Practice Essentials
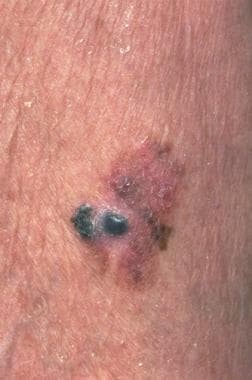
Malignant melanoma (see the image below) is a neoplasm of melanocytes or of the cells that develop from melanocytes. Although it was once considered uncommon, the annual incidence has increased dramatically over the past few decades. Surgery is the definitive treatment for early-stage melanoma, with medical management generally reserved for adjuvant treatment of high-risk locally advanced melanoma and metastatic disease.
Signs and symptoms
The history should address the following:
-
Family history of melanoma or skin cancer
-
Family history of irregular, prominent moles
-
Family history of pancreatic cancer or astrocytoma
-
Previous melanoma (sometimes multiple; patients have reported as many as 8 or more primary melanomas)
-
Lifetime sun exposure
-
Changes noted in moles (eg, size, color, symmetry, bleeding, or ulceration)
-
History or family history of multiple nevus syndrome
Physical examination includes the following:
-
Total-body skin examination, to be performed on initial evaluation and during all subsequent visits
-
Serial photography, epiluminescence microscopy, and computerized image analysis, to be considered as adjuncts
Skin examination involves assessing the number of nevi present and distinguishing between typical and atypical lesions. (The images below depict examples of melanomas.) Early melanomas may be differentiated from benign nevi by the ABCDE criteria, as follows:
-
A - Asymmetry
-
B - Border irregularity
-
C - Color that tends to be very dark black or blue and variable
-
D - Diameter ≥6 mm
-
E - Evolution over time
If a patient is diagnosed with a melanoma, examine all lymph node groups.
See Presentation for more detail.
Diagnosis
The following studies may be helpful in some cases:
-
Gene profiling assays
-
Lactate dehydrogenase
The following imaging modalities may be considered in patients with deep melanomas, or when indicated by signs or symptoms:
-
Chest radiography
-
Magnetic resonance imaging of the brain
-
Ultrasonography (possibly the best imaging study for diagnosing lymph node involvement)
-
Computed tomography of the chest, abdomen, or pelvis
-
Positron emission tomography (PET; PET-CT may be the best imaging study for identifying other sites of metastasis)
Procedures to be considered in the workup include the following:
-
Complete excisional biopsy of a suggestive lesion
-
Saucerized removal of thinner lesions
-
Sampling of each color within a large lesion, such as suspected lentigo maligna on the face
-
Surgical excision or reexcision after biopsy
-
Sentinel lymph node biopsy (SLNB; see Sentinel Lymph Node Biopsy in Patients With Melanoma)
Characteristic histologic findings include the following:
-
Bilateral symmetry
-
Poor nesting
-
Peripheral border fails to end in a nest
-
Poor maturation
-
Poor dispersion at the base
-
Cytologic atypia, with enlarged cells containing large, pleomorphic, hyperchromic nuclei with prominent nucleoli
-
Numerous or atypical mitotic figures
-
Pagetoid growth pattern with upward growth of the melanocytes
See Workup for more detail.
Management
Surgery (eg, wide local excision with SLNB and regional lymph node dissection if indicated) is the definitive treatment for early-stage melanoma. Preoperative (neoadjuvant) therapy may be appropriate in select cases; adjuvant therapy is used in patients with advanced, metastatic, unresectable, or recurrent melanoma. [1]
Adjuvant therapy for resectable stage III melanoma includes the following agents [2] :
-
Immunotherapy with checkpoint inhibitors – Nivolumab, pembrolizumab, ipilimumab, and relatlimab
-
Signal-transduction inhibitor therapy, if BRAF V600 mutation positive – BRAF and MEK inhibitors
For unresectable stage III, stage IV, and recurrent melanoma, adjuvant therapy may include the following [2] :
-
Immunotherapy
-
Signal-transduction inhibitors
-
Intralesional therapy (talimogene laherparepvec)
-
Chemotherapy
-
Palliative local therapy
Immunotherapy:
-
Interleukin-2
Cellular therapies:
-
Autologous tumor-infiltrating lymphocytes (TILs) – Lifileucel [6]
Signal-transduction inhibitors:
-
BRAF inhibitors - Vemurafenib, dabrafenib
-
MEK inhibitors - Trametinib, cobimetinib
-
KIT inhibitors - Imatinib mesylate [7]
Chemotherapy agents:
-
Dacarbazine (DTIC)
-
Temozolomide
-
Cisplatin, vinblastine, and dacarbazine (CVD)
-
Cisplatin, dacarbazine, carmustine, and tamoxifen (Dartmouth regimen)
-
Carboplatin and paclitaxel (sometimes combined with sorafenib)
The following procedures may be used to treat brain metastases:
-
Stereotactic radiosurgery (for patients with a limited number of metastases)
-
External-beam radiation
See Treatment and Medication for more detail.
This article focuses on cutaneous melanoma. For discussion of melanoma arising at other sites, see Oral Malignant Melanoma, Head and Neck Mucosal Melanomas, and Choroidal Melanoma.
Pathophysiology
Melanomas have two growth phases: radial and vertical. During the radial growth phase, malignant cells grow in a radial fashion in the epidermis. With time, most melanomas progress to the vertical growth phase, in which the malignant cells invade the dermis and develop the ability to metastasize. This is usually indicated by a dermal nest larger than the largest junctional nest, a dermal nest with mitosis, or a dermal nest within reticular dermis. (See Etiology and Workup.)
Clinically, lesions are classified according to their depth, as follows:
-
Thin: 1 mm or less
-
Moderate: 1-4 mm
-
Thick: > 4 mm
Histologic types of melanoma
There are five different forms, or histologic types, of melanoma, and specific gene mutations determine behavior and response to treatment::
-
Superficial spreading
-
Nodular
-
Acral lentiginous
-
Mucosal lentiginous
Superficial spreading melanomas
Approximately 70% of cutaneous malignant melanomas are the superficial spreading melanoma (SSM) type. Many SSMs arise from a pigmented dysplastic nevus, often one that has long been stable. Typical changes include ulceration, enlargement, or color changes. An SSM may be found on any body surface, especially the head, neck, and trunk of males and the lower extremities of females.
Nodular melanomas
Nodular melanomas (NMs) represent approximately 10-15% of melanomas and also are found commonly on all body surfaces, especially the trunk of males. These lesions are the most symmetrical and uniform of the melanomas and are dark brown or black. The radial growth phase may not be evident in NMs; however, if this phase is evident, it is short-lived, because the tumor advances rapidly to the vertical growth phase, thus making the NM a high-risk lesion. Approximately 5% of all NMs are amelanotic melanomas.
Lentigo maligna melanomas
Lentigo maligna melanomas (LMMs) also account for 10-15% of melanomas. They typically are found on sun-exposed areas (eg, hand, neck). LMMs may have areas of hypopigmentation and often are quite large. LMMs arise from a lentigo maligna precursor lesion. (See the image of lentigo maligna melanoma below.)
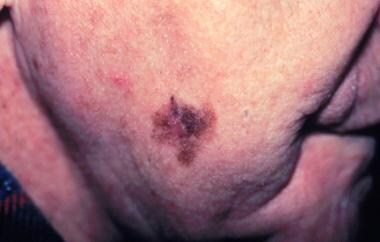 Lentigo maligna melanoma, right lower cheek. The centrally located erythematous papule represents invasive melanoma with surrounding macular lentigo maligna (melanoma in situ). Image courtesy of Susan M. Swetter, MD.
Lentigo maligna melanoma, right lower cheek. The centrally located erythematous papule represents invasive melanoma with surrounding macular lentigo maligna (melanoma in situ). Image courtesy of Susan M. Swetter, MD.
Acral lentiginous melanomas
Acral lentiginous melanomas (ALMs) are the only melanomas that have an equal frequency in Blacks and Whites. They occur on the palms, soles, and subungual areas. Subungual melanomas often are mistaken for subungual hematomas (splinter hemorrhages). Like NM, ALM is extremely aggressive, with rapid progression from the radial to vertical growth phase.
Mucosal lentiginous melanomas
Mucosal lentiginous melanomas (MLMs) develop from the mucosal epithelium that lines the respiratory, gastrointestinal, and genitourinary tracts. MLMs are rare, accounting for only for 0.8–1.8% of all melanomas in the United States; in Chinese populations, the relative incidence has been reported as high as 23%, likely because of the lower prevalence of cutaneous melanoma in Asian populations. The head and neck — principally the nasal cavity and paranasal sinuses — is the most common site for MLMs, accounting for approximately half of all cases, but MLMs may occur on any mucosal surface, including the conjunctiva, oral cavity, esophagus, vagina, female urethra, penis, and anus. [8, 9]
Noncutaneous melanomas commonly are diagnosed in patients of advanced age. MLMs appear to have a more aggressive course than cutaneous melanomas, although this may be in part because they commonly are diagnosed at a later stage of disease than the more readily apparent cutaneous melanomas. In addition, the genetic alterations and oncogenic drivers in mucosal melanomas differ from those in cutaneous melanomas. The BRAF and NRAS mutations that are common drivers in cutaneous melanoma are less often found in mucosal melanoma, whereas SF3B1 and KIT mutations are more often found. [8]
Sites other than the skin
The majority of melanomas are in the skin, but other sites include the eyes, mucosa, gastrointestinal tract, genitourinary tract, and leptomeninges. Metastatic melanoma with an unknown primary site may be found in lymph nodes only.
Etiology
Melanomas originate from melanocytes, which arise from the neural crest and migrate to the epidermis, uvea, meninges, and ectodermal mucosa. The melanocytes, which reside in the skin and produce a protective melanin, are contained within the basal layer of the epidermis, at the junction of the dermis and epidermis.
Melanomas may develop in or near a previously existing precursor lesion or in healthy-appearing skin. A malignant melanoma developing in healthy skin is said to arise de novo, without evidence of a precursor lesion. Many of these melanomas are induced by solar irradiation, either chronic or intermittent. Melanoma also may occur in unexposed areas of the skin, including the palms, soles, and perineum.
Certain lesions are considered to be precursor lesions of melanoma. These include the following nevi:
-
Common acquired nevus
-
Dysplastic nevus, especially those with a broad lentiginous growth pattern
-
Congenital nevus
-
Cellular blue nevus
-
Deep penetrating nevus
Genetics
Many genes are implicated in the development of melanoma, including TERT, CDKN2A (p16), CDK4, RB1, CDKN2A (p19), PTEN/MMAC1, and ras. CDKN2A (p16) appears to be especially important in both sporadic and hereditary melanomas. This tumor suppressor gene is located on band 9p21, and its mutation plays a role in various cancers.
Ultraviolet radiation
Exposure to ultraviolet (UV) radiation is a critical factor in the development of most melanomas. Ultraviolet A (UVA), wavelength 320-400 nm, and ultraviolet B (UVB), 290-320 nm, potentially are carcinogenic and actually may work in concert to induce a melanoma.
UV radiation appears to be an effective inducer of melanoma through many mechanisms, including suppression of the immune system of the skin, induction of melanocyte cell division, free radical production, and damage of melanocyte DNA.
Interestingly, melanoma does not have a direct relationship with the amount of sun exposure, because it is more common in white-collar workers than in those who work outdoors.
Sunburn
Acute, intense, and intermittent blistering sunburns, especially on areas of the body that only occasionally receive sun exposure, are the greatest risk factor for the development of sun exposure–induced melanoma on the trunk and legs, whereas lentigo maligna is associated with chronic sun exposure. This sun-associated risk factor is different from that for squamous and basal cell skin cancers, which are associated with prolonged, long-term sun exposure.
Parkinson disease
Retrospective case-control analyses from the Mayo Clinic concluded that patients with Parkinson disease (PD) have about a 4-fold increased risk of having preexisting melanoma, and patients with melanoma have a similar risk of developing PD. The results support studies by other researchers showing an increased risk for melanoma in patients with PD. [10]
In the Mayo Clinic study, Dalvin et al found 32 cases of melanoma in 974 patients with PD (with 26 of the 32 diagnosed before the onset of PD), versus 63 cases in the control group of 2922 persons without PD; thus, the likelihood of having a history of melanoma was 3.8-fold higher in patients with PD compared with controls (95% confidence interval [CI], 2.1 - 6.8; P< 0.001).
In a second analysis, Dalvin et al found 43 cases of PD in 1544 patients diagnosed with melanoma, compared with 14 cases of PD in a control group of 1544 persons without melanoma. This translated to a 4.2-fold increased risk for PD after being diagnosed with melanoma, compared with controls (95% CI, 2.0 - 8.8; P< 0.001). [10]
Additional risk factors
Importantly, other factors exist that may predispose an individual to melanoma; chemicals and viruses are etiologic agents that also have been implicated in the development of melanoma.
Greatly elevated risk factors for cutaneous melanoma include the following:
-
Changing mole
-
Dysplastic nevi in familial melanoma
-
More than 50 nevi, 2 mm or greater in diameter
Moderately elevated risk factors for cutaneous melanoma include the following:
-
One family member with melanoma
-
Previous history of melanoma
-
Sporadic dysplastic nevi
-
Congenital nevus
Slightly elevated risk factors for cutaneous melanoma include the following:
-
Immunosuppression
-
Sun sensitivity
-
History of acute, severe, blistering sunburns
-
Freckling
Epidemiology
Occurrence in the United States
The American Cancer Society estimates that 100,640 cases of invasive cutaneous melanoma will be diagnosed in the United States in 2024 (59,170 in men and 41,470 in women), along with 99,700 cases of in situ melanoma. Since the early 2000s, incidence rates of melanoma in persons younger than age 50 years have stabilized in women and declined by about 1% per year in men; in adults age 50 and older, rates increased in women by about 3% per year but stabilized in men. [11]
Although melanoma accounts for only about 1% of skin cancers, it is responsible for the vast majority of deaths from skin cancers. The American Cancer Society estimates that 8290 people in the US (5430 men and 2860 women) will die of melanoma in 2024. [11]
However, a review of Surveillance, Epidemiology, and End Results (SEER) data from 1975 to 2014 identified discrepancies in incidence and mortality trends that suggest considerable overdiagnosis of melanoma in White persons. During that period, in Blacks, the incidence of melanoma increased by almost 25%, while mortality due to melanoma decreased by approximately 25%. In Whites, melanoma incidence increased approximately 4-fold in women and 6-fold in men, while mortality was stable in women and increased by less than 50% in men. These researchers calculate that had medical care not improved, estimated mortality would have increased 60% in White women and more than doubled in White men. They estimate that 59% of White women and 60% of White men with melanoma were overdiagnosed in 2014. [12]
International statistics
Worldwide, the incidence of malignant melanoma has increased rapidly over the past 50 years, with the highest incidence in fair-skinned populations and in geographic areas closest to the equator. Australia and New Zealand have the highest incidence of melanoma in the world, at an age-standardized rate of 32.5 cases per 100,000 population. [13]
Racial demographics
Melanoma is more common in Whites than in Blacks and Asians. The rate of melanoma in Blacks is estimated to be one twentieth that of Whites. White people with dark skin also have a much lower risk of developing melanoma than do those with light skin. The typical patient with melanoma has fair skin and a tendency to sunburn rather than tan. White people with blond or red hair and profuse freckling appear to be most prone to melanomas. In Hawaii and the southwestern United States, Whites have the highest incidence, approximately 20-30 cases per 100,000 population per year.
Sex- and age-related demographics
Overall, melanoma is the fifth most common malignancy in the US population, accounting for 6% of all new cancer cases in men and 4% of all new cases in women. However, the relative incidence of melanoma in men and women varies by age: in people younger than 50 years of age, incidence rates are higher in women than in men, but thereafter rates are much higher in men. Those differences presumably reflect historical differences in occupational and recreational exposure to UV radiation, as well as higher use of indoor tanning by young women. [11] Women tend to have lesions that are nonulcerated and thinner than those in men.
Melanoma may occur at any age, although children younger than age 10 years rarely develop a de novo melanoma. The median age at diagnosis is 66 years, and 80% of patients are 45 to 84 years old. [14] As a percentage of cancers, the incidence rates of melanoma in US adolescents and young adults from 1973-2015 were as follows [15] :
-
Age 15-19 years: Males, 4.0; females, 3.8
-
Age 20-24 years: Males 9.3; females, 11.9
-
Age 25-29 years: Males, 18.1; females, 21.0
-
Age 30-34 years: Males, 28.3; females, 28.4
-
Age 35-39 years: Males, 40.3; females, 34.9
Prognosis
Malignant melanomas usually present at two extremes: at one end of the spectrum are patients with small skin lesions that are easily curable by surgical resection, and at the other are patients with widely metastatic disease, in whom the therapeutic options are limited and the prognosis is dismal, with a median survival of only 6-9 months. For this reason, physicians must be aware of the clinical characteristics of melanoma to make an early diagnosis.
Prognosis also is related to the type of melanoma. Superficial spreading and nodular types of melanoma are the 2 most common fatal melanomas, based on a review of data from the original 9 registries of the Surveillance, Epidemiology, and End Results (SEER) program from 1978-2007. [16] This confirms prior studies.
The most important prognostic factors include the following [2] :
-
Thickness and/or level of invasion
-
Mitotic index (mitoses per millimeter)
-
Ulceration or bleeding at the primary site
-
Number of regional lymph nodes involved, with distinction of macrometastasis and micrometastasis
-
Systemic metastasis, including the site (nonvisceral versus lung versus all other visceral sites) and serum lactate dehydrogenase (LDH) level elevation
In general, positive prognostic factors include the following [2] :
-
Younger age
-
Female sex
-
Melanoma located on the extremities
In a review of 3,872 cases of lymph node–positive melanoma, the proportion of examined lymph nodes found to be positive (the lymph node ratio) independently predicted disease-specific survival. These researchers concluded that the lymph node ratio consistently improved the prognostic accuracy of the TNM system. [17]
Prognosis is also worse in patients with immune compromise (eg, organ transplant recipients, persons with HIV infection). [18] A study of patients who developed melanoma after solid organ transplantation found that their overall survival was worse than the rate reported in a national sample of patients with melanoma. In transplant recipients with thicker melanomas, disease-specific survival was significantly poorer than in patients without a prior history of transplantation. [19]
In patients with mucosal melanoma, a multivariable analysis determined that anatomic primary site was an independent predictor of overall survival and disease-specific survival. Tumors in the nasal cavity and oral cavity were associated with survival superior compared with tumors in the nasopharynx and paranasal sinuses. Age older than 70 years, tumor size, nodal status, and distant metastasis status were also predictive of outcome. [20]
Stage and prognosis
Prognosis depends on the disease stage at diagnosis. According to SEER data from 2013-2019, 5-year relative survival rates are as follows [18] :
-
Localized (confined to primary site) - > 99.5%
-
Regional (spread to regional lymph nodes) - 74%
-
Distant (metastatic) - 35%
For stage IV melanoma, prognosis varies according to the site of metastasis. Sandru et al reported that median overall survival by metastasis site was as follows [21] :
-
Subcutaneous and lymph nodes: 20.8 months
-
Lungs: 13 months
-
Liver and digestive tract: 5.5 months
-
Central nervous system: 2.5 months
Also see Malignant Melanoma Staging.
Patient Education
The focus of melanoma prevention and patient education is avoidance of sun exposure; see Treatment/Prevention. For patient education information, see Melanoma.
-
A 1.5-cm melanoma with characteristic asymmetry, irregular borders, and color variation.
-
Malignant melanoma. Image courtesy of Hon Pak, MD.
-
Lentigo maligna melanoma, right lower cheek. The centrally located erythematous papule represents invasive melanoma with surrounding macular lentigo maligna (melanoma in situ). Image courtesy of Susan M. Swetter, MD.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Approach Considerations
- Biopsy of a Suggestive Lesion
- Surgical Excision or Reexcision After Biopsy
- Histologic Findings
- Elective Lymph Node Dissection
- Sentinel Lymph Node Biopsy
- Complete Chemistry Panel
- Lactate Dehydrogenase Assay
- Chest Radiography
- Magnetic Resonance Imaging
- Computed Tomography
- Positron Emission Tomography
- Staging
- Show All
- Treatment
- Guidelines
- Medication
- Questions & Answers
- Media Gallery
- References