Practice Essentials
Ovulation is the result of a maturation process that occurs in the hypothalamic-pituitary-ovarian (HPO) axis and is orchestrated by a neuroendocrine cascade terminating in the ovaries. Any alteration results in a failure to release a mature ovum, leading to anovulatory cycles. Anovulation may manifest in a variety of clinical presentations, from luteal insufficiency to oligomenorrhea.
Anovulation is a not a disease but a sign, in much the same way that polycystic ovaries are the manifestation of a much larger disease process.
Education for these patients should focus on an understanding of the underlying disorders to ensure compliance with both medical therapy and lifestyle modifications.
Pathophysiology
To understand anovulation, one must first understand what occurs during a normal ovulatory cycle. In normal physiology, ovulation is dependent on the presence of a functioning hypothalamic-pituitary-ovarian (HPO) axis. The arcuate nucleus within the hypothalamus is composed of a collection of neurons and, when stimulated, releases GnRH into the portal vessels of the pituitary stalk in a pulsatile fashion. GnRH stimulates receptors in the anterior pituitary gland to produce and secrete both LH and FSH. In women, FSH induces maturation of ovarian follicles and eventual production of estrogen, while LH modulates the secretion of androgens from the ovarian theca cells. [1] Estrogen, in turn, produces negative feedback on the pituitary gland.
As the follicle grows through accumulation of follicular fluid, the cohort of granulosa cells acquire the necessary receptors to respond to LH with increased formation of cyclic adenosine monophosphate (cAMP). During the midcycle, the estrogen levels in the circulation reach a concentration that causes a positive feedback action on LH secretion. This is called the LH surge. Generally speaking, approximately 16-24 hours after the LH peak, ovulation occurs with the extrusion of a mature oocyte from the graafian follicle and the formation of the corpus luteum. [2] These events are the culmination of a well-coordinated interplay between hormones and their appropriate receptors and proteolytic enzymes and prostaglandins acting in concert with one another, all directed by the HPO axis.
The system is so sensitive that even the slightest alteration in any of these factors can disrupt its fluidity and lead to anovulation.
When problems arise at any of the many different levels involved in the normal menstrual cycle, it is sometimes helpful to separate the levels by organ system. The hypothalamus and the anterior pituitary can be considered the neuroendocrine components by virtue of their proximity to each another, while the ovaries are a separate compartment. The third aspect that can be defective is the signaling process that occurs between these 2 areas. [2]
The initial stimulus must come from the hypothalamus in the form of gonadotropin-releasing hormone (GnRH); this decapeptide must be secreted in a pulsatile fashion within a critical range. For example, sexual maturity is not attained until the onset of regular ovulatory cycles, which may take months to years to occur. This maturation process is orchestrated by a neuroendocrine cascade and modified by autocrine and paracrine events in the ovaries, in which GnRH is the principal mediator. [3]
Any alteration in the GnRH pulse generator alters the hormonal milieu necessary for gonadotropin secretion and eventual response at the level of the ovary. Several entities (eg, hyperprolactinemia) are known to cause this type of dysregulation. Increasing levels of prolactin can cause a woman to progress from a deficient luteal phase to overt amenorrhea, usually associated with complete GnRH suppression. More common causes of dysregulation include stress, anxiety, and eating disorders, which are also associated with an inhibition of normal GnRH pulsatility through excessive hypothalamic activity of corticotrophin-releasing hormone and stimulation of beta-endorphins. [4]
How polycystic ovary syndrome (PCOS) is associated with anovulatory cycles has not been completely elucidated. Two associations with this disease entity are theorized to be at least somewhat responsible for its development. The first is the persistent elevation of LH levels in these patients; the second is the apparent arrest of antral follicle development at the 5- to 10-mm stage and consequent failure to enter the preovulatory phase of the cycle. [5] This evidence indicates that the disturbance is mainly a central defect that initiates the cascade of events leading to its onset.
Similarly, any condition, whether primary or secondary, that results in either a persistent elevation or an insufficient attainment of estrogen levels can inhibit ovulation through a disruption of the mechanisms that induce the LH surge. To achieve the corresponding changes within the cycle, estradiol levels must rise and fall appropriately. [2]
Etiology
Volumes have been written on the different clinical entities associated with anovulation. Based on serum gonadotropins and ovarian hormones, clinicians are usually able to discern whether the ovulatory dysfunction is of central or ovarian origin. In the presence of PCOS, hormone levels are usually within the reference range, but they are accompanied by a wide array of clinical manifestations that may signal the presence of this disorder. The following describes the most important causes of anovulation and elaborates on their clinical and biochemical manifestations:
First described in 1935 by Stein and Leventhal, PCOS is the most common endocrinopathy in women of reproductive age, with a prevalence of approximately 6.5%. Its cardinal features are hyperandrogenism and polycystic ovaries. [6] Clinically, PCOS is characterized by menstrual irregularities, hyperandrogenism, hyperinsulinemia, and long-term metabolic disturbances, such as diabetes mellitus, cardiovascular disease, and dyslipidemias. [7] Risk factors include history of premature adrenarche, family history of PCOS, and history of weight gain during the perimenarchal time. [8]
During the Rotterdam Conference of 2003, a revision of the diagnostic criteria the US National Institutes of Health conference initially proposed in 1990 was made to include the findings of polycystic ovaries. In 2006, the Androgen Excess Society has proposed a new set of criteria for the diagnosis of PCOS where the patient must demonstrate clinical or biological signs of hyperandrogenism plus additional criteria.
2003 consensus statement on PCOS
Any 2 of 3 of the following criteria must be present:
-
Clinical and/or biochemical signs of hyperandrogenism and exclusion of other etiologies (eg, congenital adrenal hyperplasia [CAH], androgen-secreting tumors, Cushing syndrome)
-
Oligo-ovulation or anovulation
-
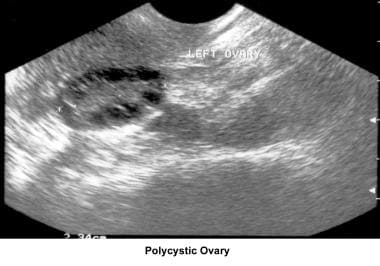
Polycystic ovaries - Presence of 12 or more follicles in each ovary measuring 2-9 mm in diameter and/or increased ovarian volume greater than 10 mL [9] (See the image below.)
2015 American Association of Clinical Endocrinologists (AACE), American College of Endocrinology (ACE), and Androgen Excess and PCOS Society guidelines [10, 11]
-
The diagnostic criteria for PCOS should include two of the following three criteria: chronic anovulation, hyperandrogenism (clinical/biologic), and polycystic ovaries
See Guidelines for a summary of clinical practice guidelines for PCOS released in 2018 by the Australian National Health and Medical Research Council, European Society of Human Reproduction and Embryology, and American Society for Reproductive Medicine. [12]
Because of the disparity between follicular growth and steroidogenesis, Franks et al have proposed that a premature activation of LH-induced mitotic arrest, which occurs normally at the onset of the midcycle LH surge, is responsible for the ovulatory dysfunction noted in patients with PCOS. [5] Therefore, a combination of elevated LH levels with enhanced LH action may be responsible not only for the arrested growth of the follicles but also the increased production of estradiol. [13, 5]
Franks et al further hypothesize that these mechanisms may be intimately related to overproduction of cAMP at the level of the granulosa cell, whereby follicular arrest occurs simultaneously with excess androgen production. [5]
Hyperinsulinemia and insulin resistance
Insulin resistance, independent of obesity, has also been described as being pathognomonic of PCOS. [14] Impaired glucose tolerance has a prevalence of 30% and undiagnosed type 2 diabetes mellitus has a prevalence of 8-10% in women with PCOS. [8] Mechanisms have been proposed to account for the excess insulin production by the pancreas: (1) decreased insulin binding, (2) decreased insulin receptor numbers (down-regulation), and (3) postreceptor failures due to serine phosphorylation and eventual increase in pancreatic beta-cell stimulation.
Regardless of the cause, insulin resistance is predominantly seen peripherally, at the level of skeletal muscle, where 85-90% of circulating insulin is used. The compensatory hyperinsulinemia that occurs contributes to androgen excess by directly stimulating androgen production in the adrenal cortex and through a direct effect on granulosa cells and a simultaneous decrease of sex hormone–binding globulin (SHBG) in the ovaries. [15]
Increased insulin levels can lead to hyperplasia of the basal layer of the epidermis, resulting in velvety hyperpigmented areas of skin usually located on the back of the neck, the axilla, under the breasts, and inner thighs. These lesions are known as acanthosis nigricans. [8]
Obesity
A centripetal, or apple-shaped, distribution of adipose tissue (waist-hip ratio, >0.88) is associated with a greater risk for hypertension, diabetes, and dyslipidemia.
Although many of the metabolic abnormalities can be alleviated with weight loss, they are still present. PCOS is neither caused by obesity nor cured by weight reduction. [16]
A randomized, controlled trial by Bloom et al that included 1200 women who were attempting to conceive found that obesity was associated with a greater risk of anovulatory cycles. Higher baseline adiposity, as indicated by body mass index, hip and waist circumferences, and skinfold measurements, was a modest predictor of anovulation, even in women who did not have a clinical diagnosis of infertility or PCOS. [17]
Hirsutism and acne
Hirsutism is the excessive growth of terminal hair, usually in a midline distribution or male-type pattern. The condition is characterized by a response of the pilosebaceous unit to androgens, causing a transformation of vellus to terminal hair in androgen-dependent areas. [18] Hirsutism is classified into the following groups: (1) idiopathic, (2) drug-induced, and (3) due to androgen excess. Most cases of hirsutism result from a combination of mildly increased androgen production and increased skin sensitivity to androgens. Cases that are not androgen-dependent (eg, those caused by medications or familial hypertrichosis) are best diagnosed by physical examination.
Approximately 95% of women with androgen-dependent hirsutism have both adrenal and ovarian causes. The most common cause of hirsutism is PCOS. Additionally, different cultures and races tend to have or display hair in different amounts and locations. These patients are generally classified into the first group in the presence of normal levels of circulating androgens.
If excess androgens are the primary cause of the hirsutism, androgen receptors are found in the anagen (active) phase in the follicles' dermal papillae and associated sebaceous glands, where stimulation is provided by the more potent androgens testosterone and dihydrotestosterone. In excessive amounts, they cause hirsutism, except on the scalp, where androgenic alopecia is induced. [19]
A clinical tool that offers an objective assessment of the hirsute patient is the modified Ferriman-Gallwey scale proposed by Hatch and coworkers. It takes into account 9 of the 11 sites originally described, each receiving a score of 0-4 (maximum). A score of 8 or more is considered significant.
The presence of hirsutism in conjunction with other signs of virilization should alert the physician to the possibility of androgen-producing tumors of adrenal or ovarian origin.
Acne vulgaris, usually seen in adolescence, is directly associated with the level of circulating androgen concentration. In a study of postpubertal women with the chief complaint of acne, PCOS was diagnosed in 37% of participants. [20]
Chronic anovulation
Chronic anovulation with estrogen present can occur in a variety of endocrine disorders. It can occur in women with PCOS and other functional abnormalities, including those with Cushing syndrome, hyperthyroidism, hypothyroidism, late-onset adrenal hyperplasia resulting from 21-hydroxylase deficiency, 11-alpha-hydroxylase deficiency, or 3-beta-hydroxysteroid dehydrogenase deficiency.
Chronic anovulation may also involve tumors of the ovary, including granulosa-theca cell tumors, Brenner tumors, cystic teratomas, mucinous cystadenomas, and Krukenberg tumors. These tumors secrete excess estrogen or androgens that undergo aromatization in extraglandular sites.
Premature ovarian failure
Premature ovarian failure (POF), also known as hypergonadotropic hypogonadism, occurs when oocytes and the surrounding cells are lost prior to 40 years of age. Because the ovaries do not respond to FSH and LH (hypogonadism), there is no negative feedback creating a hypergonadotropic state. POF is diagnosed with 2 serum FSH levels greater than 40 mIU/mL at least 1 month apart. The incidence of POF has been estimated by Coulam in 1986 as 1 in 1,000 women younger than 30 years, and 1 in 100 women younger than 40 years. Gonadal dysgenesis is the most frequent cause of POF, two thirds of which are a result of a deletion on an X chromosome. Although a normal complement of germ cells is present in the early fetal ovary, oocytes undergo accelerated atresia, and the ovary is replaced by a fibrous streak. [21]
Cushing syndrome
Cushing syndrome is characterized by hypercortisolism. Its clinical manifestations encompass a spectrum of symptoms, the severity of which is often influenced by the presence or absence of androgen excess. Progressive obesity and abnormal waist/hip ratio are not uncommon, although the limbs are often spared. Frequently, patients experience proximal muscle weakness and cannot rise from a sitting position. Patients can also be hypertensive as a result of mineralocorticoid excess.
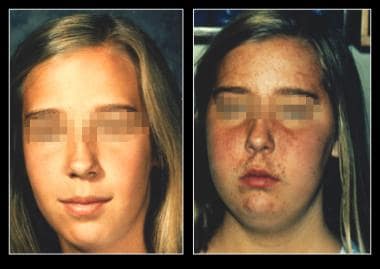 Anovulation. On the left is an unaffected patient aged 12 years. On the right is the same patient aged 13 years after developing Cushing disease.
Anovulation. On the left is an unaffected patient aged 12 years. On the right is the same patient aged 13 years after developing Cushing disease.
Normally, cortisol-releasing hormone and hypothalamic factors are released into the hypophyseal portal blood and carried to the anterior pituitary, where they stimulate the release of adrenocorticotropic hormone (ACTH). In Cushing disease, the increased plasma ACTH concentrations stimulate increased adrenocorticoid secretion, thus inhibiting hypothalamic corticotropin-releasing hormone (CRH) secretion and other factors from the pituitary. This aberration in the cycle, as well as changes in secretory patterns, are thought to influence the mechanisms involved in ovulatory function. Although the exact mechanisms have not been elucidated, it is believed that hypercortisolism and adrenal hyperandrogenism suppress gonadotropin secretion with impaired LH response to GnRH.
Adrenal insufficiency
Adrenal insufficiency can be due to a variety of causes, including deficient hypothalamic secretion of CRH, deficient pituitary secretion of ACTH, or destruction of the adrenal cortex (Addison disease, primary adrenal insufficiency).
Adrenal androgen deficiency results in loss of axillary and pubic hair in women and decreased libido. Women with autoimmune adrenal insufficiency are at increased risk of premature ovarian failure and anovulation.
Congenital adrenal hyperplasia
CAH encompasses a group of autosomal recessive disorders caused by an inherited deficiency of enzymes involved in cortisol synthesis in the adrenal cortex.
These disorders, listed in decreasing order of frequency, include a deficiency of 21-hydroxylase, 11-alpha-hydroxylase, and 3-beta-hydroxysteroid dehydrogenase. Hyperandrogenism induces a disruption of the HPO axis and consequently leads to menstrual irregularities or anovulatory cycles.
Some women experience oligomenorrhea. Increased thyroid hormone levels raise SHBG production and therefore serum levels, reflecting increased tissue response to these hormones. Total estrogen and testosterone circulating levels are also increased.
The levels of active or free fractions of these sex steroids are often reduced. Treatment of hyperthyroidism results in regular ovulatory menstrual cycles and fertility. Mid luteal phase progesterone levels in thyrotoxic women improve after treatment.
Women with hypothyroidism often experience the spectrum of menorrhagia and metrorrhagia. Patients with hypothyroidism have reduced levels of SHBG and decreased levels of circulating estrogens and testosterone. Follicle-stimulating hormone (FSH) and LH levels are also reduced. Hypothyroidism can cause aberrations in coagulation. It is believed that a hypothyroid state leads to decreased levels of factors VII, VIII, IX, and XI. Hypothyroidism is the most common endocrinologic condition associated with anovulation.
In addition, hypothyroidism alters steroid metabolism and clearance, which may lead to endometrial dysfunction. This evidence supports the fact that menorrhagia is a symptom of hypothyroidism. These symptoms are easily treated with thyroid hormone replacement. Hypothyroidism leads to increased levels of thyroid-releasing hormone and therefore increased levels of thyroid-stimulating hormone (TSH [ie, thyrotropin]) and prolactin. Hyperprolactinemia from long-standing primary hypothyroidism may be responsible for varying degrees of ovulatory dysfunction.
Prolactin excess manifests clinically as infertility, oligomenorrhea, and amenorrhea. The mechanism seems to involve the inhibition of pituitary gonadotropins via suppression of GnRH pulsatility. As a result of this inhibition, serum gonadotropin levels are significantly decreased, causing secondary hypogonadism. Mild hyperprolactinemia may cause infertility, even in the presence of a regular menstrual cycle, while elevated levels of prolactin may cause galactorrhea.
Patients with hyperprolactinemia must always have a complete history and physical examination to rule out easily correctable causes of hyperprolactinemia. Some of these causes include medication usage (eg, exogenous estrogens, neuroleptics, antidepressants, some antipsychotic medications). MRI should be obtained to rule out a mass lesion in the hypothalamic-pituitary region (see image below). Usually, imaging is not warranted unless levels surpass 100 ng/mL after a negative pregnancy test result. Normalization of prolactin levels through the adjustment of current medications or addition of bromocriptine restores ovulation and fertility.
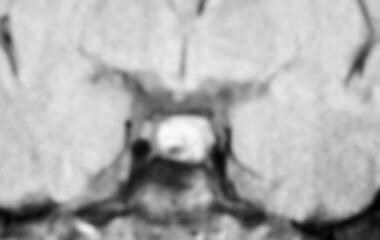 Anovulation. MRI showing a nonenhancing area in the pituitary consistent with a microadenoma in a patient with hyperprolactinemia.
Anovulation. MRI showing a nonenhancing area in the pituitary consistent with a microadenoma in a patient with hyperprolactinemia.
Eating disorders and aggressive exercise habits
Anorexia is defined as a serious, usually chronic psychiatric disorder of an indolent nature that can be life-threatening if unrecognized. The condition is characterized by a patient's inability to maintain a weight of at least 85% of her ideal body weight. Other essential features of the disorder include an intense fear of gaining weight, a distorted body image (body dysmorphic syndrome), and amenorrhea.
Below-average body weight is not in itself enough to cause amenorrhea; the numerous mechanisms intimately related to profound weight loss are also factors. Frisch and McArthur have suggested that a minimum of 17% body fat is required for the initiation of menses, and at least 22% body fat is necessary for menstrual cyclicity. [22] Leptin, a serum hormone secreted by adipose tissues in proportion to total body lipid stores, has recently received much attention for its role in the pathogenesis of weight and menstrual control in these patients.
A diurnal secretion pattern has been established in women of reproductive age who have a normal body mass index (BMI). Athletes with exercise-induced amenorrhea exhibit leptin levels similar to those of prepubertal girls and consequently lose the diurnal release pattern. Andrico et al demonstrated that leptin levels are significantly lower in patients with functional hypothalamic amenorrhea compared with those of controls who were matched for both age and weight. [23] These investigators suggest that energy balance can interfere with the ratios of body weight to leptin and BMI to leptin in functional hypothalamic amenorrhea.
The consequences of exercise-induced amenorrhea are similar to those of estrogen deficiency. These patients are at an increased risk of osteopenia and, eventually, osteoporosis if not treated expeditiously. Vaginal atrophy and infertility issues are generally the rule. A multidisciplinary approach is therefore necessary when treating these patients in order to address underlying issues and physical manifestations.
Amenorrhea is a consequence of multiple alterations in female hormonal pathways. Anorexic women show a 24-hour pattern of FSH and LH similar to that observed in prepubescent children. They may also exhibit altered ACTH levels and characteristically low triiodothyronine (T3) levels.
The mechanisms involved in amenorrhea caused by excessive exercise or anorexia nervosa are not well understood. However, links seem to exist among malnutrition, chronic disease, and hormonal alterations. Excessive levels of endogenous opioids, with a concomitant increase in CRH secretion, inhibit GnRH release as well. Patients can expect an increased risk of infertility, vaginal and breast atrophy, and osteopenia.
Bulimia nervosa is a another eating disorder in which a female consumes large amounts of food followed by inappropriate behavior to avoid weight gain, [24] most commonly by inducing vomiting, otherwise known as the binge-and-purge cycle. Fluctuations or extreme weight loss are not common. However, these patients usually experience menstrual irregularities or anovulatory cycles. Commonly, they have halitosis and dental caries. They may also exhibit strange behavior when eating in a group setting. Bulimic patients usually prefer to eat during times that do not coincide with those of other members of the household.
The pathophysiology of anovulation in bulimic women is very similar to that of anorexic women, although the neuroendocrine disturbances are significantly milder than in anorexia. LH levels are reduced because of a blunting of the GnRH pulse generator, which leads to the menstrual disturbances commonly observed. Thyroid function is altered, as well as glucose tolerance, with accompanying hypercortisolemia.
Hypothalamic and pituitary causes
The presence of GnRH is essential; studies have shown that the administration of a GnRH antagonist to women at midcycle prevents the LH surge. The physiologic capabilities of the hypothalamic GnRH neurons and pituitary gland are subject to disruption by several pathologic entities, including tumors, trauma, and irradiation.
Hypothalamic causes include tumors, trauma, and irradiation
Note the following:
-
Hypothalamic tumors can produce space-occupying lesions that are frequently suprasellar. These tumors can cause abnormally low levels of circulating pituitary hormone by compression of the hypothalamus or pituitary stalk, essentially causing delayed puberty, anovulation, and amenorrhea. Craniopharyngioma is an epithelial cell tumor of nonpituitary origin that accounts for approximately 3% of intracranial neoplasms; it is the most common neoplasm associated with delayed puberty. Craniopharyngiomas arise from remnants of Rathke pouch epithelium and result from incomplete closure of the hypophyseal or craniopharyngeal duct.
-
The clinical features are dependent upon the rate of growth of the tumor and can include visual impairment (ie, bitemporal hemianopsia [70%] due to compression of the optic chiasm), pituitary-adrenal dysfunction (50%), hypothyroidism (25%), somnolence (20%), and diabetes insipidus (10%). [4, 25] The evaluation of craniopharyngioma includes the use of CT scanning and MRI. Most lesions are evidenced by enlargement of the pituitary fossa, and suprasellar extension and calcifications are common. Treatment involves surgical extirpation followed by intensive postoperative radiation.
-
A major complication is both hypothalamic and pituitary deficiency despite a conservative surgical approach. After surgical management with tumor irradiation, the 10-year survival rate for people with craniopharyngioma is 70-80%. [25]
-
An endodermal sinus tumor is a highly malignant germ cell tumor of the hypothalamus commonly observed in the first and second decades of life. Believed to be derived from antecedents of the yolk sac, it produces alpha-fetoprotein. It is a highly vascular neoplasm that metastasizes early to various regions of the CNS.
-
Hand-Schüller-Christian disease (histiocytosis X) is a rare destructive lesion in children that causes delayed puberty, growth retardation, and diabetes insipidus. These tumors are composed of mononuclear phagocytic cells (histiocytes) that infiltrate and destroy hypothalamic tissue. Clinical features include visual disturbances, hyperprolactinemia, hypopituitarism, anovulation, obesity, and growth retardation. The first presenting symptom is usually growth retardation in a child. Diagnosis is established by biopsy of the lesion, and subsequent treatment is with radiation therapy and growth hormone (GH) replacement.
-
Head injury can cause hypothalamic damage and result in secondary hypopituitarism (ie, a lack of appropriate hypothalamic-releasing factors). The presence of hyperprolactinemia resulting from the traumatic disruption of dopamine inhibition provides evidence of the location of the lesion and helps the clinician differentiate hypothalamic damage from panhypopituitarism in the presence of normal or even low levels of other pituitary hormones.
-
Motor vehicle accidents are thought to cause transection of the hypothalamic-pituitary stalk during forward motion of the head. Transection usually manifests first as diabetes insipidus and then as secondary hypopituitarism and hyperprolactinemia.
-
The hypothalamus is radiosensitive; therefore, external irradiation can cause damage to the hypothalamus and impair its function. In contrast, pituitary cells are relatively radioresistant. Therefore, hypopituitarism that occurs after the administration of external irradiation to the brain typically results from hypothalamic damage.
-
Similarly, irradiation of the head and neck for treatment of nasopharyngeal cancer frequently results in hypopituitarism as early as 1 year after therapy. [25]
Pituitary causes include trauma and irradiation.
Note the following:
-
The most common cause of pituitary trauma is iatrogenic, occurring during neurosurgery. Other causes include birth trauma, intracranial hemorrhage, fetal asphyxia, and breech delivery, all of which may lead to direct pituitary damage. A sella turcica fracture or pituitary stalk transsection can cause panhypopituitarism and/or diabetes insipidus (if the posterior pituitary is involved). The degree of hypopituitarism varies and typically manifests within a year after trauma has occurred. Many patients do not experience signs of posttraumatic pituitary failure for several decades.
-
Irradiation can cause a significant degree of reproductive dysfunction and anovulation, usually due to hypothalamic damage because pituitary cells exhibit little radiosensitivity. Anticancer treatments are also associated with psychologic stress and weight loss, which can magnify the degree of menstrual dysfunction. The effects of cranial irradiation are dose related, and 70% of women undergoing cranial irradiation experience menstrual irregularities. [26]
Nonneoplastic causes of hypopituitarism include empty sella syndrome, Sheehan syndrome, pituitary apoplexy, and pituitary adenomas.
Note the following:
-
Empty sella syndrome is a benign cause of hyperprolactinemia in 4-16% of women who present with amenorrhea and galactorrhea. [2] It is characterized by an abnormal relationship between the sella turcica and the sellar diaphragm that results in the herniation of the subarachnoid space into the pituitary fossa. A flattening of the pituitary gland and concomitant separation of the gland from the hypothalamus occurs, which can result in the inhibition of GnRH and subsequent anovulation. The degree of flattening of the pituitary also plays a role in the anovulation. Typically, if 90% of the gland is compressed, pituitary failure ensues.
-
The characteristic changes in the relationships between the sellar contents and the suprasellar arachnoid space can be congenital (primary empty sella syndrome) or can result from surgery, radiation, or spontaneous infarction of pituitary adenomas (secondary empty sella syndrome). The diagnosis of empty sella syndrome is usually incidental in patients being evaluated for symptoms of headache and/or an unusual presentation of rhinorrhea. The preferred radiologic imaging modality, MRI, reveals a symmetrically enlarged sella turcica from cerebrospinal fluid (CSF) and demonstrable pituitary flattening with lateral stalk deviation, which can make the fossa initially appear empty.
-
CT-guided intrathecal injection of contrast (metrizamide) can be also be used to demonstrate abnormalities in intracranial CSF distribution. The resultant endocrine abnormalities are varied. Because empty sella syndrome is associated with compression of the infundibular stalk, the disruption of dopamine reaching the pituitary leads to concomitant hyperprolactinemia (5%). Other endocrine abnormalities are rare; however, isolated ACTH deficiency and GH hypersecretion or deficiency have also been described. Treatment for empty sella syndrome is focused on hormone replacement and/or dopamine agonists for hyperprolactinemia in the anovulatory patient.
-
Sheehan syndrome was initially described by Sheehan in 1939 as postpartum pituitary necrosis that results from significant intrapartum or postpartum hemorrhage or shock. It is considered the most common cause of panhypopituitarism in women of childbearing age. [27] During pregnancy, the anterior pituitary lactotrophs double in size. As a result, the demand for oxygen is greater, and the response to hypotension from hemorrhage is magnified.
-
Rapid hypotension from hemorrhage can interrupt the venous return to the gland and result in necrosis. The initial presenting sign of panhypopituitarism is usually failure to lactate postpartum, followed by amenorrhea, loss of pubic or axillary hair, secondary adrenal insufficiency, anorexia, lethargy, and weight loss. These symptoms are often delayed, and the degree of hypopituitarism varies. Slow clinical progression could suggest other factors involved in the pathogenesis of the disease, such as autoimmunity from antigens released from an infarcted gland. [28]
-
The diagnosis is confirmed based on the history and the results from the tests of anterior pituitary reserve: GH, ACTH, prolactin, TSH, LH, and FSH, all of which are found in very low levels. GH deficiency is the most common (90%), followed by ACTH (66%), LH/FSH (65%), and TSH (42%). Posterior pituitary involvement is rare, with central diabetes insipidus occurring in fewer than 5% of cases. [29] The treatment of Sheehan syndrome is based on appropriate hormone replacement. In patients receiving gonadotropins, successful ovulation and eventual pregnancy is common.
-
Pituitary apoplexy is a life-threatening disorder characterized by an acute massive infarction of the pituitary gland; it is a medical emergency that usually results from preexisting, endocrinologically inactive adenomas or tumors of the pituitary gland. The extent of hemorrhage, edema, and infarction of the gland determines the degree of compression of adjacent structures and subsequent neurologic damage. Patients typically present with acute onset of the triad of excruciating, localized retro-orbital headache (95%); nausea and vomiting (65%); and ophthalmoplegia (78%) secondary to compression and stretching of the meninges adjacent to the sella. The tumor expansion can also cause visual field defects and loss of pituitary function, demonstrable by low levels of GH, ACTH, LH/FSH, and TSH.
-
Management of this condition involves immediate administration of cortisol, supportive measures, and surgical intervention and decompression of the sella through a transsphenoidal approach. Studies have shown that patients who undergo surgical decompression within 1 week of the infarction have significant return of their visual defects compared with those undergoing delayed intervention. [30] The mortality rate accompanying this condition is high when exogenous cortisol is not administered after recognition of an ACTH deficiency. For patients with anovulation, treatment is supportive. Studies of amenorrheic adolescents who undergo surgical intervention for infarcted microadenomas have shown an 80% postsurgical improvement in endocrine function. [31]
-
Approximately 10% of all intracranial tumors are pituitary adenomas, with hypersecretion of biologically active hormones. The most common endocrinopathies are acromegaly due to GH excess, Cushing disease due to ACTH excess, and amenorrhea/galactorrhea syndrome due to prolactin excess (prolactinoma). Elevated gonadotropin levels can also result from gonadotropin-secreting pituitary adenomas. However, these rare adenomas are not associated with amenorrhea and usually secrete the beta subunit of FSH and rarely LH. The most common of the pituitary adenomas are prolactinomas.
-
These tumors are rarely life threatening; they result in amenorrhea, galactorrhea, and/or visual defects due to impingement of the optic chiasm from the space-occupying characteristics of the tumor. Previously, prolactinomas were thought to arise from dysregulation of prolactin due to the idiopathic attenuation of hypothalamic dopamine. Recent studies have described a genetic basis for tumor development. Prolactinomas are now thought to arise from proliferation of a single mutated lactotroph (prolactin-producing cell), usually in the lateral wing of the pituitary gland. These tumors have been found to occur in 20% of patients with multiple endocrine neoplasia syndrome, type 1 (MEN 1). [32]
-
Women who present with amenorrhea/galactorrhea syndrome should undergo laboratory testing for prolactin. levels of more than 250 ng/mL usually indicate prolactin-secreting adenomas, and levels of less than 150 ng/mL are usually secondary to large nonsecreting tumors of the pituitary. MRI or CT scanning with contrast can be used to help confirm diagnosis; however, both techniques have a high false-positive rate because they detect small nonsecreting tumors, cysts, or infarcts. Visual field testing is performed on symptomatic patients.
-
Treatment is reserved for women who experience either effects of tumor size or effects of hyperprolactinemia. [32] Most patients with microadenomas (< 10 mm) are managed conservatively with serial prolactin levels and follow-up MRI if they become symptomatic with headache or if prolactin levels rise significantly. Macroadenomas (>10 mm) may require more aggressive interventions, such as surgery or irradiation. Medical therapy with dopamine agonist therapy (eg, bromocriptine, cabergoline) can be used in patients with anovulatory menstrual cycles, decreased libido, sexual dysfunction, hirsutism, and osteoporosis. For women desiring fertility, bromocriptine is typically used until the fourth week of pregnancy. Cabergoline is not used for ovulation induction because of the lack of significant data on its possible adverse effects or teratogenicity in pregnancy.
-
Other indications for medical treatment include decreased libido, sexual dysfunction, hirsutism, and galactorrhea.
Epidemiology
United States statistics
Almost all women experience anovulatory cycles at some point in their reproductive lives. Yet, to attempt to determine the frequency of chronic anovulation in the general population is quite difficult because of underreporting. Estimates of chronic anovulation rates range from 6-15% of women during the reproductive years.
Interestingly, an article by Rasgon introduced a certain subset of the population as being at an increased risk for anovulatory disorders, stating that reproductive endocrine disorders, such as PCOS, hypothalamic amenorrhea, premature menopause, and hyperprolactinemia, are reportedly more common in women with epilepsy than in the general female population. The article further elaborates on the frequency of PCOS in patients who endure epilepsy independent of the use of antiepileptic therapy. [33] The risk of developing PCOS during valproate (VPA) treatment seems to be higher in women with epilepsy than in women with bipolar disorders; this might be due to an underlying neuroendocrine dysfunction. Gynecologists must be aware of the possibility that PCOS might be related to VPA use in this population of patients, and the risks and benefits of this treatment should be weighed in the presence of PCOS. [34]
Race-, sex-, and age-related demographics
Race
In one study, the frequency of anovulation was greater among white women (9 of 63 [14.3%]) than black women (4 of 56 [7.1%]) or Hispanic women (7 of 102 [6.9%]), although these differences were not statistically significant. [35]
Sex
Anovulation occurs only in women of reproductive age.
Age
Anovulation is physiologic at the extremes of reproductive age. During menarche, absence of ovulation is due to immaturity of the HPO axis, leading to an uncoordinated secretion of GnRH (pulsatility).
During perimenopause, ovarian factors and a dysregulation of feedback mechanisms are responsible.
When anovulation occurs outside of the perimenarchal or perimenopausal years, extrinsic and intrinsic causes must be excluded.
Prognosis
Morbidity/mortality
Prognosis is generally favorable with appropriate and timely treatment.
Morbidities associated with chronic anovulation include hyperinsulinemia, insulin resistance, early onset of type 2 diabetes mellitus, dyslipidemia, cardiovascular disease, hypertension, infertility, endometrial hyperplasia, and endometrial cancer.
Complications
Complications of anovulation include the following:
-
Endometrial hyperplasia
-
Insulin resistance or type 2 diabetes mellitus
-
Cardiovascular disease
-
Venous thromboembolism secondary to estrogen therapy
-
Electrolyte derangements (anorexia nervosa)
-
Arrhythmias (anorexia nervosa)
Pregnancy complications
Women with PCOS who conceive are at increased risk for gestational diabetes, preeclampsia, cesarean delivery, and preterm and post-term delivery. Their newborns are at increased risk of being large for gestational age but are not at increased risk of stillbirth or neonatal death. [36]
-
Anovulation. Polycystic ovary. Courtesy of Jairo E. Garcia, MD.
-
Anovulation. On the left is an unaffected patient aged 12 years. On the right is the same patient aged 13 years after developing Cushing disease.
-
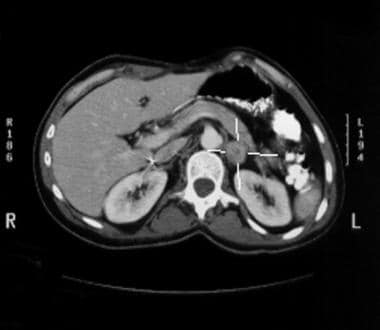
Anovulation. Left adrenal mass discovered incidentally.
-
Anovulation. MRI showing a nonenhancing area in the pituitary consistent with a microadenoma in a patient with hyperprolactinemia.