Practice Essentials
Vascular birthmarks [1] encompass a group of vascular disorders that for a long time included conditions of various presentation and etiology. In 1996, the Workshop of the International Society for the Study of Vascular Anomalies (ISSVA) presented a new classification of this vast group of conditions, which divided them into vascular tumors and vascular malformations. [2, 3] This allowed more systematic classification and management with less confusion in diagnosis of these often complex lesions. The ISSVA classification of vascular anomalies has subsequently been modified and refined. [4]
Arteriovenous fistula (AVF), by definition, describes an abnormal communication between an artery and a vein. These communications are congenital; can occur at any point in the vascular system; and vary in size, length, location, and number. AVF is a term reserved for a singular communication between an artery and a vein that usually has an acquired etiology.
Most vascular tumors can be observed through their typical phases of development until they involute. Children should be evaluated for the extent of the tumors and involvement of vital structures.
Lesions in endangering locations are best treated with corticosteroids, interferon alfa, laser ablation, and embolization therapy. Most AVMs can be medically managed and controlled; only a few demonstrate progressive growth and warrant surgical intervention. Indications for surgical intervention of vascular malformations include hemorrhage, painful ischemia, congestive heart failure, nonhealing ulcers, functional impairment, and limb-length inequality.
Promising advances are being made in the diagnosis and treatment of AVMs. These advances provide hope to patients who are affected by these sometimes debilitating conditions.
Pathophysiology
In the classification of vascular anomalies currently employed, a division is made between tumors and malformations. [4] The first group is characterized by high turnover of endothelium, whereas the second group is characterized by the presence of dysmorphogenesis with no evidence of abnormal epithelial turnover.
Vascular tumors
The most common vascular tumor is infantile hemangioma. It is seen shortly after birth, is more common in girls and in Whites, and is congenital. These tumors are usually solitary but not infrequently may be multiple and involve the liver, the gastrointestinal (GI) tract, and the central nervous system (CNS). They are characterized by three phases of growth, as follows [5, 6] :
-
Proliferation
-
Quiescence or plateau
-
Involution
In the proliferating phase, tumors typically grow rapidly, are bright red or bluish in color, and are firm and tense. They may develop surface ulcerations and episodes of bleeding.
The involuting phase usually begins at the end of first year of life and is characterized by decreased tempo of the tumor growth, with the gradual color fading from the center of the mass and less tense consistency.
Transition to the involuted phase occurs during the second half of the first decade of life. Normal skin is restored in half of patients, and persistent skin changes such as telangiectasias, skin thinning and scarring may occur.
Other congenital tumors include tufted angioma, congenital hemangioma, and spindle cell hemangioma. [4]
Vascular malformations
Generally, congenital vascular malformations are inborn errors in embryologic development. Woollard described the development of the vascular system in three stages, as follows:
-
First stage - Condensation of undifferentiated cells into capillary blood spaces
-
Second stage - Formation of a retiform plexus in which blood flows from an arterial to a venous side; the channels in the retiform plexus combine and regress to form the vessels in the vascular system
-
Third stage - Development of axial arteries in the limb buds
The final product is a complex interplay among genetic, hormonal, biochemical, and chemical factors. Developmental arrest can occur at any point during vessel formation, creating different types of vascular malformations. The exact cause for the arrest is not entirely known. All AVMs are present at birth, but they are not always clinically evident. Stimuli during puberty or pregnancy or following minor trauma can precipitate clinical features of the malformation.
Vascular malformations are characterized by normal epithelial turnover and are usually sporadic; however, some of them have been linked to genetic disorders. They can be subdivided according to the type and size of vascular bed involved, as well as the rate of flow through them.
Capillary malformations
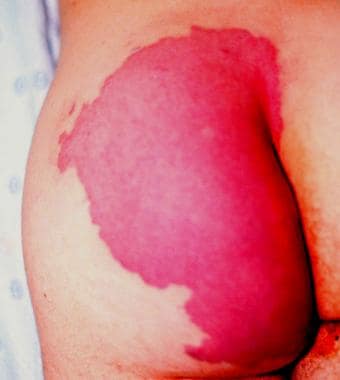
Capillary malformations are usually sporadic; they can be divided into slow-flowing Klippel-Trenaunay, Maffucci, and Proteus syndromes and fast-flowing Parkes Weber and Bannayan-Riley-Ruvalcaba syndromes. These malformations are frequently associated with dilated capillary vessels in the dermis, asymmetric overgrowth of the involved limbs, and sometimes multiple soft-tissue tumors, abnormal development of venous and arterial systems, and the presence of port-wine stains (see the image below).
Lymphatic malformations
Lymphatic malformations are usually related to genomic mutations and include Milroy disease, Meige syndrome, yellow-nail syndrome, and Noonan syndrome. They are usually characterized by abnormal development of different portions of the lymphatic system.
Venous malformations
Venous malformations are the most common vascular malformations. They are usually sporadic and may be present at birth but are not always clinically obvious; they predominantly occur in the skin and soft tissue (see Presentation). These malformations include glomuvenous malformation and blue rubber bleb nevus syndrome.
Arteriovenous malformations
AVMs have the presence of arteriovenous shunts in multiple capillary beds both on the skin and involving internal organs. They are usually accompanied by a bruit and hyperemia with prominent venous outflow (see Presentation). They are represented by hereditary hemorrhagic telangiectasia (HHT) syndrome, [7] also known as Rendu-Osler-Weber disease.
Although the pathogenetic mechanisms of AVMs are not completely understood, the hemodynamic alterations that lead to the clinical manifestations of AVMs have been described well.
Mulliken described a system of classifying AVMs on the basis of structural criteria, as follows:
-
Truncal AVMs - These are usually hemodynamically active and tend to present in the upper limb, head and neck, and pelvis; the lesions are localized and composed of a mass of enlarged vessels
-
Diffuse AVMs - These are usually found in the limbs, more frequently in the lower limbs than in the upper; in contrast to the truncal type, the connections are small and numerous; they are hemodynamically less active
-
Localized AVMs - These are usually inconsequential hemodynamically because of higher resistance in the connections; the lesions are composed mainly of abnormal intercalated tissues, not masses of enlarged vessels
Any structural type of AVM can be hemodynamically active or stable.
Arteriovenous fistulas
An abnormal communication causes shunting of blood from the high-pressure arterial side to the low-pressure venous side. This creates an abnormal low-resistance circuit that steals from the high-resistance normal capillary bed (see the image below).
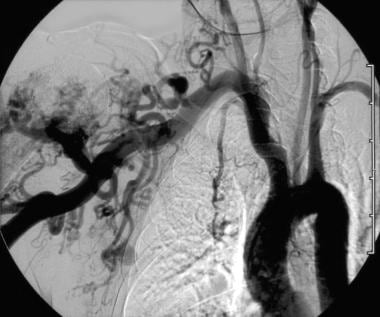 Hypertrophied subclavian artery resulting from low-resistance high-volume flow through an upper extremity arteriovenous malformation (AVM).
Hypertrophied subclavian artery resulting from low-resistance high-volume flow through an upper extremity arteriovenous malformation (AVM).
Blood follows the path of least resistance. Flow in the afferent artery and efferent vein increases, causing dilatation, thickening, and tortuosity of the vessels. If the resistance in the fistula is low enough, the fistulous tract steals from the distal arterial supply, actually causing a reversal of arterial flow in the segment distal to the AVF. This is known as a parasitic circulation. The parasitic circulation causes decreased arterial pressures in the distal capillary beds and can cause tissue ischemia.
The increased flow into the venous circulation does not necessarily cause higher venous pressures. However, it can cause vessel wall abnormalities, such as thickening of the media and fibrosis of the wall. These changes are known as arterialization.
The blood flow into the venous circulation causes turbulence, which is responsible for the palpable thrill. The thrill is dependent on the geometry of the fistula and does not represent volume of flow accurately.
In addition to the decreased distal arterial pressures, which might cause distal ischemia, peripheral venous pressures are increased, leading to swelling, visible veins (varicosities), and even ulcers in the limb.
The heart responds to the decreased peripheral vascular resistance by increasing stroke volume and cardiac output. This leads to tachycardia, left ventricular dilatation, and, eventually, heart failure.
Etiology
The majority of AVMs are developmental errors that occur between weeks 4 and 10 of embryogenesis. The factors that cause these errors are unknown. Potential exogenous causes, such as viral infections, toxins, and drugs, have been implicated but not yet proven. Almost all AVMs are sporadic and nonfamilial, though a few syndromes (eg, Sturge-Weber, Klippel-Trenaunay) include inherited vascular abnormalities.
The most common etiology for acquired AVFs is penetrating trauma. AVFs also can occur from nontraumatic causes, such as erosion of an aneurysm into a neighboring vein, or as a consequence of surgery performed for therapeutic purposes (eg, access for hemodialysis). [8]
Epidemiology
All AVMs are present at birth, but they are not always clinically evident. Stimuli during puberty or pregnancy or following minor trauma can precipitate clinical features of the malformation. [9] AVMs occur with equal frequency among males and females.
Prognosis
Many congenital AVMs/AVFs may regress spontaneously. The large ones, over the years, may lead to cardiac decompensation and death. In patients with acquired AVFs, death can occur from cardiac failure, infection (bacterial endocarditis), or rupture if the AVF is between a large artery and vein (like iliac or renal AVFs or aortocaval fistulas); however, the prognosis is excellent once the AVF is corrected.
Yakes et al obtained follow-up studies in 19 of 20 patients with AVMs treated with ethanol embolization. [10] All patients showed persistent occlusion of the malformation radiographically after as long as 24 months of follow-up. Widlus et al treated 11 patients with cyanoacrylate embolization. [11] During the 40-month follow-up period, 82% reported complete resolution of their symptoms and the remaining patients showed improvement. No patients reported worsening of their symptoms with embolization in these two series.
Do et al treated 12 patients for pelvic AVMs with combined endovascular and embolosclerotherapy. [12] During the follow-up period (median, 33.2 mo), 10 showed no residual lesion and complete symptomatic relief; two experienced partial remission.
Pearce reported experience in 15 patients with vascular malformations treated surgically. Five patients were lost to follow-up. On the assumption that those patients did well and did not seek further intervention, two thirds of those undergoing excision improved; 13% were unchanged after surgical excision, and 20% were worse.
Prompt recognition is important for outcome. Brinjikji et al reviewed spinal dural AVFs (lesions that are commonly missed on imaging or misdiagnosed) diagnosed at their institution between January 1, 2000, and November 1, 2014. [13] They found that delays in diagnosing these AVFs led to a high incidence of additional disability that frequently could not be reversed by surgical or endovascular treatment.
In a systematic review and meta-analysis (16 studies; N = 368), Batista et al found transvenous embolization of brain AVMs to be promising in terms of safety and effectiveness. [14] The rate of complete occlusion after the procedure was 91%, and the overall rate of good postdischarge outcomes was 89%. Only one patient died. The ischemic complication rate was 1%, the hemorrhagic complication rate was 6%, and the technical complication rate was 8%.
Patient Education
Physicians must be aware of the subtle signs of AVF in order to make the correct diagnosis. Prominent veins in the leg in a young individual following trauma, which may be mistakenly diagnosed as simple varicose veins, and rapid onset of heart failure in an otherwise healthy person, which may be diagnosed as cardiomyopathy, are examples for the need to look further for the presence of AVFs.
-
Buttock port-wine stain.
-
Lower extremity venous malformation.
-
Upper extremity arteriovenous malformation (AVM).
-
Hypertrophied subclavian artery resulting from low-resistance high-volume flow through an upper extremity arteriovenous malformation (AVM).
-
Hand angiogram demonstrating arteriovenous connections. Note the steal of blood from the fingertips.