Practice Essentials
Anthrax is a zoonotic infection caused by the gram-positive rod Bacillus anthracis. Most cases of anthrax are cutaneous (95%); the remaining cases are inhalational (5%) and gastrointestinal (< 1%). Anthrax caused by inhalation is usually fatal, and symptoms usually begin days after exposure. Bioterrorism must be suspected in any case of inhalational anthrax.
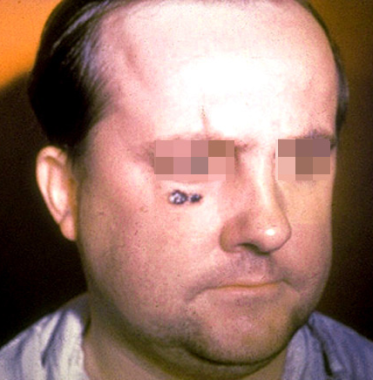 Skin lesion of anthrax on face. Image courtesy of the Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Skin lesion of anthrax on face. Image courtesy of the Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
See Clues on the Skin: Acute Poisonings, a Critical Images slideshow, to help diagnose patients based on their dermatologic presentations.
Signs and symptoms
Depending on the route of exposure to B anthracis spores, patients may present with cutaneous, respiratory, or GI complaints. Exposure may be via handling of sick animals or contaminated wool, hair, or hides; inhalation; or ingestion of contaminated meat.
Cutaneous anthrax
-
Cutaneous anthrax develops 2-5 days (range, 1-7 days) postexposure.
-
Lesions most commonly develop at lacerations, abrasions, or insect bites on exposed areas of skin; most commonly affects upper extremities but may arise anywhere on the body.
-
Infection begins as a pruritic papule that enlarges within 24-48 hours to form a 1-cm vesicle; this then becomes an ulcer surrounded by an edematous halo. [1]
-
Resultant lesions are usually 2-3 cm in diameter with a round, regular, raised edge.
-
Lesions may become edematous and necrotic but are usually not purulent.
-
They are painless but on occasion are slightly pruritic.
-
Regional lymphadenopathy may occur and may be painful.
-
The ulcer and edema evolve into a black eschar within 7-10 days and then last for 7-14 days before separating and leaving a scar.
-
Lymphadenopathy may be persistent.
-
With neck lesions, edema and lymphadenopathy may impinge on the airway and cause stridor and respiratory compromise.
Oropharyngeal anthrax
-
Oropharyngeal anthrax develops 2-7 days after ingestion.
-
Presents as neck swelling and fever in the presence of an oral lesion
-
The lesion starts as an edematous area that becomes necrotic and forms a pseudomembrane within 2 weeks.
-
Sore throat, dysphagia, respiratory distress, and oral bleeding may also occur, depending on the lesion site.
-
Soft-tissue edema and dramatic cervical lymph node enlargement usually follow.
Intestinal anthrax
-
Intestinal anthrax develops 2-5 days after ingestion.
-
Commonly presents as nonspecific abdominal pain with fever; may be associated with nausea, vomiting, malaise, anorexia, hematemesis, dysentery, and/or diarrhea
-
Distributive or hypovolemic shock may develop depending on the severity of illness.
Inhalational anthrax
-
Inhalational anthrax begins abruptly 1-3 days (range, hours to 60 days) postexposure and follows a biphasic course
-
Typically begins as fever with nonproductive cough and may feature myalgia, fatigue, or retrosternal chest pain
-
Transient clinical improvement may occur after the first few days, followed by rapid progression and clinical deterioration including the following:
- High-grade fever
- Symptoms of respiratory failure: severe dyspnea, tachypnea, hypoxemia
- Hematemesis or hemoptysis
- Chest pain, which may be severe enough to mimic acute coronary syndrome
- Decreased level of consciousness, meningismus, and coma (with meningeal involvement)
See Clinical Presentation for more detail.
Diagnosis
B anthracis is present in high numbers in appropriate specimens and can be demonstrated by staining or culture. Laboratory personnel should take biosafety level 2 precautions.
Diagnostic studies may include the following:
-
Staining of cutaneous ulcer exudate with methylene blue or Giemsa stain
-
Punch biopsy at the edge of the lesion, examined by silver staining and immunohistochemical testing (for patients with prior antibiotic treatment)
-
Blood culture and Gram stain (for patients with systemic symptoms)
-
ELISA serology for B anthracis toxins or IgG response to B anthracis protective antigen
-
Chest radiography – In inhalational anthrax, typically shows widening of the mediastinum and pleural effusions, whereas the parenchyma may appear normal
-
Chest CT – In inhalational anthrax, detects hemorrhagic mediastinal and hilar lymph nodes and edema, peribronchial thickening, and pleural effusions
-
Lumbar puncture (for patients with meningeal symptoms) – CSF is grossly hemorrhagic, with grossly hemorrhagic with few polymorphonuclear neutrophils (PMNs) and numerous gram-positive bacilli
See Workup for more detail.
Management
Treatment of anthrax varies. See Treatment and Medication for more detail.
Background
Anthrax is a zoonotic infection caused by Bacillus anthracis. Most anthrax is cutaneous (95%). The remaining cases of the disease are inhalational (5%) and gastrointestinal (< 1%). Cutaneous anthrax results from exposure to the spores of B anthracis while handling sick animals or contaminated wool, hair, or animal hides. Pulmonary anthrax results from inhaling anthrax spores. GI anthrax results from ingesting meat products that contain anthrax. Anthrax is present in areas where animals, particularly herbivores, graze. Anthrax caused by inhalation is usually fatal, and symptoms usually begin days after exposure. This delay makes the initial exposure to B anthracis difficult to track.
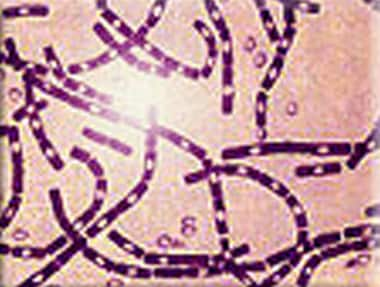 Polychrome methylene blue stain of Bacillus anthracis. Image courtesy of Anthrax Vaccine Immunization Program Agency, Office of the Army Surgeon General, United States.
Polychrome methylene blue stain of Bacillus anthracis. Image courtesy of Anthrax Vaccine Immunization Program Agency, Office of the Army Surgeon General, United States.
Anthrax was described in the early literature of the Greeks, Romans, Egyptians, and Hindus. The term anthrakis means coal in Greek, and the disease is named after the black appearance of its cutaneous form. [2] The fifth plague described in the Old Testament book of Genesis may be among the earliest descriptions of anthrax. At the end of the 19th century, Robert Koch's experiments with anthrax led to the original theory of bacteria and disease. John Bell's work in inhalational anthrax led to wool disinfection processes and the term woolsorter's disease.
A modern concern is use of anthrax as a biologic warfare agent. During the first Gulf War, Iraq reportedly produced 8500 L of anthrax. A total of 150,000 US troops were vaccinated with anthrax toxoid. In the weeks following the terrorist attacks of September 11, 2001, 22 confirmed or suspected cases of anthrax infection were disseminated via the US postal system; the spores mailed in these letters were ultimately traced to a US army medical research institute. Since there have been no cases of naturally occurring inhalational anthrax in the US since 1976, alarm should be raised for the occurrence of even a single infection.
For patient education information, see the Bioterrorism and Disaster Medicine Center, as well as Biological Warfare, Anthrax, and Personal Protective Equipment.
Pathophysiology
Anthrax is primarily a disease of herbivores (eg, cattle, sheep, goats, horses). Pigs are not immune, but they are more resistant, as are dogs and cats. Birds are usually naturally resistant to anthrax. Buzzards and vultures are naturally resistant to anthrax but may transmit the spores on their talons and beaks.
Anthrax (B anthracis) is a large, spore-forming, gram-positive rod. Persistence of spores is aided by nitrogen and organic soil content, environmental pH greater than 6, and ambient temperature greater than 15°C. Spores can exist indefinitely in the environment. Optimal growth conditions result in a vegetative phase and bacterial multiplication. Drought or rainfall can trigger anthrax spore germination, while flies and vultures spread the spores.
Virulence depends on the bacterial capsule and the toxin complex. The capsule is a poly-D-glutamic acid that protects against leukocytic phagocytosis and lysis. Experiments by Sterne demonstrated that the capsule is vital for pathogenicity.
Anthrax toxins
Anthrax toxins are composed of 3 entities: a protective antigen, a lethal factor, and an edema factor. The protective antigen is an 83-kd protein that binds to cell receptors within a target tissue. Once it is bound, a fragment is cleaved free to expose an additional binding site. The binding of edema factor at this site results in the formation of edema toxin; the binding of lethal factor results in the formation of lethal toxin.
Edema toxin acts by converting adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). Cellular cAMP levels are increased, leading to cellular edema within the target tissue. Lethal factor is not well understood; it may inhibit neutrophil phagocytosis, lyse macrophages, and cause release of tumor necrosis factor and interleukin-1. Death from anthrax occurs as a result of the effects of lethal toxin. Near death or just after death, animals bleed from all body orifices.
Cutaneous anthrax
Humans are relatively resistant to cutaneous invasion by B anthracis, but the organisms may gain access through microscopic or gross breaks in the skin. In cutaneous anthrax, a malignant pustule develops at the infection site. This pustule is a central area of coagulation necrosis (ulcer) surrounded by a rim of vesicles filled with bloody or clear fluid. A black eschar forms at the ulcer site. Extensive edema surrounds the lesion.
The organisms multiply locally and may spread to the bloodstream or other organs (eg, spleen) via the efferent lymphatics. B anthracis remains in the capillaries of invaded organs, and the local and fatal effects of the infection are due, in large part, to the toxins elaborated by B anthracis. Dissemination from the liver, spleen, and kidneys back into the bloodstream may result in bacteremia. Secondary hemorrhagic intestinal foci of anthrax result from B anthracis bacteremia.
Intestinal anthrax
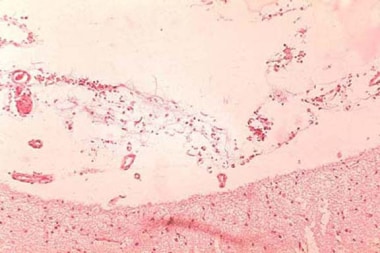
Primary intestinal anthrax predominantly affects the cecum and produces a local lesion similar to the lesion produced in the cutaneous form. In this illness, spores invade the GI mucosa. In some cases, necrosis and ulceration at the site of infection produce GI hemorrhage.
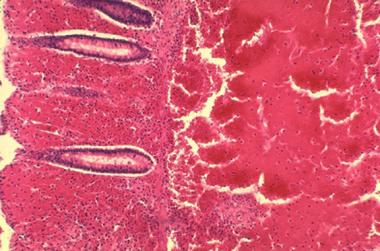 Histopathology of large intestine showing marked hemorrhage in the mucosa and submucosa. Image courtesy of Marshall Fox, MD, Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Histopathology of large intestine showing marked hemorrhage in the mucosa and submucosa. Image courtesy of Marshall Fox, MD, Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
As spores are transported to mesenteric lymph nodes, replication and bacteremia begin. Ascites and ileus follow as the lymphatic system becomes occluded with the large number of bacilli. Peritoneal fluid is turbid with the presence of leukocytes and red blood cells from hemorrhagic adenitis. Vascular stasis occurs, and the stomach and intestine become edematous.
Oropharyngeal anthrax
Oropharyngeal anthrax is a variant of intestinal anthrax and occurs in the oropharynx after ingestion of meat products contaminated by anthrax. Oropharyngeal anthrax is characterized by throat pain and difficulty in swallowing. The lesion at the site of entry into the oropharynx resembles the cutaneous ulcer.
Inhalational anthrax
Inhalational anthrax occurs after a person inhales spores into the lungs. Primate studies suggest that the minimum infective dose ranges from 4000-8000 inhaled spores. Inhaled spores are ingested by pulmonary macrophages and then carried to hilar and mediastinal lymph nodes. The incubation period is 1-6 days.
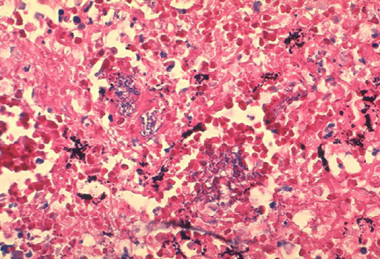 Histopathology of mediastinal lymph node showing a microcolony of Bacillus anthracis on Giemsa stain. Image courtesy of Marshall Fox, MD, Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
Histopathology of mediastinal lymph node showing a microcolony of Bacillus anthracis on Giemsa stain. Image courtesy of Marshall Fox, MD, Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
The spores undergo germination and multiplication and begin to elaborate toxins. Anthrax in the lungs does not cause pneumonia, but it does cause hemorrhagic mediastinitis and pulmonary edema. Hemorrhagic pleural effusions frequently accompany inhalational anthrax. After the lymph nodes become overwhelmed, bacteremia and death quickly ensue. Without treatment, the mortality rate of inhalational anthrax is approximately 95%.
Bacteremic anthrax with hematogenous spread most commonly follows inhalational anthrax. In bacteremic anthrax, hemorrhagic lesions may develop anywhere on the body. Septicemic anthrax refers to overwhelming infection resulting from bloodstream invasion secondary to inhalation or intestinal anthrax.
Anthrax meningitis
Anthrax meningitis may complicate any form of anthrax, with bacteremia and hematogenous spread to the CNS. It also has occurred without a primary focus. The meninges are characteristically hemorrhagic and edematous. The mortality rate is near 100%.
Etiology
Anthrax is caused by B anthracis, a gram-positive bacillus. B anthracis has a diameter of 1-1.5 µm and a length of 3-10 µm. It is usually straight but may be slightly curved. The ends of the bacilli are truncated, not rounded. Anthrax bacilli tend to form into long chains and may appear similar to streptobacilli on cultures.
B anthracis produces a capsule that is easily visualized using a methylene blue or India ink stain. Ground-glass–appearing colonies are adherent and appear gray or white on blood agar. Colonies measure 4-5 mm in diameter and have characteristic comma-shaped protrusions.
 Polychrome methylene blue stain of Bacillus anthracis. Image courtesy of Anthrax Vaccine Immunization Program Agency, Office of the Army Surgeon General, United States.
Polychrome methylene blue stain of Bacillus anthracis. Image courtesy of Anthrax Vaccine Immunization Program Agency, Office of the Army Surgeon General, United States.
Bacilli grow optimally in enhanced carbon dioxide and are nonmotile. The organism shows preferential growth on phenylethyl alcohol blood agar with characteristic gelatin hydrolysis and salicin fermentation. B anthracis is catalase positive. Capsule formation may help differentiate B anthracis from other nonpathogenic bacilli. Anthrax is differentiated from other gram-positive rods on culture by lack of motility in broth and lack of hemolysis on blood agar.
Table 1. Microbiological Differences Between B anthracis and Non– B anthracis Bacilli (Open Table in a new window)
B anthracis |
Non–B anthracis bacilli (pseudoanthrax bacilli) |
Nonmotile long chains |
Generally motile short chains |
Capsule formation on bicarbonate agar |
No capsule formation in bicarbonate |
No growth on penicillin agar (10 mcg/mL) |
Usually good growth on penicillin agar |
Growth in gelatin resembles inverted fir tree |
Growth in gelatin absent or resembles atypical fir tree |
Gelatin liquefaction slow |
Gelatin liquefaction usually rapid |
No hemolysis of sheep RBCs |
Hemolysis of sheep RBCs |
Ferments salicin slowly or not at all |
Usually ferments salicin rapidly |
Pathogenic to laboratory animals |
Nonpathogenic to laboratory animals |
Adapted from Cunha CB. Anthrax: Ancient Plague, Persistent Problem. Infect Dis Pract. 1999;23(4):35-9. |
|
Anthrax toxins
Anthrax exotoxins are produced in the vegetative phase and are composed of proteins (see Table 2 below). Lethal toxin is the single most important virulence factor and is the primary cause of death. Lethal toxin is a combination of protective antigen and lethal factor. Edema factor and lethal toxin inhibit phagocytosis and polymorphonuclear neutrophil (PMN) function. The other major anthrax virulence factor is its antiphagocytic poly-D-glutamic acid capsule.
Table 2. Toxins and Protein Toxins of Bacillus anthracis (Open Table in a new window)
Edema factor (EF) + lethal factor (LF) = Host cell penetration by B anthracis |
EF + protective antigen (PA) = Edema toxin |
LF + PA = Lethal toxin (primary virulence factor of B anthracis) |
Edema toxin + lethal toxin = Inhibited PMN function and phagocytosis |
Risk factors
Studies of inhalational anthrax based on several cases in patients with no known exposure in the October 2001 postal anthrax release found the following determinants of risk:
-
Bacterial virulence factors
-
Balance between infectious aerosol production and removal
-
Pulmonary ventilation rate
-
Duration of exposure
-
Host susceptibility factors
Dilution ventilation of the indoor environment is an important determinant of the risk for infection. Enhanced room ventilation, germicidal irradiation with ultraviolet light, and other engineering control measures may be used to decrease the risk for infection.
Important host susceptibility risk factors for inhalational anthrax include the following:
-
Prior exposure to radiation
-
Alcoholism
-
Underlying pulmonary disease
Epidemiology
United States statistics
Natural incidence is rare, but infection is an occupational hazard among veterinarians, farmers, and individuals who handle animal wool, hair, hides, or bone meal products. During the last 30 years, the indigenous US incidence of any anthrax infection has been less than 1 case per year. From 1955–1994, US cases totaled 235, with 224 cases of cutaneous anthrax, 11 cases of inhalational anthrax, and 20 fatalities. The last fatal case during this period occurred in 1976, when a home craftsman died of inhalational anthrax after working with yarn imported from Pakistan.
Before October 2001, the Centers for Disease Control and Prevention (CDC) investigated several threats in the United States, including Indiana, Kentucky, Tennessee, and California. In October 2001, 22 confirmed or suspected cases of anthrax infection were identified. Cases were reported from Florida, New York, New Jersey, the District of Columbia, and Connecticut. There were 11 confirmed cases of inhalational anthrax (5 deaths) and 7 confirmed and 4 suspected cases of cutaneous anthrax (no deaths).
Seven cases were associated with occupational exposures in the postal service, and 2 cases had documented exposures to contaminated mail in the business office of a media company. No sources of exposure were identified for 2 women who were presumably exposed to secondarily contaminated mail. No reports in the literature have documented direct human-to-human transmission.
International statistics
Anthrax is uncommon in Western Europe, but the disease is not uncommon in the Middle East, the Indian subcontinent, [3] Africa, Asia, and Latin America. In 1958, approximately 100,000 cases of anthrax occurred worldwide. Exact figures do not exist because of reporting difficulties in Africa. Anthrax is endemic in Africa and Asia despite vaccination programs.
Sporadic outbreaks have occurred as a result of both agricultural and military disruptions. During the 1978 Rhodesian civil war, failure of veterinary vaccination programs led to a human epidemic, causing 6500 anthrax cases and 100 fatalities. A mishap at a military microbiology facility in Sverdlovsk in the former Soviet Union in 1979 resulted in at least 66 deaths. [2] Human anthrax often is associated with agricultural or industrial workers who come in contact with infected animal tissue.
Race-, sex-, and age-related differences in incidence
There is no racial, sexual, or age predilection for anthrax. However, because anthrax is often related to industrial exposure and farming, the disease most often affects young and middle-aged adults. Persons of any age can of course be affected if anthrax is used as a bioterrorist weapon.
Prognosis
Most cases of anthrax are the cutaneous type, are mild, and resolve with or without treatment. If treated early with appropriate antibiotics, the mortality rate of cutaneous anthrax is less than 1%-2%. [4]
However, other forms of anthrax are potentially fatal, with inhalational anthrax carrying the worst prognosis. Inhalational anthrax and its subsequent systemic infection (eg, septicemia, hemorrhagic leptomeningitis) have a mortality rate approaching 100%. Even with present-day critical care capabilities and modern medical technology, the mortality rates of GI and inhalational anthrax remain 40% and 45%, respectively. [5] If treatment is initiated during the incubation period of 1-6 days and before the manifestation of symptoms, mortality can decrease to 1%.
Oropharyngeal or intestinal anthrax carries a less favorable prognosis than cutaneous anthrax but a more favorable prognosis than inhalational anthrax. Patients with oropharyngeal anthrax may develop airway obstruction (as may those with inhalational anthrax or cutaneous anthrax involving the neck). Intestinal anthrax is more difficult to diagnose given its initial nonspecific syndrome and is associated with higher morbidity and mortality rates. [5]
-
Polychrome methylene blue stain of Bacillus anthracis. Image courtesy of Anthrax Vaccine Immunization Program Agency, Office of the Army Surgeon General, United States.
-
Histopathology of mediastinal lymph node showing a microcolony of Bacillus anthracis on Giemsa stain. Image courtesy of Marshall Fox, MD, Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
-
Cutaneous anthrax. Image courtesy of Anthrax Vaccine Immunization Program Agency, Office of the Army Surgeon General, United States.
-
Skin lesion of anthrax on face. Image courtesy of the Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
-
Skin lesions of anthrax on neck. Cutaneous anthrax showing the typical black eschar. Image courtesy of the Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
-
Histopathology of large intestine showing marked hemorrhage in the mucosa and submucosa. Image courtesy of Marshall Fox, MD, Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
-
Histopathology of the large intestine showing submucosal thrombosis and edema. Image courtesy of Marshall Fox, MD, Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
-
Inhalation anthrax. Chest radiograph with widened mediastinum 22 hours before death. Image courtesy of P.S. Brachman, MD, Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
-
Histopathology of mediastinal lymph node showing mediastinal necrosis. Image courtesy of Marshall Fox, MD, Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
-
Hemorrhagic meningitis resulting from inhalation anthrax. Image courtesy of the Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
-
Anthrax infection. Histopathology of hemorrhagic meningitis in anthrax. Image courtesy of Marshall Fox, MD, Public Health Image Library, US Centers for Disease Control and Prevention, Atlanta, Georgia.
-
Microscopic picture of anthrax showing gram-positive rods. Image courtesy of Ramon E. Moncada, MD.
-
Seven-month-old infant with anthrax. In this infant, the infection progressed rapidly with significant edema developing the day after exposure. This large hemorrhagic lesion developed within 3 more days. The infant was febrile and was admitted to the hospital on the second day after the symptoms appeared.On September 28, 2001, the infant had visited the mother's workplace. On September 29, nontender massive edema and a weeping erosion developed. On September 30, a 2-cm sore developed over the edematous area. (Note that edema preceded the primary lesion.) On October 2, an ulcer or eschar formed, and the lesion was diagnosed as a spider bite. Hemolytic anemia and thrombocytopenia developed, and the patient was hospitalized. Serum was drawn on October 2; the polymerase chain reaction results were positive for Bacillus anthracis. On October 13, skin biopsy results were positive with immunohistochemical testing for the cell wall antigen.Note that the initial working diagnosis was a Loxosceles reclusa spider bite with superimposed cellulitis. Courtesy of American Academy of Dermatology with permission of NEJM.
-
Fourth patient with cutaneous anthrax in New York City, October 2001. This dry ulcer was present. Photo used with permission of the patient. Courtesy of American Academy of Dermatology. Courtesy of Sharon Balter of the New York City Department of Health.
-
Note the hemorrhage that is associated with cutaneous anthrax lesions. The early ulcer has a moist base. Courtesy of American Academy of Dermatology.
-
Note the central ulcer and eschar. Courtesy of American Academy of Dermatology.
-
An example of a central ulcer and eschar with surrounding edema. Courtesy of American Academy of Dermatology with permission from Boni Elewski, MD.
-
Note the black eschar. Courtesy of American Academy of Dermatology. Courtesy of Gorgas Course in Clinical Tropical Medicine.
-
Anthrax with facial edema. Courtesy of American Academy of Dermatology.