Practice Essentials
Nephrolithiasis specifically refers to calculi in the kidneys, but renal calculi and ureteral calculi (ureterolithiasis) are often discussed in conjunction (see the images below). Ureteral calculi almost always originate in the kidneys, although they may continue to grow once they lodge in the ureter. The majority of renal calculi contain calcium. The pain generated by renal colic is primarily caused by dilation, stretching, and spasm because of the acute ureteral obstruction.
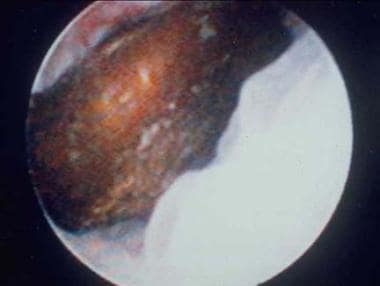 Distal ureteral stone observed through a small, rigid ureteroscope prior to ballistic lithotripsy and extraction. The small caliber and excellent optics of today's endoscopes greatly facilitate minimally invasive treatment of urinary stones.
Distal ureteral stone observed through a small, rigid ureteroscope prior to ballistic lithotripsy and extraction. The small caliber and excellent optics of today's endoscopes greatly facilitate minimally invasive treatment of urinary stones.
Signs and symptoms
The classic presentation for a patient with acute renal colic is the sudden onset of severe pain originating in the flank and radiating inferiorly and anteriorly; at least 50% of patients will also have nausea and vomiting. Patients with urinary calculi may report pain, infection, or hematuria. Patients with small, nonobstructing stones or those with staghorn calculi may be asymptomatic or experience moderate and easily controlled symptoms.
The location and characteristics of pain in nephrolithiasis include the following:
-
Stones obstructing ureteropelvic junction: Mild to severe deep flank pain without radiation to the groin; irritative voiding symptoms (eg, frequency, dysuria); suprapubic pain, urinary frequency/urgency, dysuria, stranguria, bowel symptoms
-
Stones within ureter: Abrupt onset of severe, colicky pain in the flank and ipsilateral lower abdomen; radiation to testicles or vulvar area; intense nausea with or without vomiting
-
Upper ureteral stones: Pain radiates to flank or lumbar areas
-
Midureteral calculi: Pain radiates anteriorly and caudally
-
Distal ureteral stones: Pain radiates into groin or testicle (men) or labia majora (women)
-
Stones passed into bladder: Mostly asymptomatic; rarely, positional urinary retention
See Presentation for more detail.
Diagnosis
The diagnosis of nephrolithiasis is often made on the basis of clinical symptoms alone, although confirmatory tests are usually performed.
Examination of patients with nephrolithiasis includes the following findings:
-
Dramatic costovertebral angle tenderness; pain can move to upper/lower abdominal quadrant with migration of ureteral stone
-
Generally unremarkable abdominal evaluation: Possibly hypoactive bowel sounds; usually, absence of peritoneal signs; possibly, painful testicles but normal-appearing
-
Constant body positional movements (eg, writhing, pacing)
-
Tachycardia
-
Hypertension
-
Microscopic hematuria
Testing
The European Association of Urology (EAU) recommends the following laboratory tests in all patients with an acute stone episode [1] :
-
Urinary sediment/dipstick test: To demonstrate blood cells, with a test for bacteriuria (nitrite) and urine culture in case of a positive reaction
-
Serum creatinine level: To measure renal function
Other laboratory tests that may be helpful include the following:
-
CBC with differential in febrile patients
-
Serum electrolyte assessment in vomiting patients (eg, sodium, potassium, calcium, phosphorus)
-
Serum and urinary pH level: May provide insight regarding patient’s renal function and type of calculus (eg, calcium oxalate, uric acid, cystine), respectively
-
Microscopic urinalysis
-
24-hour urine profile
Imaging studies
The following imaging studies are used in the evaluation of nephrolithiasis:
-
Noncontrast abdominopelvic CT scan: The imaging modality of choice for assessment of urinary tract disease, especially acute renal colic; can determine stone diameter and density
-
Renal ultrasonography: To determine presence of a renal stone and the presence of hydronephrosis or ureteral dilation; used alone or in combination with plain abdominal radiography
-
Plain abdominal radiograph (flat plate or KUB): To assess total stone burden, as well as size, shape, composition, location of urinary calculi; often used in conjunction with renal ultrasonography or CT scanning
-
IVP (urography) (historically, the criterion standard): For clear visualization of entire urinary system, identification of specific problematic stone among many pelvic calcifications, demonstration of affected and contralateral kidney function
-
Plain renal tomography: For monitoring a difficult-to-observe stone after therapy, clarifying stones not clearly detected or identified with other studies, finding small renal calculi, and determining number of renal calculi present before instituting a stone-prevention program
-
Retrograde pyelography: Most precise imaging method for determining the anatomy of the ureter and renal pelvis; for making definitive diagnosis of any ureteral calculus
-
Nuclear renal scanning: To objectively measure differential renal function, especially in a dilated system for which the degree of obstruction is in question; reasonable study in pregnant patients, in whom radiation exposure must be limited
See Workup for more detail.
Management
Pharmacotherapy and supportive care
Medical treatment of acute stone attacks (renal colic) involves supportive care and administration of agents such as the following:
-
IV hydration
-
NSAIDs (eg, ketorolac, ketorolac intranasal, ibuprofen)
-
Nonnarcotic analgesics (eg, acetaminophen [APAP])
-
PO/IV narcotic analgesics (eg, codeine, morphine sulfate, oxycodone/APAP, hydrocodone/APAP, dilaudid, fentanyl)
-
Alpha blockers (eg, tamsulosin, terazosin) to facilitate stone passage
-
Antiemetics (eg, metoclopramide, ondansetron)
-
Antibiotics (eg, ampicillin, gentamicin, trimethoprim-sulfamethoxazole, ciprofloxacin, levofloxacin, ofloxacin)
The following drug classes are used for stone prevention/chemolysis:
-
Uricosuric agents (eg, allopurinol)
-
Alkalinizing agents (eg, potassium citrate, sodium bicarbonate) - for uric acid and cysteine calculi
-
Thiazide diuretics - help treat hypercalcicuria
Surgical options
Stones that are 7 mm and larger are unlikely to pass spontaneously and require some type of surgical procedure, such as the following:
-
Stent placement
-
Percutaneous nephrostomy
-
Extracorporeal shockwave lithotripsy (ESWL)
-
Ureteroscopy
-
Percutaneous nephrostolithotomy (PCNL) or mini PNCL
-
Open nephrostomy - largely supplanted by less-invasive techniques
-
Anatrophic nephrolithotomy - for large. complex staghorn calculi that cannot be cleared by an acceptable number of PCNLs; typically done via laparoscopic or robotic approach
See Treatment and Medication for more detail.
Background
Nephrolithiasis is a common disease that affects 1 in 11 people in the United States. [2] Incidence and prevalence rates have been increasing over the last several decades. [3] Based on claims data from the Urologic Disease in America Project, costs associated with a diagnosis of nephrolithiasis in 2000 were estimated at $3,494 per individual, thereby resulting in a total direct cost of nephrolithiasis at $4.5 billion among the employed population. [4]
Urinary tract stone disease has been a part of the human condition for millennia; in fact, bladder and kidney stones have even been found in Egyptian mummies. Some of the earliest recorded medical texts and figures depict the treatment of urinary tract stone disease.
Acute renal colic is probably the most excruciatingly painful event a person can endure. Striking without warning, the pain is often described as being worse than childbirth, broken bones, gunshot wounds, burns, or surgery. Renal colic affects approximately 1.2 million people each year and accounts for approximately 1% of all hospital admissions.
Most active emergency departments (EDs) manage patients with acute renal colic every day, depending on the hospital’s patient population. Initial management consists of proper diagnosis, prompt initial treatment, and appropriate consultations, but concurrently, efforts should be directed towards patient education, including initial preventive therapy measures.
Although nephrolithiasis is not a common cause of renal failure, certain problems, such as preexisting azotemia and solitary functional kidneys, clearly present a higher risk of additional renal damage. Other high-risk factors include diabetes, struvite and/or staghorn calculi, and various hereditary diseases such as primary hyperoxaluria, Dent disease, cystinuria, and polycystic kidney disease. Spinal cord injuries and similar functional or anatomical urological anomalies also predispose patients with kidney stones to an increased risk of renal failure.
Recurrent obstruction, especially when associated with infection and tubular epithelial or renal interstitial cell damage from microcrystals, may activate the fibrogenic cascade, which is mainly responsible for the actual loss of functional renal parenchyma.
For other discussions on urolithiasis and nephrolithiasis, see Pediatric Urolithiasis, as well as Imaging Urinary Calculi, Hypercalciuria, Hyperoxaluria, and Hypocitraturia.
Anatomy
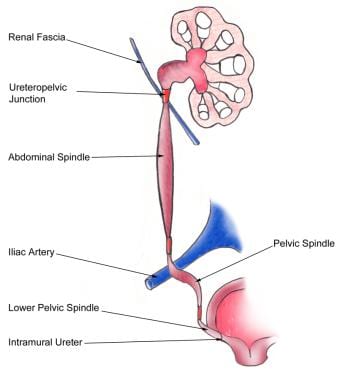
The basic anatomy of the ureter is as follows (see the image below).
Most of the pain receptors of the upper urinary tract responsible for the perception of renal colic are located submucosally in the renal pelvis, calices, renal capsule, and upper ureter. Acute distention seems to be more important in the development of the pain of acute renal colic than spasm, local irritation, or ureteral hyperperistalsis.
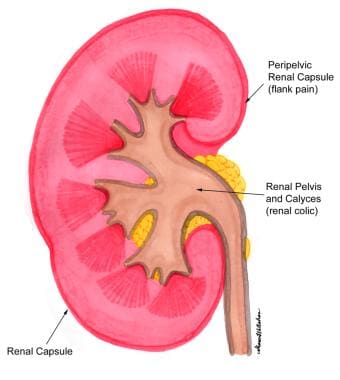
Stimulation of the peripelvic renal capsule causes flank pain, while stimulation of the renal pelvis and calices causes typical renal colic (see the image below). Mucosal irritation can be sensed in the renal pelvis to some degree by chemoreceptors, but this irritation is thought to play only a minor role in the perception of renal or ureteral colic.
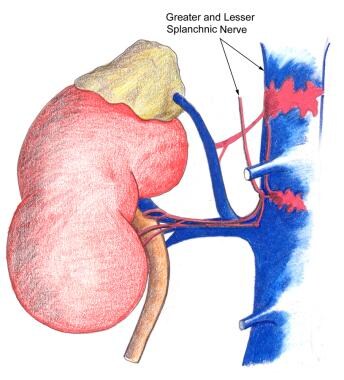
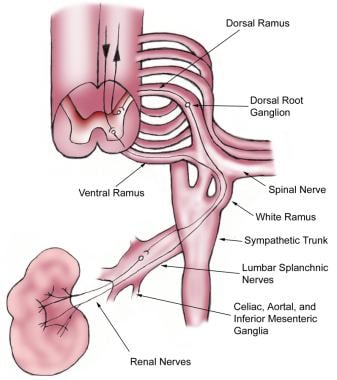
Renal pain fibers are primarily preganglionic sympathetic nerves that reach spinal cord levels T-11 to L-2 through the dorsal nerve roots (see the images below). Aortorenal, celiac, and inferior mesenteric ganglia are also involved. Spinal transmission of renal pain signals occurs primarily through the ascending spinothalamic tracts.
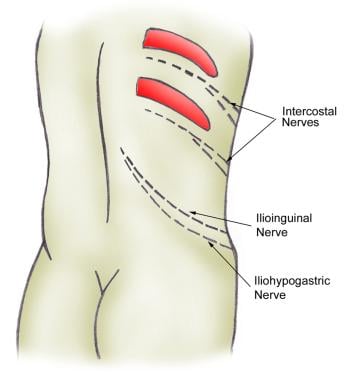
In the lower ureter, pain signals are also distributed through the genitofemoral and ilioinguinal nerves (see the image below). The nervi erigentes, which innervate the intramural ureter and bladder, are responsible for some of the bladder symptoms that often accompany an intramural ureteral calculus.
Pathophysiology
Formation of stones
Urinary tract stone disease is likely caused by two basic phenomena. The first phenomenon is supersaturation of the urine by stone-forming constituents, including calcium, oxalate, and uric acid. Crystals or foreign bodies can act as nidi, upon which ions from the supersaturated urine form microscopic crystalline structures. The resulting calculi give rise to symptoms when they become impacted within the ureter as they pass toward the urinary bladder.
The overwhelming majority of renal calculi contain calcium. Uric acid calculi and crystals of uric acid, with or without other contaminating ions, comprise the bulk of the remaining minority. Other, less frequent stone types include cystine, ammonium acid urate, xanthine, dihydroxyadenine, and various rare stones related to precipitation of medications in the urinary tract. Supersaturation of the urine is likely the underlying cause of uric and cystine stones, but calcium-based stones (especially calcium oxalate stones) may have a more complex etiology.
The second phenomenon, which is most likely responsible for calcium oxalate stones, is deposition of stone material on a renal papillary calcium phosphate nidus, typically a Randall plaque (which always consists of calcium phosphate). Evan et al proposed this model based on evidence accumulating from several laboratories. [5]
Calcium phosphate precipitates in the basement membrane of the thin loops of Henle, erodes into the interstitium, and then accumulates in the subepithelial space of the renal papilla. The subepithelial deposits, which have long been known as Randall plaques, eventually erode through the papillary urothelium. Stone matrix, calcium phosphate, and calcium oxalate gradually deposit on the substrate to create a urinary calculus.
Development of renal colic pain and renal damage
The colicky-type pain known as renal colic usually begins in the upper lateral midback over the costovertebral angle and occasionally subcostally. It radiates inferiorly and anteriorly toward the groin. The pain generated by renal colic is primarily caused by the dilation, stretching, and spasm caused by the acute ureteral obstruction. (When a severe but chronic obstruction develops, as in some types of cancer, it is usually painless.)
In the ureter, an increase in proximal peristalsis through activation of intrinsic ureteral pacemakers may contribute to the perception of pain. Muscle spasm, increased proximal peristalsis, local inflammation, irritation, and edema at the site of obstruction may contribute to the development of pain through chemoreceptor activation and stretching of submucosal free nerve endings.
The term "renal colic" is actually a misnomer, because this pain tends to remain constant, whereas intestinal or biliary colic is usually somewhat intermittent and often comes in waves. The pattern of the pain depends on the individual’s pain threshold and perception and on the speed and degree of the changes in hydrostatic pressure within the proximal ureter and renal pelvis. Ureteral peristalsis, stone migration, and tilting or twisting of the stone with subsequent intermittent obstructions may cause exacerbation or renewal of the renal colic pain.
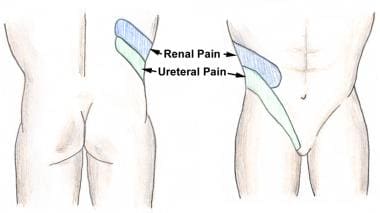
The severity of the pain depends on the degree and site of the obstruction, not on the size of the stone. A patient can often point to the site of maximum tenderness, which is likely to be the site of the ureteral obstruction (see the image below).
A stone moving down the ureter and causing only intermittent obstruction actually may be more painful than a stone that is motionless. A constant obstruction, even if high grade, allows for various autoregulatory mechanisms and reflexes, interstitial renal edema, and pyelolymphatic and pyelovenous backflow to help diminish the renal pelvic hydrostatic pressure, which gradually helps reduce the pain.
The interstitial renal edema produced stretches the renal capsule, enlarges the kidney (ie, nephromegaly), and increases renal lymphatic drainage. (Increased capillary permeability facilitates this edema.) It may also reduce the radiographic density of the affected kidney’s parenchyma when viewed on a noncontrast CT scan.
Distention of the renal pelvis initially stimulates ureteral hyperperistalsis, but this diminishes after 24 hours, as does renal blood flow. Peak hydrostatic renal pelvis pressure is attained within 2-5 hours after a complete obstruction.
Within the first 90 minutes of a complete ureteral obstruction, afferent preglomerular arteriolar vasodilation occurs, which temporarily increases renal blood flow. Between 90 minutes and 5 hours after the obstruction, renal blood flow starts to decrease while intraureteral pressure continues to rise. By 5 hours after a complete obstruction, both renal blood flow and intraluminal ureteral pressure decrease on the affected side.
Renal blood flow decreases to approximately 50% of normal baseline levels after 72 hours, to 30% after 1 week, to 20% after 2 weeks, and to 12% after 8 weeks. By this point, intraureteral pressures have returned to normal, but the proximal ureteral dilation remains and ureteral peristalsis is minimal.
Interstitial edema of the affected kidney actually enhances fluid reabsorption, which helps to increase the renal lymphatic drainage to establish a new, relatively stable, equilibrium. At the same time, renal blood flow increases in the contralateral kidney as renal function decreases in the obstructed unit.
In summary, by 24 hours after a complete ureteral obstruction, the renal pelvic hydrostatic pressure has dropped because of (1) a reduction in ureteral peristalsis; (2) decreased renal arterial vascular flow, which causes a corresponding drop in urine production on the affected side; and (3) interstitial renal edema, which leads to a marked increase in renal lymphatic drainage.
Additionally, as the ureter proximal to the stone distends, some urine can sometimes flow around the obstruction, relieving the proximal hydrostatic pressure and establishing a stable, relatively painless equilibrium. These factors explain why severe renal colic pain typically lasts less than 24 hours in the absence of any infection or stone movement.
Whether calyceal stones cause pain continues to be controversial. In general, in the absence of infection, how a renal stone causes pain remains unclear, unless the stone also causes obstruction. Arguably, proving that a calyceal stone is causing an obstruction can be difficult. However, a stone trapped in a calyx plausibly could block the outflow tract from that calyx, causing an obstruction and subsequent pain.
Experimental studies in animals have suggested that renal damage may begin within 24 hours of a complete obstruction and that permanent kidney deterioration starts within 5-14 days. Whereas some practitioners wait several months for a stone to pass in an asymptomatic patient, others argue that permanent damage is occurring as long as intervention is delayed.
Based on personal experience and anecdotal cases, the author recommends waiting no longer than 4 weeks for a stone to pass spontaneously before considering intervention. Convincing asymptomatic patients of the need for surgical intervention may be difficult in the absence of a clear consensus in the urological community about the length of time to wait before surgical stone removal, fragmentation, or bypass.
If only a partial obstruction is present, the same changes occur, but to a lesser degree and over a longer period. Proximal ureteric and renal pelvic hydrostatic pressures tend to remain elevated longer, and ureteral peristalsis does not diminish as quickly. If the increased pressure is sufficient to establish a reasonable flow beyond the obstructing stone, glomerular filtration and renal blood flow approximates reference range baseline levels, although pain may be ongoing.
Etiology
A low fluid intake, with a subsequent low volume of urine production, produces high concentrations of stone-forming solutes in the urine. This is an important, if not the most important, environmental factor in kidney stone formation. The exact nature of the tubular damage or dysfunction that leads to stone formation has not been characterized.
Most research on the etiology and prevention of urinary tract stone disease has been directed toward the role of elevated urinary levels of calcium, oxalate, and uric acid in stone formation, as well as reduced urinary citrate levels.
Hypercalciuria is the most common metabolic abnormality. Some cases of hypercalciuria are related to increased intestinal absorption of calcium (associated with excess dietary calcium and/or overactive calcium absorption mechanisms), some are related to excess resorption of calcium from bone (ie, hyperparathyroidism), and some are related to an inability of the renal tubules to properly reclaim calcium in the glomerular filtrate (renal-leak hypercalciuria).
Magnesium and especially citrate are important inhibitors of stone formation in the urinary tract. Decreased levels of these in the urine predispose to stone formation.
The following are the four main chemical types of renal calculi, which together are associated with more than 20 underlying etiologies:
-
Calcium stones
-
Struvite (magnesium ammonium phosphate) stones
-
Uric acid stones
-
Cystine stones
Stone analysis, together with serum and 24-hour urine metabolic evaluation, can identify an etiology in more than 95% of patients. Specific therapy can result in a remission rate of more than 80% and can decrease the individual recurrence rate by 90%. Therefore, emergency physicians should stress the importance of urologic follow-up, especially in patients with recurrent stones, solitary kidneys, or previous kidney or stone surgery and in all children. [6]
Calcium stones
Calcium stones account for 75% of renal calculi. Recent data suggest that a low-protein, low-salt diet may be preferable to a low-calcium diet in hypercalciuric stone formers for preventing stone recurrences. [7] Epidemiological studies have shown that the incidence of stone disease is inversely related to the magnitude of dietary calcium intake in first-time stone formers, because greater fractional absorption of calcium occurs when intake is lower.
There is a trend in the urology community not to restrict dietary intake of calcium in recurrent stone formers. This is especially important for postmenopausal women in whom there is an increased concern for the development of osteoporosis. Calcium oxalate, calcium phosphate, and calcium urate are associated with the following disorders:
-
Hyperparathyroidism - Treated surgically or with orthophosphates if the patient is not a surgical candidate
-
Increased gut absorption of calcium - The most common identifiable cause of hypercalciuria, treated with calcium binders or thiazides plus potassium citrate
-
Renal calcium leak - Treated with thiazide diuretics
-
Renal phosphate leak - Treated with oral phosphate supplements
-
Hyperuricosuria - Treated with allopurinol, low purine diet, or alkalinizing agents such as potassium citrate
-
Hyperoxaluria - Treated with dietary oxalate restriction, oxalate binders, vitamin B-6, or orthophosphates
-
Hypocitraturia - Treated with potassium citrate
-
Hypomagnesuria - Treated with magnesium supplements
Struvite (magnesium ammonium phosphate) stones
Struvite stones account for 15% of renal calculi. They are associated with chronic urinary tract infection (UTI) with gram-negative, urease-positive organisms that split urea into ammonia, which then combines with phosphate and magnesium to crystalize into a calculus. Usual organisms include Proteus, Pseudomonas, and Klebsiella species. Escherichia coli is not capable of splitting urea and, therefore, is not associated with struvite stones. Because ammonia, a base, is produced during the catalytic process, the urine pH is typically greater than 7.
Underlying anatomical abnormalities that predispose patients to recurrent kidney infections should be sought and corrected. UTI does not resolve until the stone is removed entirely.
Uric acid stones
Uric acid stones account for 6% of renal calculi. These are associated with urine pH less than 5.5, high purine intake (eg, organ meats, legumes, fish, meat extracts, gravies), or malignancy (ie, rapid cell turnover). Approximately 25% of patients with uric acid stone have gout.
Serum and 24-hour urine sample should be sent for creatinine and uric acid determination. If serum or urinary uric acid is elevated, the patient may be treated with allopurinol 300 mg daily. Patients with normal serum or urinary uric acid are best managed by alkali therapy alone.
Cystine stones
Cystine stones account for 2% of renal calculi. They arise because of an intrinsic metabolic defect resulting in failure of renal tubular reabsorption of cystine, ornithine, lysine, and arginine. Urine becomes supersaturated with cystine, with resultant crystal deposition.
Cystine stones are treated with a low-methionine diet (unpleasant), binders such as penicillamine or a-mercaptopropionylglycine, large urinary volumes, or alkalinizing agents. A 24-hour quantitative urinary cystine determination helps to titrate the dose of drug therapy to achieve a urinary cystine concentration of less than 300 mg/L.
Drug-induced stone disease
A number of medications or their metabolites can precipitate in urine causing stone formation. These include the following [8, 9, 10] :
-
Indinavir
-
Atazanavir
-
Guaifenesin
-
Triamterene
-
Silicate (overuse of antacids containing magnesium silicate)
-
Sulfa drugs, including sulfasalazine, sulfadiazine, acetylsulfamethoxazole, acetylsulfasoxazole, and acetylsulfaguanidine
-
Ceftriaxone (rarely) [11]
A population-based case-control study from the United Kingdom found that use of any of the following five oral antibiotic classes 3–12 months before the index date was associated with nephrolithiasis:
-
Sulfas - Adjusted odds ratio (OR) 2.33 (95% confidence interval [CI] 2.19-2.48)
-
Cephalosporins – OR 1.88 (95% CI 1.75-2.01)
-
Fluoroquinolones – OR 1.67 (95% CI 1.54-1.81)
-
Nitrofurantoin/methenamine – OR 1.70 (95% CI 1.55-1.88)
-
Broad-spectrum penicillins – OR 1.27 (9% CI 1.18-1.36)
Associations were greatest for exposure at younger ages (P< 0.001) and exposure 3–6 months before the index date (P< 0.001). With all but broad-spectrum penicillins, the risk remained statistically significant 3–5 years from exposure. [12]
Genetic factors
Nephrolithiasis is known to have a familial nature and significant heritability, and genes that may be involved in renal stone formation have been identified. Genome-wide association studies and candidate gene studies have implicated genes involved in renal tubular handling of lithogenic substrates, such as calcium, oxalate, and phosphate, and of inhibitors of crystallization, such as citrate and magnesium. [13]
Using whole-exome sequencing, Daga et al detected monogenic causative mutations in 15 of 51 families with members who presented before age 25 years with at least one renal stone or with a renal ultrasound finding of nephrocalcinosis. Identified mutations were in seven recessive genes (AGXT, ATP6V1B1, CLDN16, CLDN19, GRHPR, SLC3A1, SLC12A1), one dominant gene (SLC9A3R1), and one gene (SLC34A1) with both recessive and dominant inheritance. [14]
In patients with idiopathic hypercalciuria and calcium-containing kidney stones, genetic screens of nephrolithiasis determinants have identified candidates involved in renal calcium handling, such as the transient rec eptor potential vanilloid 5 (TRPV5), which is an important player in Ca2+ homeostasis. TheTRPV5 channel also plays a role in renal calcium transport, and may be the future target of therapies for individuals at risk for nephrolithiasis. [15]
Epidemiology
United States statistics
The lifetime prevalence of nephrolithiasis is approximately 11% for men and 7% for women in the United States, and it is currently rising. Having a family member with a history of stones doubles these rates. Approximately 30 million people are at risk in the United States. Roughly 2 million patients present on an outpatient basis with stone disease each year in the United States, which is a 40% increase from 1994. [16]
The likelihood that a White man will develop stone disease by age 70 years is 1 in 8. Stones of the upper urinary tract are more common in the United States than in the rest of the world. Recurrence rates after the first stone episode are 14%, 35%, and 52% at 1, 5, and 10 years, respectively.
Between 2005 and 2015, the overall annual incidence of nephrolithiasis in US adults increased from 0.6% to 0.9%. [17] The increasing incidence of kidney stone disease in the United States seems to be related to the socioeconomic status of the patient population. The lower the economic status, the lower the likelihood of renal stones. Other parts of the world with lower standards of living tend to have lower incidences of kidney stones but higher rates of bladder calculi.
Blacks have a lower incidence of stones than Whites, and people living in the South and Southwest have higher incidences of stones than people living in other parts of the United States. The increased incidence noted in the southeastern United States has prompted the use of the term “stone belt” for this region of the country. [18]
According to a population-based, repeated cross-sectional study, the estimated mean annual incidence of nephrolithiasis in South Carolina increased 1% annually from 1997 to 2012, reaching 239 per 100,000 population. Adjusting for age and race, the incidence increased 15% in females but remained stable for males. The incidence in Blacks increased 15% more compared with Whites. Among age groups, the greatest increase was observed among 15–19 year olds, in whom in whom the incidence increased 26%. [19]
International statistics
Nephrolithiasis occurs in all parts of the world. The incidence of urinary tract stone disease in developed countries is similar to that in the United States; the annual incidence of urinary tract stones in the industrialized world is estimated to be 0.2%. Stone disease is rare in only a few areas, such as Greenland and the coastal areas of Japan. A lifetime risk of 2-5% has been noted for Asia, 8-15% for the West, and 20% for Saudi Arabia.
In developing countries, bladder calculi are more common than upper urinary tract calculi; the opposite is true in developed countries. These differences are believed to be diet-related.
Age distribution for nephrolithiasis
Most urinary calculi develop in persons aged 20-49 years. Peak incidence occurs in people aged 35-45 years, but the disease can affect anyone at any age. Patients in whom multiple recurrent stones form usually develop their first stones while in their second or third decade of life. An initial stone attack after age 50 years is relatively uncommon.
Nephrolithiasis in children has historically been rare, with approximately 5-10 children aged 10 months to 16 years being seen annually for the condition at a typical US pediatric referral center. Over the last 25 years, however, the incidence of nephrolithiasis in children has increased by approximately 6-10% annually. In adolescents, the incidence has reached 50 per 100,000. [20]
Sex distribution for nephrolithiasis
In general, urolithiasis is more common in males (male-to-female ratio of 3:1). Stones due to discrete metabolic/hormonal defects (eg, cystinuria, hyperparathyroidism) and stone disease in children are equally prevalent between the sexes. Stones due to infection (struvite calculi) are more common in women than in men. Female patients have a higher incidence of infected hydronephrosis. Symptomatic nephrolithiasis occurs in less than 1% of pregnancies. [21]
In recent decades, the male-to-female ratio in urolithiasis has been narrowing, possibly as a result of lifestyle changes and increasing obesity in women. In addition, women are more often experiencing kidney stones that necessitate emergency department visits and are showing a higher mortality rate than men, especially with stone disease associated with urosepsis and requiring intensive care unit admission. [22]
Racial differences in incidence
Urinary tract calculi are far more common in Asians and Caucasians than in Native Americans, Africans, African Americans, and some natives of the Mediterranean region. Caucasian males are affected 3-4 times more often than African American males, though African Americans have a higher incidence of infected ureteral calculi than Caucasians. With uric acid stones, however, non-Caucasian have a higher frequency of stone formation than Caucasians. Some groups, such as the Hmong, have frequencies up to 50%.{ref80)
Although some differences may be attributable to geography (stones are more common in hot and dry areas) and diet, heredity also appears to be a factor. This is suggested by the finding that, in regions with both Caucasian and non-Caucasian populations, stone disease is much more common in Caucasians.
Prognosis
Approximately 80-85% of stones pass spontaneously. Approximately 20% of patients require hospital admission because of unrelenting pain, inability to retain enteral fluids, proximal UTI, or inability to pass the stone.
The most morbid and potentially dangerous aspect of stone disease is the combination of urinary tract obstruction and upper urinary tract infection. Pyelonephritis, pyonephrosis, and urosepsis can ensue. Early recognition and immediate surgical drainage are necessary in these situations.
Because the minimally invasive modalities for stone removal are generally successful in removing calculi, the primary consideration in managing stones is not whether the stone can be removed but whether it can be removed in an uncomplicated manner with minimum morbidity.
The usually quoted recurrence rate for urinary calculi is 50% within 5 years and 70% or higher within 10 years, although a large, prospective study published in 1999 suggested that the recurrence rate may be somewhat lower at 25-30% over a 7.5-year period. Recurrence rates after an initial episode of ureterolithiasis have also been reported to be 14%, 35%, and 52% at 1, 5, and 10 years, respectively.
Metabolic evaluation and treatment are indicated for patients at greater risk for recurrence, including those who present with multiple stones, who have a personal or family history of previous stone formation, who present with stones at a younger age, or who have residual stones after treatment.
Medical therapy is generally effective at delaying (but perhaps not completely stopping) the tendency for stone formation. The most important aspect of medical therapy is maintaining a high fluid intake and subsequent high urinary volume. Without an adequate urinary volume, no amount of medical or dietary therapy is likely to be successful in preventing stone formation.
According to estimates, merely increasing fluid intake and regularly visiting a physician who advises increased fluids and dietary moderation can cut the stone recurrence rate by 60%. This phenomenon is known as the “stone clinic” effect. In contrast, optimal use of metabolic testing with proper evaluation and compliance with therapy can completely eliminate new stones in many patients and significantly reduces new stone formation in most patients.
Patient Education
A patient who tends to develop stones should be counseled to seek immediate medical attention if he or she experiences flank or abdominal pain or notes visible blood in the urine.
Although discovering the underlying cause of a patient’s stones and starting preventive therapy is not the primary responsibility of the physician treating a patient with acute renal colic (such measures are best addressed once the immediate problem has been addressed), this physician should, at the very least, educate the patient and family members about the availability of preventive testing and treatment. When properly performed and evaluated, preventive treatment plans can improve the situation in most patients with stones.
Note that failure to offer stone-prevention advice could actually be a source of medicolegal liability. Numerous patients have claimed they have not been told about stone-prevention options.
One anecdotal example from the practice of one of the editors is that of a 65-year-old man with a 5-year history of more than 60 stones. Although he underwent two open surgeries for stone removal, his stones were not evaluated for chemical composition. Eventually, the stones were analyzed and found to be pure uric acid. Although his uric acid excretion rate was normal, he had highly acidic urine, which led to the uric acid calculi formation. After starting oral therapy of allopurinol and potassium citrate, he remained free of stones for 10 years.
Even patients who develop single stones may be strongly motivated to follow a program for maximum kidney stone prophylaxis. Discussing the pros and cons of a comprehensive stone-prevention program with all patients who have documented kidney stone disease—not with just those who are obviously at high risk—may be prudent.
For patient education information, see the Kidney Stones Health Center. In addition, numerous Internet sites offer kidney stone information, including the National Institutes of Health (NIH) and the Urology Care Foundation.
-
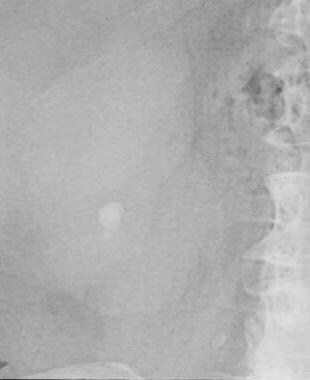
Small renal calculus that would likely respond to extracorporeal shockwave lithotripsy.
-
Complete staghorn calculus that fills the collecting system of the kidney (no intravenous contrast material in this patient). Although many staghorn calculi are struvite (related to infection with urease-splitting bacteria), the density of this stone suggests that it may be metabolic in origin and is likely composed of calcium oxalate. Percutaneous nephrostolithotomy or perhaps even open surgical nephrolithotomy is required to remove this stone.
-
Distal ureteral stone observed through a small, rigid ureteroscope prior to ballistic lithotripsy and extraction. The small caliber and excellent optics of today's endoscopes greatly facilitate minimally invasive treatment of urinary stones.
-
Two calculi in a dependent calyx of the kidney (lower pole) visualized through a flexible fiberoptic ureteroscope. In another location, these calculi might have been treated with extracorporeal shockwave lithotripsy (ESWL), but, after being counseled regarding the lower success rate of ESWL for stones in a dependent location, the patient elected ureteroscopy. Note that the image provided by fiberoptics, although still acceptable, is inferior to that provided by the rod-lens optics of the rigid ureteroscope in the previous picture.
-
Nephrolithiasis: acute renal colic. Anatomy of the ureter.
-
Nephrolithiasis: acute renal colic. Distribution of nerves in the flank.
-
Nephrolithiasis: acute renal colic. Nerve supply of the kidney.
-
Nephrolithiasis: acute renal colic. Renal colic and flank pain.
-
Nephrolithiasis: acute renal colic. Nerve supply of the kidney.
-
Nephrolithiasis: acute renal colic. Distribution of renal and ureteral pain.
-
Noncontrast helical CT scan of the abdomen demonstrating a stone at the right ureterovesical junction.
-
Intravenous pyelogram (IVP) demonstrating dilation of the right renal collecting system and right ureter consistent with right ureterovesical stone.