Practice Essentials
Malaria is an ancient and continuously unmatched parasitic cause of human suffering throughout the world. Plasmodiumspp, an obligate intracellular protozoon using the mosquito as its vector, permeates the tropical and subtropical world. Historically, it has crushed societies, devastated militaries, and hampered economic growth. It continues to wreak havoc, targeting and killing the most vulnerable in our global society.
Signs and Symptoms
-
Malaria may range from mild to severe disease complicated by profound end-organ damage.
-
Typically beginning with a flu-like illness, the hallmark symptom of malaria is its paroxysmal fever that can last up to 10 hours at a time. The classic description is a quartan (every 72 hours) or tertian (every 48 hours) fever periodicity synchronized with Plasmodium merozoites bursting from red blood cells during malaria’s asexual erythrocytic phase. These paroxysms occur with abrupt cold “chills” that transitions after about an hour to profuse sweating, high fever, headache, malaise, and myalgias – then subsequent defervescence.
-
Infection with malaria can result in several physiologic derangements such as anemia, acidosis, and hypoglycemia to name a few. Due to its tendency to transition to an adherent phenotype, infection with P falciparum predisposes patients to thrombosis and thrombocytopenia throughout the body.
-
Cerebral malaria is characterized by its variable presentation of neurologic decline, often leading to coma and convulsions.
Diagnosis
-
The gold-standard diagnostic test for malaria remains direct visualization of the parasite by an experienced microscopist from thick & thin blood smears.
-
Rapid tests that evaluate for the presence of malarial antigens in the blood are available but may have lower sensitivities and depending on the region and genetics of the prevalent species; they cannot differentiate species accurately.
-
Polymerase chain reaction (PCR) also is available at reference laboratories for molecular species-level identification.
Management
-
The treatment of malaria depends on clinical severity, local medical resources, geography, local Plasmodium spp. prevalence, patient genetics, historical use of malaria chemoprophylaxis by the patient, knowledge of local anti-malarial drug resistance, and the patient’s pregnancy status.
-
Most treatment regimens for Plasmodium falciparum, the deadliest species, involve artemisinin combination therapy.
-
Clinically severe disease requires intravenous therapy with eventual step-down to completion of an oral regimen.
-
Patients with P vivax or ovale infections require anti-relapse therapy with an 8-aminoquinolone (primaquine or tafenoquine) after initial treatment; this is due to the species’ abilities to form hypnozoites in the liver, which are untouched by the typical schizontocides used for acute treatment.
Background
In 2022 the World Health Organization’s “World Malaria Report” indicated that between 2000-2019 deaths per year from the parasitic disease had declined from 897,000 to 568,000 with overall cases declining from 245 million to 232 million. Concurrent with the COVID-19 pandemic, malaria’s death toll and case count unfortunately increased to 619,000 and 247 million in 2021 respectively – 76% of whom were children and 52% of whom occurred in just 4 countries: Nigeria, the Democratic Republic of Congo, the Republic of Niger, and the United Republic of Tanzania. [1] The present-day devastation caused by malaria sadly is nothing new to the world.
History
Implications on civilization include the following:
-
Malaria has affected the development of human civilization for millennia. [2] Likely accounts of the disease have been found in most developed ancient societies such as Mesopotamia, India, China, Egypt, Greece, and Rome; these accounts have been covered extensively by both modern popular and scientific writings. [3] Malaria even has been postulated to have protected against invaders while simultaneously accelerating the demise of ancient Rome via its presence in the Pontine Marshes. The presence of Plasmodium falciparum on the Apennine peninsula may have led to the many reports of outbreaks of deadly fevers between the 1 st and 2 nd centuries AD. [4]
-
The efficient Anopheles mosquito vector enabled malaria to suppress economic productivity and development in the subtropical regions around the globe, especially within sub-Saharan Africa. Plasmodium falciparum’s deadly course repelled European traders for centuries and was powerful and persistent enough to potentially select for different genetic profiles such as “sickle-cell trait” in African natives. [2]
-
At the turn of the 20 th century, Colonel William Gorgas and the United States military led the charge in malaria prevention, proving efficacy of environmental vector control for the Panama Canal Commission. Through swamp drainage, oil spraying, employing “mosquito swatters,” installing screens in living quarters, and instituting the use of anti-malarial prophylaxis with quinine, malaria case incidence was reduced from 800 per 1,000 workers to 16 per 1,000 in a matter of 3 years. [5] Malaria eventually was eradicated from the United States in 1951, yet the Anopheles vector persists. [6] Granting that treatment advanced over the first half of the century, malaria has continued to wreak havoc on a global scale. It exposed itself in the Second World War as the US Navy and Marine Corps alone suffered over 100,000 cases and 3.3 million “sick-days” due to malaria. [7] Most intensely, in the South Pacific on the island of Guadalcanal there was an astonishing case incidence of 1,781 per 1,000 per year in November 1942 amongst US and Allied forces. Lower incidence rates and case numbers occurred in the Korean and Vietnam conflicts; however, malaria remains a major issue for forward deployed forces globally, with many modern cases requiring telemedicine consultation from infectious disease specialists in the states. [8]
Discovery
The following were significant in the discovery of malaria:
-
In 1880, while stationed in Algeria, the French Army physician Charles Louis Alphonse Laveran discovered the protozoal cause of malaria using a microscope with dry objective at 400x magnification. [9] As this discovery refuted the miasma theory as well as the leading germ theory of the time that espoused a bacterial cause of the disease, it took the better part of a decade to convince other leading researchers in the field that a protozoa was the pathogenic agent. In 1907, Laveran received the Nobel prize in medicine for his discoveries.
-
Using the English Army physician Ronald Ross’ work with avian malaria as a foundation, a series of Italian physician scientists - notably Giovanni Grassi, Amico Bignami, and Giuseppe Bastianelli incriminated the Anopheles mosquito as the vector for human malaria and further elucidated the protozoan’s life cycle.
Etiology
There are 4 common Plasmodium species of concern to humans. There is a fifth in Southeast Asia that rarely can infect humans and cause severe disease, Plasmodium knowlesi; however, this species typically causes simian malaria. The overwhelming majority of worldwide morbidity from malaria is caused by P falciparum and to a lesser degree, P vivax. [1, 10]
Biology
Figure 1. Malaria life cycle
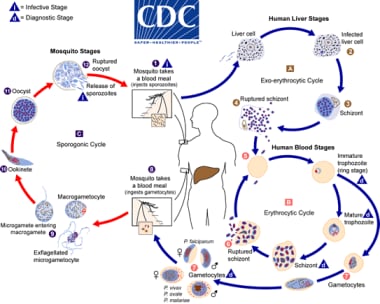 Malaria life cycle. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].
Malaria life cycle. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].
Stages of Infection
Infection
-
Infection occurs during the mosquito’s blood meal as the female Anopheles inserts her proboscis into human skin and injects up to 100 sporozoites (sporo- meaning “spores,” -zoite meaning “animal”) per bite with her saliva, each of them gliding at speeds up to 2 micrometers per second. After waiting 1 to 3 hours in the dermis, the successful sporozoites reach the bloodstream and are swiftly carried off through the blood to the liver, where they must cross the sinusoidal barrier to access and invade their target hepatocytes.
Exoerythrocytic Schizogeny – “Liver Stage”
-
Once they have transitioned from migratory to invasive phenotypes, the sporozoites mature into spherical multinuclear schizonts (schiz- meaning “divided,” -ont meaning “type”). After 5-21 days depending on species, each liver schizont ruptures into 2,000 to 40,000 uninucleate merozoites (mero- meaning “part,” -zoite meaning “animal”).
Erythrocytic Schizogeny - “Blood Stage”
-
At this point, each merozoite may infect a red blood cell; differences between the species at specific stages are noted below. The parasites begin to feed off hemoglobin within the erythrocytes, with the parasites first appearing as ring forms, then forming trophozoites (tropho- meaning “nourishment,” -zoite meaning “animal”). Hemozoin is the brown pigment biocrystal waste product generated from the parasite’s hemoglobin meal; this results in safe disposal of the free-radical-generating heme molecule that otherwise would be toxic to the Plasmodium. Trophozoites again mature into schizonts, which rupture into merozoites, thus continuing the erythrocytic or “blood” stage. Some trophozoites mature into sexual stage gametocytes rather than schizonts. These male and female gametocytes then may be ingested by another feeding Anophelese mosquito.
Sporogony - “Mosquito Stage”
-
Male and female gametocytes ingested by a mosquito undergo sexual reproduction in the gut of the mosquito, producing motile ookinetes (oo- meaning “egg,” -kinete meaning “relating to motion”), which invade the wall of the mosquito midgut, forming oocysts. The oocysts subsequently rupture, releasing sporozoites that invade their way to the mosquito’s salivary glands and await a new human host.
Species Specific Nuances
P falciparum
-
Typical incubation period: 8-11 days [11]
-
Classic fever periodicity: 36-48 hours
-
Unique syndromes and mechanisms
- A main feature of P falciparum is its ability to convert to its cytoadherent phenotype by insertion of P falciparum erythrocyte membrane protein 1 (pfEMP1) into the membrane of erythrocytes, which leads to the negative effects of infected erythrocyte sequestration. [12] Sequestration of infected erythrocytes can throw off the accuracy of microscopy determined parasitemia. pfEMP1 is the product of var gene transcription, of which there are ~60 copies, making it a highly variable antigen that also contributes to immune evasion.
P vivax
-
Typical incubation period: 8-17 days (but may be 1 year or more due to hypnozoite formation) [11]
-
Classic fever periodicity: 48 hours
-
Unique syndromes and mechanisms
- Both P vivax and P ovale are known to variably enter quiescence after hepatocyte invasion. These hypnozoites (hypno- meaning “sleeping”) result in primary infection weeks to months (up to 1 year or more) about 50% of the time. [13] This serves as a mechanism for relapsed infection despite treatment of the patient with schizonticidal agents; when P vivax or P ovale are suspected pathogens, the affected patient must receive presumptive anti-relapse therapy (PART) with primaquine or tafenoquine to target the hypnozoites. Other antimalarials are ineffective against hypnozoites, but if the patient is unable to receive PART, chloroquine prophylaxis should be provided for 1 year from the acute infection, as most of the relapses resulting from hypnozoite reactivation occur within this timeframe. [14]
- P vivax infects reticulocytes preferentially via the Duffy antigen present on red blood cells. [15, 16] Incidentally, most Africans are Duffy-negative, and there is a low prevalence of P vivax on that continent. Ethiopia, India, Indonesia, and Pakistan account for more than 75% of all cases.
P ovale
-
Typical incubation period: 10-17 days (but may be 1 year or more due to hypnozoite formation) [11]
-
Classic fever periodicity: 48 hours
-
Unique syndromes and mechanisms
- Like P vivax, P ovale also produces hypnozoites in the hepatic phase of its life cycle (see above).
- P ovale typically produces a milder infection compared to P falciparum or P vivax and may self-resolve after 6-10 paroxysms, but it does cause significant morbidity in endemic areas (ie, West Africa). [10, 11]
P malariae
-
Typical incubation period: 18-40 days
-
Classic fever periodicity: 72 hours
-
Unique syndromes and mechanisms
- P malariae infections typically cause lower parasitemia and account for a minority of the malaria burden in the world. [10] P malariae infection also is associated with proteinuria and membranoproliferative glomerulonephritis secondary to immune complex deposition. [11] Because of the slow maturation process as indicated by the long incubation period, low parasitemia infections may persist for long periods of time with recrudescence up to 50 years.
P knowlesi
-
Typical incubation period: 9-12 days [11]
-
Classic fever periodicity: 24-27 hours
-
Unique syndromes and mechanisms
- P knowlesi is a simian malaria that has been found to cross into humans in Southeast Asia – particularly on the island of Borneo. [1] It resembles P falciparum in early erythrocytic stages and as it matures it resembles P malariae on microscopy; this unfortunately puts the patient at risk of being diagnosed with a less severe infection. In contrast to P malariae, P knowlesi infections progress to severe disease at a high rate (~8%); therefore, if a patient is diagnosed with high parasitemia malaria identified as P malariae by microscopy, it should be assumed that the patient has a severe P knowlesi infection until proven otherwise.
Epidemiology
Per the WHO, as of 2021 malaria was endemic in 84 countries, placing nearly half the world’s population at risk of contracting the disease. [1] Modern US travelers acquire almost 90% of malaria by traveling to Africa; almost 9% of the disease is acquired in Asia, whereas South and Central America/ the Caribbean make up the remainder. [17] In these patients, there is a 14% risk for severe disease, and 13% of the cases initially are misdiagnosed.
Epidemiologic measures [15]
-
Entomologic inoculation rate: sporozoite-positive mosquito bites per unit time
-
Annual parasite incidence: number of new parasite-confirmed cases per 1000 population
-
Spleen rate: proportion of individuals of a given age with enlarged spleens
Transmission
-
The female Anopheles mosquito requires the protein from a blood meal to produce its eggs. This genus serves as the vector for human malaria; there are more than 400 species of Anopheles. However, only 40 species of Anopheles transmit malaria to humans. [18] Varying amongst species-specific preferences, the mosquitos lay their eggs in water ranging from large open bodies such as ponds to much smaller collections like puddles from footprints. The eggs hatch into larvae, then molt several times to become pupal stages of adults. When protein for egg production is required, the adult female is attracted to many chemical indicators of human activity: CO 2, lactic acid, odors, and moisture. Typically, they feed at night or in the late evening or early morning, and after feeding, the mosquitos display different preferences in where and how long they rest before biting again. All these differences in species-specific preferences are of importance when it comes to vector control.
-
Transmission intensity typically is measured by parasite incidence. The varying degrees are characterized as follows:
- High transmission: >450 cases per 1,000 people annually and P falciparum prevalence >35%
- Moderate transmission: 250-450 cases per 1,000 people annually and P falciparum/ vivax prevalence 10-35%
- Low transmission: 100-250 cases per 1,000 people annually and P falciparum/ vivax prevalence 1-10%
- Very low transmission: < 100 cases per 1,000 people annually and P falciparum/ vivax prevalence 0-1%
-
Epidemiology of clinical cases is affected by the background of acquired protective immunity to the parasite. In areas with continuously moderate to high transmission, repeated exposure to the parasite reduces the risk of adolescents and adults contracting severe disease. If environmental control measures effectively limit transmission in a given area, the population’s acquired immunity decreases over time; this necessitates sustainment of the transmission-control efforts to prevent a subsequent increased risk for epidemic severe malaria. The same premise applies to previously immune people who have traveled away from the endemic for an extended period of time (ie, 1 year or more).
Geography of modern disease burden
-
Several geographic traits influence transmission levels and burden of disease: altitude, humidity, annual rainfall, proximity to bodies of water, land use, and temperature.
The maps below were generated using data obtained from the WHO 2022 World Malaria Report.
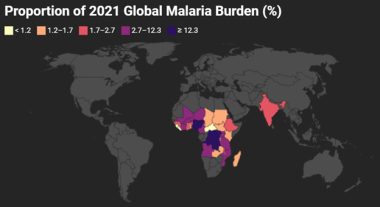 Proportion of 2021 Global Malaria Burden. Gray area accounts for the remaining estimated 4.4% of worldwide malaria burden. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
Proportion of 2021 Global Malaria Burden. Gray area accounts for the remaining estimated 4.4% of worldwide malaria burden. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
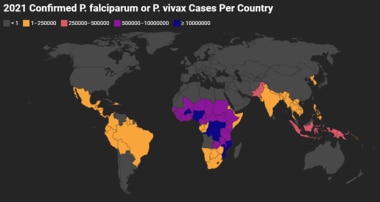 Confirmed P falciparum or P vivax Cases Per Country 2021. The map accounts for the total of the cases per country where either species were confirmed as the primary infection. The map does not include confirmed “mixed infections.” Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
Confirmed P falciparum or P vivax Cases Per Country 2021. The map accounts for the total of the cases per country where either species were confirmed as the primary infection. The map does not include confirmed “mixed infections.” Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
North America
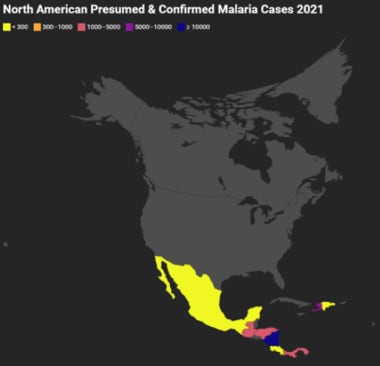 North American Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
North American Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
South America
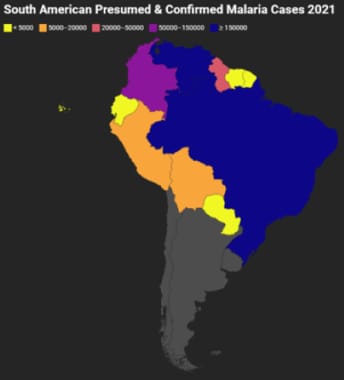 South American Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
South American Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
Europe
Per the WHO 2022 World Malaria Report, no 2021 case data exists for the European region. [1] Malaria was eradicated from Europe in the 1970s through insecticide spraying, drug therapy, and environmental engineering; however, climatic conditions are becoming more conducive to malaria transmission and the large influx of migrant populations may serve as an adequate parasite reservoir. [19]
Africa
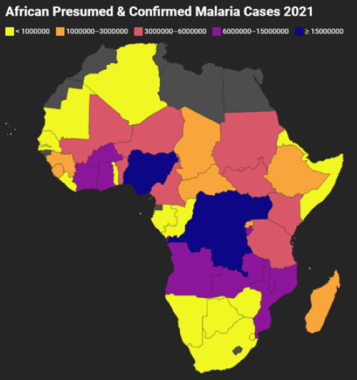 African Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
African Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
Asia & South Pacific
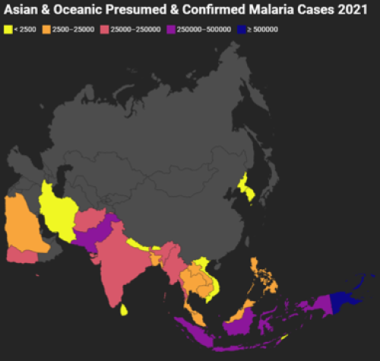 Asian and Oceanic Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
Asian and Oceanic Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
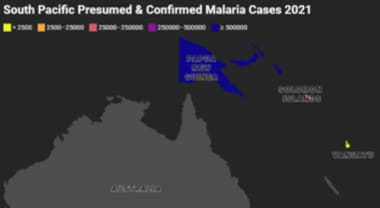 South Pacific Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
South Pacific Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
Species-Specific Epidemiology
P falciparum and vivax are the most common species of malaria that cause disease in humans – see the map below for proportional comparison of the cases caused by the 2 species from 2021. [1]
-
Americas – P vivax (76%), P falciparum & mixed (24%)
-
Eastern Mediterranean – P vivax (24%), P falciparum & mixed (74%), other (2%)
-
South-East Asia – P vivax (44%), P falciparum & mixed (55%), other (1%)
-
Western Pacific – P vivax (32%), P falciparum & mixed (68%)
-
High transmission countries in East & Southern Africa – P vivax (< 1%), P falciparum (>99%)
-
Low transmission countries in East & Southern Africa – P vivax (8%), P falciparum & mixed (92%)
-
Central Africa – P falciparum (100%)
-
West Africa – P falciparum (>99%), other (< 1%)
-
P vivax infects reticulocytes preferentially via the Duffy antigen present on red blood cells. [15, 16] Incidentally, most Africans are Duffy-negative, and there is a low prevalence of P vivax on that continent. Note that Ethiopia, India, Indonesia, and Pakistan account for more than 75% of all P vivax cases. As previously mentioned, P ovale is endemic to West Africa, and P knowlesi has been identified in South-East Asia. P malariae is found throughout the tropics; however, most cases seen in US travelers are mixed infections in returning travelers from Africa. [10]
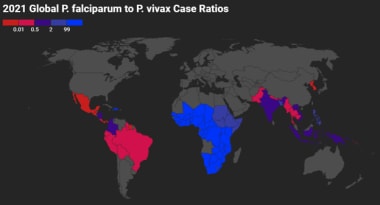 Global P falciparum to P vivax Case Ratios 2021. Gray indicates that there were either no data available or there were zero endemic cases. Red indicates higher proportion of P vivax cases, whereas blue indicates higher proportion of P falciparum cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
Global P falciparum to P vivax Case Ratios 2021. Gray indicates that there were either no data available or there were zero endemic cases. Red indicates higher proportion of P vivax cases, whereas blue indicates higher proportion of P falciparum cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
Prognosis
Most patients with uncomplicated malaria exhibit marked improvement within 48 hours after the initiation of treatment and are fever free after 96 hours. P falciparum infection carries a poor prognosis with a high mortality rate if untreated. However, if the infection is diagnosed early and treated appropriately, the prognosis is excellent.
Mortality
In 2021, 247 million malaria cases occurred globally with 619,000 deaths, 96% of which occurred in the African region. [1] Roughly 80% of the deaths occurred in children younger than 5 years. Although preventable and treatable, poverty, war, and economic/ social instability in endemic areas historically have resulted in close to 1 million deaths each year.
Population Protective & Risk Factors
Genetic variation and population dynamics play a prominent role in the global patterns of malaria prognosis and epidemiology.
Age-Related
-
The peak age of uncomplicated malaria declines as transmission intensity declines – however, the youngest age groups (specifically those younger than 5 years) are at most risk for hospitalization and death despite the epidemiology of transmission. [20] Furthermore, the peak age of death caused by malaria is in children younger than 1 year. This has led to age-targeted mitigation strategies such as intermittent preventive treatment of infants and children as well as vaccine development.
-
As peak age also declines with increasing severity of disease, it suggests that immunity develops against more severe forms of malaria more swiftly than immune protection against clinical disease. This singularity may be exaggerated by the tendency for those unable to mount strong immune responses to die from malaria at an earlier age.
Sex-Related
-
Recent public health data from a malaria endemic region of Africa (Uganda) suggest that females of child-bearing age (15-39 years) are at increased risk for suspected malaria compared with other sex/age demographics, although males in that same age group and older have significantly higher test positivity. [21] Females in the study had almost twice the incidence of malaria compared with males. However, the authors noted that females in the study were also more than twice as likely to visit their local health facility for a recent fever as compared with males. Finally, there was also a higher incidence of non-malaria-related healthcare visits among females in general.
-
Previous studies have reported a male bias in incidence and prevalence of malaria infection, and another study out of Uganda showed that this was potentially secondary to females clearing asymptomatic infections at a faster rate than males. [22] This has lent strong evidence to the theory that males are less able to control parasite densities (anti-parasite immunity), leading to higher male prevalence of infection, whereas females are less able to tolerate higher parasite densities without fever (anti-disease immunity), leading to a higher likelihood of experiencing symptoms. [21] This evidence suggests the presence of sex differences regarding acquisition of immunity to malaria.
Genetics-Related
-
Hemoglobinopathies
- Starting with the association of HbS (sickle cell trait) and less severe disease and less parasitemia discovered by AC Allison published in 1954, several different polymorphisms in the hemoglobin genes have been linked to protection against severe malaria. [23] This is especially true in geographic regions of Africa where malaria transmission is higher. [24] Apart from HbS, case control studies have shown that alpha and beta-thalassemia as well as HbC disease are protective against malaria – all 3 conferring greater than 50% protection against severe forms of malaria. [25] The mechanisms of protection differ amongst the hemoglobinopathies and likely are multifactorial; reduced erythrocyte invasion and maturation, reduced development of adherent phenotypes, and increased erythrocyte clearance in response to oxidative stress all may be contributory mechanisms.
-
Glucose-6-Phosphate Dehydrogenase
- Glucose-6-phosphate dehydrogenase (G6PD) is an enzyme required for NADPH production and subsequent glutathione reduction to protect red blood cell membranes against oxidative damage. A deficiency of G6PD is the most common inherited red blood cell abnormality, affecting over 400 million people, with up to 30% of them residing in sub-Saharan Africa. [24] It is X-linked (more so affecting males), and homozygosity of the deficiency can lead to severe episodes of hemolysis. Despite its potential downside, it has been shown to be highly protective against severe malaria via increased splenic phagocytosis of ring-form infected erythrocytes. [26] The prevalence of the protective polymorphisms of G6PD deficiency also are associated with malaria transmission intensity in endemic countries.
-
Blood Group Antigens
- Apart from P vivax requiring the Duffy antigen for erythrocyte entry, the risk conferred by other blood groups has been elucidated. [16] The rosetting phenomenon, which occurs through the cytoadherent phenotype of P falciparum-infected erythrocytes clumping and clogging of microvessels, is much less likely to occur in patients with group O RBCs. This is because A and B antigens serve as rosetting receptors for uninfected RBCs. This leads to a much lower likelihood of severe malaria in patients with group O blood; a study in Mali showed an odds reduction for severe versus uncomplicated malaria in group O patients of 66%. [27]
Pathophysiology
Malaria Pathogenesis
Despite the many morphologies of the parasite in its life cycle, only a few stages cause clinical disease in humans, the most severe of which are typically P falciparum and P vivax. [12] The initial schizont broods rupture out of the liver phase, release thousands of merozoites into the bloodstream, and attempt to establish periodicity within the erythrocytic phase (time periods vary per species) where level of parasitemia increases exponentially.
-
Fever
- Once the parasite concentration is high enough, a fever is mounted (pyrogenic threshold). This pyrogenic threshold may be anywhere from 0.05 to 1.25 parasites/1,000 red blood cells (0.005 - 0.125% parasitemia) – immune patients may require 0.2% parasitemia to experience fever. [11, 28] The most theorized mechanism for the cyclic fevers is the human immune response to the hemozoin (waste product generated from Plasmodium’s diet of hemoglobin) that bursts into the bloodstream during each cycle of asexual erythrocytic schizogony. [29] Hemozoin is known to be a Plasmodium pathogen associated molecular pattern (PAMP) recognized by toll-like receptors (TLRs) that induces intense immune response. Other malarial toxins such as malaria glycophosphatidylinositol (GPI) likely are culpable through similar mechanisms. The temperature fluctuation with the human febrile response is expected to play a role in guiding the broods of parasites toward the classic paroxysmal fever-associated periodicity of schizont rupture.
-
Anemia
- Severe malaria-associated anemia typically is seen in the young in areas of the world with poor health infrastructure and diets poor in essential vitamins. [30] Schizont rupture from erythrocytes is an obvious mechanism of anemia; however, the more profound loss of red blood cell mass is seen in the population of uninfected erythrocytes. Their decline is thought to occur via oxidative damage of the erythrocyte membranes, eventually leading to hemolysis. Another mechanism of anemia lies within bone marrow; both insufficient production and function of erythropoietin as well as possible apoptosis induction of erythrocyte precursors lead to dyserythropoiesis.
-
Splenomegaly
- During acute malaria infection, the spleen serves as the main driver of infected erythrocyte clearance, immune cell activation, and extramedullary hematopoiesis. [31] The extraordinary burden on the spleen causes the red pulp to become congested with infected and uninfected red blood cells, but splenomegaly also occurs by massive cellular expansion in both the red and white pulp. Impressively, the organ is able to revert back to a normal size after clearance of the infection (at least in mice). Because of P vivax’ tropism for reticulocytes (prevalent in the spleen), splenic rupture is a characteristic severe complication of P vivax, and there is evidence to suggest a separate splenic life cycle for this species. [32]
-
Acidosis
- End-organ damage from microvascular sequestration, respiratory depression, and production of Plasmodium lactate dehydrogenase (pLDH) by the malaria parasite all lower the pH of the blood. [12]
-
Renal Injury
- Cytoadherence with thrombi formation in glomeruli is a common mechanism of acute kidney injury. [15] If anuria or hemoglobinuria (“blackwater fever”) occur, it likely is secondary to an abundance of cell-free hemoglobin that results in reduced renal perfusion. Although contributing to morbidity of the acute illness, most patients do not require long-term dialysis.
-
Cerebral malaria
- After infecting a red blood cell, P falciparum can insert adhesive proteins into the erythrocyte membrane, thus creating a cytoadherant phenotype (further described below in ‘species specific nuances’). [12] The ability to stick to endothelium, uninfected blood cells, and platelets causes more than 20% of brain capillaries to be filled with P falciparum parasites, resulting in vascular congestion, retinopathy, capillary leakage, thrombi, hemorrhage, axonal injury, coma, and death from cerebral malaria within 48 hours (especially in children).
-
Placental malaria
- Occurring with highest incidence in Africa secondary to P falciparum due to its ability to form the cytoadherent phenotype, placental malaria is characterized by the accumulation of infected red blood cells within the intervillous space and ensuing infiltration of maternal macrophages. [33] In severe cases, the resulting chronic intravillositis leads to decreased transplacental nutrient transport, fetal growth restriction and low birth weight babies, as well as increased risk for preterm birth and preeclampsia for the mother.
Patient Education
Individuals traveling to malarial regions must be provided with adequate information regarding prevention strategies, as well as tailored and effective antiprotozoal medications.
Avoid mosquitoes by limiting exposure during times of typical blood meals (ie, dawn, dusk). Wearing long-sleeved clothing and using insect repellants also may prevent infection. Avoid wearing perfumes and colognes.
Adult-dose 95% DEET lasts up to 10-12 hours, and 35% DEET lasts 4-6 hours. In children, use concentrations of less than 35% DEET. Use sparingly and only on exposed skin. Remove DEET when the skin no longer is exposed to potential mosquito bites. Consider using bed nets that are treated with the insecticide permethrin. Although this is an effective method of preventing malaria transmission in endemic areas, an increasing incidence of pyrethroid resistance in Anopheles spp has been reported. [34] Seek out medical attention immediately upon contracting any tropical fever or flulike illness.
-
Malarial merozoites in the peripheral blood. Note that several of the merozoites have penetrated the erythrocyte membrane and entered the cell.
-
This micrograph illustrates the trophozoite form, or immature-ring form, of the malarial parasite within peripheral erythrocytes. Red blood cells infected with trophozoites do not produce sequestrins and, therefore, are able to pass through the spleen.
-
An erythrocyte filled with merozoites, which soon will rupture the cell and attempt to infect other red blood cells. Notice the darkened central portion of the cell; this is hemozoin, or malaria pigment, which is a paracrystalline precipitate formed when heme polymerase reacts with the potentially toxic heme stored within the erythrocyte. When treated with chloroquine, the enzyme heme polymerase is inhibited, leading to the heme-induced demise of non–chloroquine-resistant merozoites.
-
A mature schizont within an erythrocyte. These red blood cells (RBCs) are sequestered in the spleen when malaria proteins, called sequestrins, on the RBC surface bind to endothelial cells within that organ. Sequestrins are only on the surfaces of erythrocytes that contain the schizont form of the parasite.
-
Malaria life cycle. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].
-
Proportion of 2021 Global Malaria Burden. Gray area accounts for the remaining estimated 4.4% of worldwide malaria burden. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
-
Confirmed P falciparum or P vivax Cases Per Country 2021. The map accounts for the total of the cases per country where either species were confirmed as the primary infection. The map does not include confirmed “mixed infections.” Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
-
North American Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
-
South American Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
-
African Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
-
Asian and Oceanic Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
-
South Pacific Presumed and Confirmed Malaria Cases 2021. Gray indicates that there were either no data available or there were zero endemic cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
-
Global P falciparum to P vivax Case Ratios 2021. Gray indicates that there were either no data available or there were zero endemic cases. Red indicates higher proportion of P vivax cases, whereas blue indicates higher proportion of P falciparum cases. Map created using data adapted from WHO 2022 World Malaria Report [https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022].
-
Thin blood smear showing the ring forms of P falciparum that look like headphones with double chromatin dots. Note how P falciparum is seen infecting erythrocytes of all ages – a trait that can be utilized by the microscopist by noting the similar size of infected erythrocytes to other surrounding uninfected erythrocytes. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].
-
Thick blood smear depicting the banana shaped gametocyte of P falciparum. Multiple ring-form trophozoite precursors are also visible in the background. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].
-
Thin blood smear of the ring forms of P vivax. Note that P vivax typically has a single chromatin dot vs the two chromatin dots in P falciparum. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].
-
The diagnostic form of P vivax is the amoeboid trophozoite form where the cytoplasm has finger-like projections (pseudopods) without a typical round/oval structure. These pseudopods are unique to P vivax. Numerous small pink-red dots are also seen in both P vivax and P ovale; these are known as caveola-vesicle complexes (CVCs or Schüffner’s dots) and are composed of numerous flask-like indentations on infected reticulocytes membrane skeleton associated with tube-like vesicles. CVCs are thought to play a role in nutrient uptake or release of metabolites from parasite-infected erythrocytes. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].
-
Thin smear of P ovale in ring stage. Note that typically there is a single chromatin dot, larger cells are infected indicative of reticulocytes, and multiple ring forms may be present intracellularly. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].
-
Thin smear of P ovale trophozoite. Note that this species is difficult to differentiate from P vivax as it contains CVCs (Schüffner’s dots) and infects reticulocytes; a notable unique characteristic of P ovale is the presence of fimbriae on the reticulocyte membrane, which are even more likely to be seen in gametocyte infected red blood cells. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].
-
Thin blood smear of “band form” trophozoite of P malariae. Note that the infected erythrocyte is smaller than surrounding cells, indicating that P malariae infects older erythrocytes. As the trophozoite matures, the cytoplasm elongates and dark pigment granules of hemozoin are visualized toward the periphery. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].
-
Thin blood smear of P knowlesi trophozoites. An immature ring form is seen on the right next to the mature band form trophozoite on the left. Note the small size of the infected red blood cells and how the band form is similar in appearance to P malariae. Courtesy of the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/dpdx/malaria/index.html].






