, particularly with HPIV-1.
Parainfluenza viruses are responsible for disease throughout the year, but winter outbreaks of respiratory tract infections, especially croup, in children throughout the temperate zones of the northern and southern hemispheres represent peak periods of prevalence. Most infections are endemic, but sharp small epidemics involving HPIV-1 and HPIV-2 occasionally occur.
The first reported outbreak of HPIV-4 infection occurred in Hong Kong in the autumn of 2004, involving 38 institutionalized children and 3 staff members during a 3-week period in a developmental disabilities unit. [10] For the influenzalike illnesses reported, the main etiologic agents in the early epidemic period were noninfluenza viruses, and among these noninfluenza viruses, HPIV accounted for about 24% of the infections. [12] In a study from Southern China, seasonal peaks due to HPIV-3 and HPIV-1 were observed during autumn, whereas the HPIV-2 and HPIV-4 were detected less frequently, with their incidence increasing with the decline in the frequency of HPIV-3 and HPIV-1. [4]
A 15-year analysis of HPIV from England and Wales revealed that most HPIV isolates detected were HPIV-3; detection was seen throughout the year but peaked between March and June. HPIV-1 and HPIV-2 circulated in low numbers, with a relative peak in the last quarter of the year and a biennial cycle. [13]
Sentinel Surveillance for Severe Acute Respiratory tract Infection (SARI) data from the Mediterranean region revealed HPIV isolations of 1.7% for HPIV-1, 0.9% for HPIV-2, and 3.9% for HPIV-3. [14]
A study on community-acquired pneumonia from Italy using the novel Luminex technology revealed that HPIV-related pneumonia accounted for 11% of cases, with most (7%) caused by HPIV-4. [15]
Age-, sex-, and race-related demographics
HPIVs are ubiquitous and infect most people during childhood. The highest rates of serious HPIV illnesses occur among young children.
HPIV-1 can cause LRTIs in young infants but is rare in those younger than 1 month due to maternal antibody protection. [38] However, recently an outbreak of HPIV-3 infection among 6 preterm infants was reported in a neonatal nursery. [16] The full burden of HPIV-1 in adults and elderly persons has not been determined, but studies have shown that this virus causes yearly hospitalizations in healthy adults and may play a role in bacterial pneumonias and death in nursing-home residents.
HPIV-2 accounts for 60% of all infections that develop in children younger than 5 years, with a peak incidence between ages 1 and 2 years. Young infants (< 6 months) are particularly vulnerable to infection with HPIV-3. Unlike other HPIV infections, 40% of HPIV-3 infections occur in the first year of life.
HPIVs have minor predilection for either sex or race. PIV-associated bronchiolitis reportedly occurs more often in non-White males [38] . However, a recent study has shown that HPIVs were more commonly isolated from male patients than females. [4]
Prognosis
Approximately 41,000 individuals are admitted to the hospital for parainfluenza virus infections each year. Precautions are necessary within hospitals to prevent further spread. [17] Only 1-5% of patients admitted to the hospital need artificial airway support.
HPIV infections in older children and adults generally are mild. Occasionally, bronchiolitis or viral pneumonia in children and tracheobronchitis in adults has been reported. Generally, pediatric patients with parainfluenza infections do well, with symptoms typically resolving in 7 to 10 days.
On occasion, the infection spreads to the lower respiratory tract, causing bronchiolitis or viral pneumonia. Denudation of respiratory epithelium places patients at a slightly increased risk for bacterial superinfection. Evaluate any patient recovering from croup who deteriorates suddenly for possible bacterial tracheitis.
In developed countries, mortality induced by HPIV is unusual, occurring almost exclusively in young infants or immunocompromised or elderly people. In developing countries, however, the preschool population is at considerable risk for HPIV-induced death. Whether because of primary viral disease or because of the facilitation of secondary bacterial infections in malnourished children, LRTI causes 25-30% of the death in this age group, and HPIV causes at least 10% of LRTIs.
It was reported that detection of the HPIV in lungs of infected patients was associated with worse outcomes than viral detection in the upper respiratory tract samples alone. This suggests that viral detection in the lungs of infected patients can be used to predict poor outcome. [18]
-
Transmission electron micrograph of parainfluenza virus. Two intact particles and free filamentous nucleocapsid.