Practice Essentials
Non-Hodgkin lymphomas (NHLs) are tumors originating from lymphoid tissues, mainly of lymph nodes. These tumors may result from chromosomal translocations, infections, environmental factors, immunodeficiency states, and chronic inflammation. See the image below.
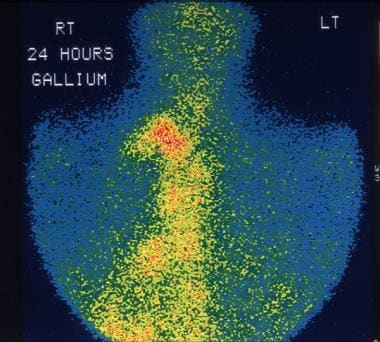 This 28-year-old man was being evaluated for fever of unknown origin. Gallium-67 study shows extensive uptake in the mediastinal lymph nodes due to non-Hodgkin lymphoma (NHL).
This 28-year-old man was being evaluated for fever of unknown origin. Gallium-67 study shows extensive uptake in the mediastinal lymph nodes due to non-Hodgkin lymphoma (NHL).
Signs and symptoms
The clinical manifestations of NHL vary with such factors as the location of the lymphomatous process, the rate of tumor growth, and the function of the organ being compromised or displaced by the malignant process.
Signs and symptoms of low-grade lymphomas include the following:
-
Peripheral adenopathy: Painless and slowly progressive; can spontaneously regress
-
Primary extranodal involvement and B symptoms: Uncommon at presentation; however, common with advanced, malignant transformation or end-stage disease
-
Bone marrow: Frequent involvement; may be associated with cytopenias(s) [1] ; fatigue/weakness more common in advanced-stage disease
Intermediate- and high-grade lymphomas have a more varied clinical presentation, including the following:
-
Adenopathy: Most patients
-
Extranodal involvement: More than one third of patients; most common sites are GI/GU tracts (including Waldeyer ring), skin, bone marrow, sinuses, thyroid, CNS
-
B symptoms: Temperature >38°C, night sweats, weight loss >10% from baseline within 6 months; in approximately 30-40% of patients
See Presentation for more detail.
Diagnosis
Examination in patients with low-grade lymphomas may demonstrate peripheral adenopathy, splenomegaly, and hepatomegaly.
Intermediate- and high-grade lymphomas may result in the following examination findings:
-
Rapidly growing and bulky lymphadenopathy
-
Splenomegaly
-
Hepatomegaly
-
Large abdominal mass: Usually in Burkitt lymphoma
-
Testicular mass
-
Skin lesions: Associated with cutaneous T-cell lymphoma (mycosis fungoides), anaplastic large-cell lymphoma, and angioimmunoblastic lymphoma [2]
Testing
Laboratory studies in a patient with suspected NHL should include the following:
-
CBC count: May be normal in early-stage disease; in more advanced stages, may demonstrate anemia, thrombocytopenia/leukopenia/pancytopenia, lymphocytosis, thrombocytosis
-
Serum chemistry studies: May show elevated LDH and calcium levels, abnormal liver function tests
-
Serum beta2-microglobulin level: May be elevated
-
HIV serology: Especially in patients with diffuse large cell immunoblastic or small noncleaved histologies
-
Human T-cell lymphotropic virus–1 serology: For patients with adult T-cell leukemia/lymphoma
-
Hepatitis B testing: In patients in whom rituximab therapy is planned, because reactivation has been reported
Other tests that may be helpful in evaluating suspected NHL include the following:
-
Immunophenotypic analysis of lymph node, bone marrow, peripheral blood
-
Cytogenetic studies: NHL occasionally associated with monoclonal gammopathy; possible positive Coombs test; maybe hypogammaglobulinemia
Imaging tests
The following imaging studies should be obtained in a patient suspected of having NHL:
-
Chest radiography
-
Upper GI series with small bowel follow-through: In patients with head and neck involvement and those with a GI primary lesion
-
CT scanning of the neck, chest, abdomen, and pelvis
-
PET scanning
-
Bone scanning: Only in patients with bone pain, elevated alkaline phosphatase, or both
-
Testicular ultrasonography: For opposite testis in male patients with a testicular primary lesion
-
Multiple gated acquisition (MUGA) scanning: To assess cardiac function, in patients being considered for treatment with anthracyclines
-
MRI of brain/spinal cord: For suspected primary CNS lymphoma, lymphomatous meningitis, paraspinal lymphoma, or vertebral body involvement by lymphoma
Procedures
The diagnosis of NHL relies on pathologic confirmation following appropriate tissue biopsy. The following are procedures in cases of suspected NHL:
-
Bone marrow aspiration and biopsy: For staging rather than diagnostic purposes
-
Excisional lymph node biopsy (extranodal biopsy): For lymphoma protocol studies
Perform lumbar puncture for CSF analysis in patients with the following conditions:
-
Diffuse aggressive NHL with bone marrow, epidural, testicular, paranasal sinus, or nasopharyngeal involvement, or 2 or more extranodal sites of disease
-
High-grade lymphoblastic lymphoma
-
High-grade small noncleaved cell lymphomas
-
HIV-related lymphoma
-
Primary CNS lymphoma
-
Neurologic signs and symptoms
See Workup for more detail.
Management
The treatment of NHL varies greatly, depending on various factors. Common therapies include the following:
-
Chemotherapy: Most common; usually combination regimens
-
Biologic agents (eg, rituximab, obinutuzumab, lenalidomide): Typically in combination with chemotherapy
-
Radiation therapy
-
Bone marrow transplantation: Possible role in relapsed high-risk disease
-
Chimeric antigen receptor (CAR) T-cell therapy: In relapsed or refractory large B-cell lymphoma
-
Radioimmunotherapy
-
Transfusions of blood products
-
Antibiotics
Pharmacotherapy
Medications used in the management of NHL include the following:
-
Cytotoxic agents (eg, chlorambucil, cyclophosphamide, doxorubicin, vincristine, fludarabine, pralatrexate, nelarabine, etoposide, mitoxantrone, cytarabine, bendamustine, carboplatin, cisplatin, gemcitabine, denileukin diftitox, bleomycin)
-
Histone deacetylase inhibitors (eg, vorinostat, romidepsin, belinostat)
-
Colony-stimulating factor growth factors (eg, epoetin alfa, darbepoetin alfa, filgrastim, pegfilgrastim)
-
Monoclonal antibodies (eg, rituximab, ibritumomab tiuxetan, alemtuzumab, ofatumumab, obinutuzumab, pembrolizumab)
-
mTOR (mechanistic [mammalian] target of rapamycin) kinase inhibitors (eg, temsirolimus)
-
Proteasome inhibitors (eg, bortezomib)
-
Immunomodulators (eg, interferon alfa-2a or alfa-2b)
-
Corticosteroids (eg, dexamethasone, prednisone)
Surgery
Surgical intervention in NHL is limited but can be useful in selected situations (eg, GI lymphoma), particularly in localized disease or in the presence of risk of perforation, obstruction, and massive bleeding. Orchiectomy is part of the initial management of testicular lymphoma.
See Treatment and Medication for more detail.
Background
The term lymphoma describes a heterogeneous group of malignancies with different biology and prognosis. In general, lymphomas are divided into 2 large groups of neoplasms: non-Hodgkin lymphoma (NHL) and Hodgkin lymphoma. About 85% of all malignant lymphomas are NHLs. The median age at diagnosis is 68 years, [3] although Burkitt lymphoma and lymphoblastic lymphoma occur in younger patients.
NHL includes many clinicopathologic subtypes, each with distinct epidemiologies; etiologies; morphologic, immunophenotypic, genetic, and clinical features; and responses to therapy. With respect to prognosis, NHLs can be divided into two groups, indolent and aggressive. [4]
Currently, several NHL classification schemas exist, reflecting the growing understanding of the complex diversity of the NHL subtypes. The Working Formulation, originally proposed in 1982, classified and grouped lymphomas by morphology and clinical behavior (ie, low, intermediate, or high grade). In the 1990s, the Revised European-American Lymphoma (REAL) classification attempted to apply immunophenotypic and genetic features in identifying distinct clinicopathologic NHL entities. The World Health Organization (WHO) classification further elaborates upon the REAL approach. This classification divides NHL into those of B-cell origin and those of T-cell and natural killer (NK)–cell origin.
A study by Shustik et al found that within the WHO classification, the subdivisions of grade 3A and 3B had no difference in outcome or curability with anthracycline-based therapy. [5]
For clinical oncologists, the most practical way of sorting the currently recognized types of NHL is according to their predicted clinical behavior. Each classification schema contributes to a greater understanding of the disease, which dictates prognosis and treatment.
Although a variety of laboratory and imaging studies are used in the evaluation and staging of suspected NHL (see Workup), a well-processed hematoxylin and eosin (H&E)–stained section of an excised lymph node is the mainstay of pathologic diagnosis. The treatment of NHL varies greatly, depending on tumor stage, grade, and type and various patient factors (eg, symptoms, age, performance status; see Treatment and Guidelines).
For discussion of individual subtypes of NHL, see the following:
Pathophysiology
NHLs are tumors originating from lymphoid tissues, mainly of lymph nodes. Various neoplastic tumor cell lines correspond to each of the cellular components of antigen-stimulated lymphoid follicles.
NHL represents a progressive clonal expansion of B cells or T cells and/or NK cells arising from an accumulation of lesions affecting proto-oncogenes or tumor suppressor genes, resulting in cell immortalization. These oncogenes can be activated by chromosomal translocations (ie, the genetic hallmark of lymphoid malignancies), or tumor suppressor loci can be inactivated by chromosomal deletion or mutation. In addition, the genome of certain lymphoma subtypes can be altered with the introduction of exogenous genes by various oncogenic viruses. Several cytogenetic lesions are associated with specific NHLs, reflecting the presence of specific markers of diagnostic significance in subclassifying various NHL subtypes.
Almost 85% of NHLs are of B-cell origin; only 15% are derived from T/NK cells, and the small remainder stem from macrophages. These tumors are characterized by the level of differentiation, the size of the cell of origin, the origin cell's rate of proliferation, and the histologic pattern of growth.
For many of the B-cell NHL subtypes, the pattern of growth and cell size may be important determinants of tumor aggressiveness. Tumors that grow in a nodular pattern, which vaguely recapitulate normal B-cell lymphoid follicular structures, are generally less aggressive than lymphomas that proliferate in a diffuse pattern. Lymphomas of small lymphocytes generally have a more indolent course than those of large lymphocytes, which may have intermediate-grade or high-grade aggressiveness. However, some subtypes of high-grade lymphomas are characterized by small cell morphology.
Etiology
NHLs may result from chromosomal translocations, infections, environmental factors, immunodeficiency states, and chronic inflammation.
Chromosomal translocations
Chromosomal translocations and molecular rearrangements play an important role in the pathogenesis of many lymphomas and correlate with histology and immunophenotype.
The t(14;18)(q32;q21) translocation is the most common chromosomal abnormality associated with NHL. This translocation occurs in 85% of follicular lymphomas and 28% of higher-grade NHLs. This translocation results in the juxtaposition of the bcl -2 apoptotic inhibitor oncogene at chromosome band 18q21 to the heavy chain region of the immunoglobulin (Ig) locus within chromosome band 14q32.
The t(11;14)(q13;q32 translocation has a diagnostic nonrandom association with mantle cell lymphoma. This translocation results in the overexpression of bcl -1 (cyclin D1/PRAD 1), a cell-cycle regulator on chromosome band 11q13.
The 8q24 translocations lead to c-myc dysregulation. This is frequently observed in high-grade small noncleaved lymphomas (Burkitt and non-Burkitt types), including those associated with HIV infection.
The t(2;5)(p23;q35) translocation occurs between the nucleophosmin (NPM) gene and the anaplastic lymphoma kinase (ALK1) gene. It results in the expression of an aberrant fusion protein found in a majority of anaplastic large cell lymphomas.
Two chromosomal translocations, t(11;18)(q21;q21) and t(1;14)(p22;132), are associated with mucosa-associated lymphoid tissue (MALT) lymphomas. The more common (ie, t[11;18][q21;q21]) translocates the apoptosis inhibitor AP12 gene with the MALT1 gene, resulting in the expression of an aberrant fusion protein. The other translocation, t(1;14)(p22;132), involves the translocation of the bcl -10 gene to the immunoglobulin gene enhancer region.
Infection
Some viruses are implicated in the pathogenesis of NHL, probably because of their ability to induce chronic antigenic stimulation and cytokine dysregulation, which leads to uncontrolled B- or T-cell stimulation, proliferation, and lymphomagenesis. Epstein-Barr virus (EBV) is a DNA virus that is associated with Burkitt lymphoma (especially the endemic form in Africa), Hodgkin disease, lymphomas in immunocompromised patients (eg, from HIV infection, [6] organ transplantation), and sinonasal lymphoma.
Human T-cell leukemia virus type 1 (HTLV-1) causes a latent infection via reverse transcription in activated T-helper cells. This virus is endemic in certain areas of Japan and the Caribbean islands, and approximately 5% of carriers develop adult T-cell leukemia or lymphoma.
Hepatitis C virus (HCV) is associated with the development of clonal B-cell expansions and certain subtypes of NHL (ie, lymphoplasmacytic lymphoma, Waldenström macroglobulinemia), especially in the setting of essential (type II) mixed cryoglobulinemia.
Kaposi sarcoma–associated herpesvirus (KSHV) is associated with body cavity–based lymphomas in patients with HIV infection and in patients with multicentric Castleman disease.
Helicobacter pylori infection is associated with the development of primary gastrointestinal (GI) lymphomas, particularly gastric mucosa-associated lymphoid tissue (MALT) lymphomas.
Environmental factors
Environmental factors linked to the development of NHL include chemicals (eg, pesticides, herbicides, solvents, organic chemicals, wood preservatives, dusts, hair dye), chemotherapy, and radiation exposure. A study by Antonopoulos et al found that maternal smoking during pregnancy may have a modest increase in the risk for childhood NHL but not HL. [7]
Immunodeficiency states
Congenital immunodeficiency states (eg, severe combined immunodeficiency disease [SCID], Wiskott-Aldrich syndrome), acquired immunodeficiency states (eg, AIDS), and induced immunodeficiency states (eg, immunosuppression) are associated with increased incidence of NHL and are characterized by a relatively high incidence of extranodal involvement, particularly of the GI tract, and with aggressive histology. Primary CNS lymphomas can be observed in about 6% of patients with AIDS.
Celiac disease has been associated with an increased risk of malignant lymphomas. The risk of lymphoproliferative malignancy in individuals with celiac disease depends on small intestinal histopathology; no increased risk is observed in those with latent celiac disease. [8]
Chronic inflammation
The chronic inflammation observed in patients with autoimmune disorders, such as Sjögren syndrome and Hashimoto thyroiditis, promotes the development of MALT and predisposes patients to subsequent lymphoid malignancies. Hashimoto thyroiditis is a preexisting condition in 23-56% of patients with primary thyroid lymphomas.
Epidemiology
The American Cancer Society estimates that approximately 80,550 new cases of NHL will be diagnosed in 2023. [9] From the early 1970s to the early 21st century, the incidence rates of NHL nearly doubled. Although some of this increase may be attributable to earlier detection (resulting from improved diagnostic techniques and access to medical care), or possibly to HIV-associated lymphomas, for the most part the rise is unexplained. Since 2015, incidence rates of NHL have declined by about 1% per year. [9]
NHL is the most prevalent hematopoietic neoplasm, representing approximately 4.1% of all cancer diagnoses and ranking eighth in frequency among all cancers. NHL is more than 5 times as common as Hodgkin lymphoma. [3]
Overall, NHL is most often diagnosed in people aged 65-74; median age at diagnosis is 68 years. [3] The exceptions are high-grade lymphoblastic and small noncleaved lymphomas, which are the most common types of NHL observed in children and young adults. At diagnosis, low-grade lymphomas account for 37% of NHLs in patients aged 35-64 years but account for only 16% of cases in patients younger than 35 years. Low-grade lymphomas are extremely rare in children.
Prognosis
The 5-year relative survival rate of patients with NHL is 74.3%. [3] The survival rate has steadily improved over the last 2 decades, thanks to improvements in medical and nursing care, the advent of novel therapeutic strategies (eg, monoclonal antibodies, chimeric antigen receptor [CAR] T-cell therapy), validation of biomarkers of response, and the implementation of tailored treatment.
The prognosis for patients with NHL depends on the following factors:
-
Tumor histology (based on Working Formulation classification)
-
Tumor stage
-
Patient age
-
Tumor bulk
-
Performance status
-
Serum lactate dehydrogenase (LDH) level
-
Beta2-microglobulin level
-
Presence or absence of extranodal disease
In general, these clinical characteristics are thought to reflect the following host or tumor characteristics:
-
Tumor growth and invasive potential (eg, LDH, stage, tumor size, beta2-microglobulin level, number of nodal and extranodal sites, bone marrow involvement)
-
Patient's response to tumor (eg, performance status, B symptoms)
-
Patient's tolerance of intensive therapy (eg, performance status, patient age, bone marrow involvement)
The International Prognostic Index (IPI), which was originally designed as a prognostic factor model for aggressive NHL, also appears to be useful for predicting the outcome of patients with low-grade lymphoma and mantle cell lymphoma. This index is also used to identify patients at high risk of relapse, based on specific sites of involvement, including bone marrow, CNS, liver, testis, lung, and spleen. These patients may be considered for clinical trials that aim at improving the current treatment standard.
An age-adjusted model for patients younger than 60 years has been proposed. In younger patients, stage III or IV disease, high LDH levels, and nonambulatory performance status are independently associated with decreased survival rates.
Pediatric and adolescent patients have better outcome than adults with CNS lymphoma. [10] An ECOG performance status score of 0-1 is associated with improved survival. Higher dose methotrexate is associated with slightly better response.
Clinical features included in the IPI that are independently predictive of survival include the following:
-
Age - Younger than 60 years versus older than 60 years
-
LDH level - Within the reference range versus elevated
-
Performance status - Eastern Cooperative Oncology Group ( ECOG) grade 0-1 versus 2-4
-
Ann Arbor stage - Stage I-II versus III-IV
-
Number of extranodal sites - Zero to 1 versus more than 1
With this model, relapse-free and overall survival rates at 5 years are as follows:
-
0-1 risk factors - 75%
-
2-3 risk factors - 50%
-
4-5 risk factors - 25%
For patients with follicular lymphoma—the second most common subtype of NHL—the Follicular Lymphoma International Prognostic Index (FLIPI) score appears to be more discriminating than the IPI. [11] The FLIPI score is calculated on the basis of 5 adverse prognostic factors, as follows:
-
Age (> 60 y)
-
Ann Arbor stage (III-IV)
-
Hemoglobin level (< 12 g/dL)
-
Number of nodal areas (> 4)
-
Serum LDH level (above normal)
Three risk groups are defined by FLIPI score:
-
Low risk (0-1 adverse factor)
-
Intermediate risk (2 factors)
-
Poor risk (3 or more adverse factors)
Biomarkers in tumor cells such as the expression of bcl- 2 or bcl- 6 proteins and cDNA microarray provide useful prognostic information.
Patients with congenital or acquired immunodeficiency have an increased risk of lymphoma and respond poorly to therapy.
Time to achieve complete remission (CR) and response duration has prognostic significance. Patients who do not achieve CR by the third cycle of chemotherapy have a worse prognosis than those who achieve rapid CR.
Immunophenotype is also a factor. Patients with aggressive T- or NK-cell lymphomas generally have worse prognoses than those with B-cell lymphomas, except the Ki-1 anaplastic large T- or null-cell lymphomas.
Cytogenetic abnormalities and oncogene expression affect prognosis. Patients with lymphomas with 1, 7, and 17 chromosomal abnormalities have worse prognoses than those with lymphomas without these changes.
Low-grade lymphomas have indolent clinical behavior and are associated with a comparatively prolonged survival (median survival is 6-10 y), but they have little potential for cure when the disease manifests in more advanced stages. They also have the tendency to transform to high-grade lymphomas.
Approximately 70% of all patients with intermediate- and high-grade NHL relapse or never respond to initial therapy. Most recurrences are within the first 2 years after therapy completion. Patients with relapsed or resistant NHL have a very poor prognosis (< 5-10% are alive at 2 years with conventional salvage chemotherapy regimens).
Drake et al found that low levels of vitamin D were associated with a decrease in clinical end points (event-free survival and overall survival) in subsets of patients with aggressive B-cell lymphoma (ie, diffuse large B-cell lymphoma or T-cell lymphoma). [12] Although the results of this study suggest an association between vitamin D levels and its metabolism with the biology of some aggressive lymphomas, further studies are needed before conclusions can be drawn.
A study by Change et al also found a protective effect associated with vitamin D and also concluded that routine residential UV radiation exposure may have a protective effect against lymphomagenesis through mechanisms possibly independent of vitamin D. [13]
Survivors of NHL are at risk for development of a second primary malignancy. A review of Surveillance, Epidemiology, and End Results data from 1992-2008 found hazard ratios for a second cancer to be 2.70 for men and 2.88 for women. [14]
Patient Education
Patients should receive a clear and detailed explanation of all the available treatment options, prognosis, and adverse effects of treatment. Advise patients to call their oncologists as necessary and educate patients about oncologic emergencies that require an immediate emergency department visit. Suggest psychosocial counseling.
For patient education information, see Lymphoma.
-
Posteroanterior (PA) chest radiograph in a man with thoracic non-Hodgkin lymphoma (NHL) shows mediastinal widening due to grossly enlarged right paratracheal and left paratracheal nodes.
-
Posteroanterior (PA) chest radiograph in a 16-year-old male adolescent with thoracic non-Hodgkin lymphoma (NHL) shows subtle enlargement of the lower paratracheal lymph nodes.
-
Nonenhanced CT scan through the mediastinum shows multiple enlarged lymph nodes in the prevascular space, in the right and left paratracheal region. Nodes in the left paratracheal region cause the trachea to be indented and narrowed on the left side. Note the small, bilateral pleural effusion
-
Nonenhanced CT scan through the mediastinum at the level of the carina shows enlarged tracheobronchial and subcarinal nodes. Note the small bilateral pleural effusion.
-
Contrast-enhanced axial CT scan in a child shows hypoattenuating, enlarged, subcarinal lymph nodes with splaying of the tracheal bifurcation.
-
Posteroanterior (PA) chest radiograph shows a large mass in the right parahilar region extending into the right upper and middle zones, with silhouetting of the right pulmonary artery. Smaller mass is seen in the periphery of the right lower zone. The masses did not respond to a trial of antibiotics. Core-needle biopsy of the larger lesion revealed NHL deposits in the lung.
-
Lateral image shows a large mass in the anterior aspect of the right upper lobe of the lung.
-
Posterior bone scan shows no abnormally increased uptake in the thoracic vertebrae. Image shows an unusual pattern of non-Hodgkin lymphoma (NHL) of the upper thoracic vertebra.
-
This 28-year-old man was being evaluated for fever of unknown origin. Gallium-67 study shows extensive uptake in the mediastinal lymph nodes due to non-Hodgkin lymphoma (NHL).
-
T1-weighted coronal MRI of the thorax in a 55-year-old woman with lower dorsal pain. Note the signal-intensity changes in the body of D12; these are associated with a right-sided, large, paravertebral soft-tissue mass involving the psoas muscle. Biopsy confirmed non-Hodgkin lymphoma (NHL).
-
T1-weighted coronal MRI of the thorax in a 55-year-old woman with lower dorsal pain (same patient as in the previous image). Note the signal-intensity changes in the body of D12; these are associated with a right-sided, large, paravertebral soft-tissue mass involving the psoas muscle. Biopsy confirmed non-Hodgkin lymphoma (NHL).
-
Positron emission tomography (PET) CT in an 80-year-old woman with diffuse, large B-cell NHL of the skin and subcutaneous tissues that recently transformed from previous low-grade non-Hodgkin lymphoma (NHL). PET shows high level of uptake in the anterior subcutaneous nodule in the chest (white arrows). CT scan of similar nodules (arrowheads) on the anterior left chest does not show PET uptake; these may represent regions of lower-grade NHL. PET image of posterior lesions shows only mild uptake (gray arrow).
-
Non-Hodgkin lymphoma of the terminal ileum. Note the doughnut sign, ie, intraluminal contrast material surrounded by a grossly thickened bowel wall. This appearance is highly suggestive of small noncleaved cell lymphoma (Burkitt type).
-
Computed tomography of the throat in highly-malignant non-hodgkin lymphoma present as lymph node swelling in a child (transverse section with contrast). DE: Computertomographie des Halses bei einem hoch-malignen Non-Hodgkin
-
Malignant lymphoma high grade_B_cell
-
Ultrasound throat lymphadenopathy non-hodgkin-lymphoma
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Approach Considerations
- Complete Blood Cell Count
- Serum Chemistry Studies
- Other Laboratory Studies
- Radiography
- CT, Bone Scan, and Gallium Scan
- Positron Emission Tomography and Ultrasonography
- Multiple Gated Acquisition Scanning
- Magnetic Resonance Imaging
- Biopsy
- Lumbar Puncture
- Histologic Findings
- Immunophenotypic Analysis
- Cytogenetic Studies
- Staging
- Show All
- Treatment
- Approach Considerations
- Management of Indolent NHL
- Management of Aggressive NHL
- Management of Indolent Recurrent NHL
- Management of Aggressive Recurrent Adult NHL
- Management of T-cell Lymphomas
- Surgical Care
- Complications of Therapy
- Dietary Modification
- Activity Restriction
- Management of NHL in Special Populations
- CAR T-cell Therapy
- Consultations
- Long-Term Monitoring
- Show All
- Guidelines
- Guidelines Summary
- Diagnosis
- Staging
- Risk Stratification
- Follicular Lymphoma
- Marginal Zone Lymphomas of MALT Type
- Mantle Cell Lymphoma
- Diffuse Large B-Cell Lymphoma
- Burkitt Lymphoma
- Primary Cutaneous B-Cell Lymphomas
- Cutaneous T-Cell Lymphoma
- Primary Cutaneous CD30+ T-Cell Lymphoproliferative Disorders
- Show All
- Medication
- Medication Summary
- Cytotoxic agents
- Antineoplastic Agents, Histone Deacetylase Inhibitors
- Antineoplastics, PI3K Inhibitors
- Monoclonal Antibodies
- Antineoplastic Agents, Proteasome Inhibitors
- Antineoplastic Agents, mTOR Kinase Inhibitors
- Antineoplastics, Angiogenesis Inhibitor
- PD-1/PD-L1 Inhibitors
- CAR T-cell Therapy
- Antineoplastics, Anti-CD19 Monoclonal Antibodies
- Colony-Stimulating Factor Growth Factors
- Immunomodulators
- Corticosteroids
- Show All
- Questions & Answers
- Media Gallery
- Tables
- References










