Overview
Lentigo maligna is a subtype of melanoma in situ that is characterized by an atypical proliferation of melanocytes within the basal epidermis; lentigo maligna that invades the dermis is termed lentigo maligna melanoma. [1] Lentigo maligna melanoma (LMM) is most often found on sun-exposed skin in the head and neck of middle-aged and elderly persons (see the image below), and is slightly more common in women. Approximately 10-30% of all cutaneous melanoma arise in this region. [2]
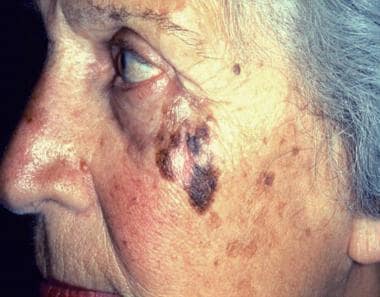 Photograph shows lentigo maligna melanoma on a patient’s cheek. Lentigo maligna melanoma most commonly occurs on sun-exposed skin, especially of the face, in elderly persons. It is characterized by a highly irregular border, heterogeneous coloration, and—because of its long radial growth phase—a large diameter. Courtesy of CDC/Carl Washington, MD, Emory University School of Medicine, and Mona Saraiya, MD, MPH.
Photograph shows lentigo maligna melanoma on a patient’s cheek. Lentigo maligna melanoma most commonly occurs on sun-exposed skin, especially of the face, in elderly persons. It is characterized by a highly irregular border, heterogeneous coloration, and—because of its long radial growth phase—a large diameter. Courtesy of CDC/Carl Washington, MD, Emory University School of Medicine, and Mona Saraiya, MD, MPH.
LMM is one of the four main subtypes of melanoma and represents 5-15% of cases. The other types are superficial spreading (70%), nodular (10-15%), and acral lentiginous melanoma (5%). [3]
The incidence of melanoma has increased steadily over the past 3 decades, and melanoma in situ has constituted a disproportionately high percentage of the rise in cases. [4] It is estimated that in 2022, 99,780 cases of invasive skin melanoma and 97,920 cases of melanoma in situ will be newly diagnosed. [5]
See Mole or Melanoma? Test Yourself With These Suspicious Lesions, a Critical Images slideshow, to help identify various skin lesions.
Sir John Hutchinson first described lentigo maligna in 1890; the disease continues to be called Hutchinson melanotic freckle on occasion. The Hutchinson melanotic freckle was originally thought to be infectious because of its slow yet progressive growth. The lesion has subsequently been termed malignant lentigo of elderly people, junctional nevus, and melanoma in situ. Most authors currently refer to it as lentigo maligna when it is confined to the epidermis and lentigo maligna melanoma when it violates the dermis.
For more information, see the following
Etiology and Pathophysiology
Risk factors for lentigo maligna melanoma include the following:
-
Ultraviolet radiation exposure: For people who live in Australia, the risk increases as the number of years spent in Australia increases, as well as with increased hours of exposure to sunlight, with the amount of actinic damage, and with a history of nonmelanoma skin cancer.
-
Increased number of melanocytic nevi, including large or giant congenital nevi
-
Fair skin
-
History of severe sunburn
-
Occupational risk
Many authors consider lentigo maligna to be a preinvasive lesion induced by long-term cumulative ultraviolet injury. Conceptually, the term melanoma is used when atypical melanocytes invade the rich vascular and lymphatic networks of the dermis, thereby establishing metastatic potential.
Most malignant melanomas arise as superficial tumors confined to the epidermis, which is often known as horizontal growth. At some point, a stepwise accumulation of genetic abnormalities leads to proliferation and progression to the vertical growth phase, which leads to dermal and deeper involvement and, subsequently, nodal metastases.
About 10% of melanomas are familial. A first-degree relative has an 8-12 times increased risk of melanoma. The major gene resides on chromosome arm 9p and encodes a tumor suppressor gene called CDKN2A or MTS1. [6] The second gene that has been noted in melanoma prone families is CDK4, and germline mutations have been identified in this group.
Epidemiology
Lentigo maligna is melanoma in situ; lentigo maligna melanoma is melanoma. The American Cancer Society estimates that 97,920 cases of in situ melanoma and 99,780 cases of invasive melanoma (about 57,180 in men and 42,600 in women) will be diagnosed in the United States in 2022. The number of deaths from melanoma is estimated at 7650 persons (about 5080 men and 2570 women). [7]
The incidence of lentigo maligna is greatest in Hawaii, intermediate in the central and southern states, and lowest in the northern states. The incidence of melanoma is highest in Australia, where lentigo maligna accounts for 10-15% of all melanomas. [8] A study in an Australian population estimated that the annual incidence of all lentigo maligna was 12.2 per 100,000 population, and that lentigo maligna occurred twice as frequently as other types of melanoma in situ. [9]
A review of the New South Wales Cancer Registry in Australia estimated that the risk of progression of lentigo maligna to lentigo maligna melanoma was 3.5% per year (95% confidence interval: 2.5-5.0). This equates to an average time of 28.3 years (95% confidence interval: 20.0-40.5) for progression of lentigo maligna to lentigo maligna melanoma. [10]
Patients with lentigo maligna tend to be older than those with superficial spreading malignant melanoma or nodular melanoma. Generally, patients with lentigo maligna are older than 40 years, with a mean age of 65 years. The peak incidence occurs in the seventh to eighth decades of life.
Lentigo maligna and lentigo maligna melanoma are associated with higher occupational exposure and lower recreational sun exposure.
Clinical Evaluation
As mentioned earlier, the risk of lentigo maligna melanoma increases with the number of years of residence in sunnier climates (eg, southern United States), and the risk increases with increased hours of exposure to sunlight, [11] increased amount of actinic damage, and a history of nonmelanoma skin cancer. Therefore, it is important to elicit this information from the patient.
In addition, associations have been reported between lentigo maligna melanoma and the following:
-
Light skin color (especially people who have red hair)
-
History of severe sunburn
-
Tyrosine-positive oculocutaneous albinism
A strong correlation exists between patients who report finding a nodule and diagnosis of melanoma. Although palpability usually indicates dermal invasion, clinical examination may be unreliable in early invasive lentigo maligna melanoma (lesions < 1 mm).
Lentigo maligna can be present for long periods (5-15 y) before invasion occurs, although rapid progression within months has been described. The percentage of lentigo maligna that progress to lentigo maligna melanoma remains unknown, but estimates of the lifetime risk of developing lentigo maligna melanoma in patients diagnosed with lentigo maligna at age 45 years appears to be 5%. The risk for progression to lentigo maligna melanoma appears to be proportional to the size of the lesion of lentigo maligna. [12]
Suspect the possibility of melanoma if a patient reports a new pigmented lesion or changes in an existing mole. About 50% of melanomas can arise from normal skin with no preexisting lesions. The review of systems should focus on symptoms pertaining to metastatic disease. Melanoma usually metastasizes to lungs, liver, and brain.
Physical assessment
Patients with melanoma need a complete and thorough physical examination, especially with focus on the skin and lymph nodes.
The most frequent findings that suggest early melanoma are changes in size or color of a new pigmented lesion or an existing mole. Lentigo maligna most commonly affects the sun-exposed skin of the head and neck, with a predilection for the nose and cheek. In fact, in Australia, the lesions occur more commonly on the right side (driver's side) of the head and neck in men and on the left side (passenger's side) in women. According to the Australian road traffic database, most Australian drivers are men, and most passengers in the front seat are women.
Less common sites of involvement include the arm, leg, and trunk. The conjunctivae and oral mucosa may become involved when a cutaneous lentigo maligna spreads onto mucosal surfaces. Signs suggestive of a more locally advanced lesion include elevation, burning, itching, pain, or bleeding.
The simple ABCDE rule of melanoma, as follows, helps patients as well as physicians to suspect and make an early diagnosis.
-
A – Asymmetry
-
B – Border irregularity
-
C – Color variegation
-
D – Diameter greater than 6 mm (tip of pencil head), although melanoma can occur in lesions less than 6 mm
-
E – Enlargement
Clinical characteristics of lentigo maligna include the following:
-
Large size: > 6 mm and often several centimeters in diameter at diagnosis
-
Irregular shape
-
Variable pigmentation: Colors may include light brown or tan, dark brown, pink, red, or white
-
Smooth surface
Lentigo maligna, the precursor lesion, has been likened to a stain on the skin. The lesion is typically tan-brown, with differing shades throughout.
Assess the total number of all types of moles. More than 50 nevi 2 mm in diameter or larger indicate an increased risk for melanoma.
Search for dysplastic (clinically atypical) melanocytic nevi. Irregular pigment patterns, such as variegation, central dark areas, or halos of pigment, may indicate the presence of dysplasia. Atypical nevi tend to be larger than common acquired neomelanocytic nevi, which rarely exceed 5 mm.
Search for congenital melanocytic nevi. People with very large or giant congenital nevi have an increased lifetime risk (>6%) of developing melanoma. Intermediate sized (>0.5 cm), raised, pigmented lesions, with or without hair, that do not have the features of clinically atypical nevi have an uncertain but elevated risk for development of melanoma.
Diagnostic Considerations
Distinguishing lentigo maligna from its invasive counterpart on a clinical basis continues to present diagnostic dilemmas, especially in patients who had previous therapeutic interventions such as cryotherapy. It is important to have a low threshold for biopsy of pigmented facial lesions. In a series of 85 excised lesions with a clinical diagnosis of lentigo maligna, more than 50% had invasive lentigo maligna melanoma.
The following conditions should also be considered when evaluating a patient for suspected lentigo maligna and lentigo maligna melanoma:
-
Common acquired nevi
-
Dysplastic nevi
-
Pigmented actinic keratosis
-
Seborrheic keratosis
-
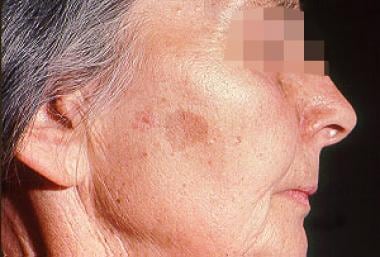
Solar lentigo (see the image below)
Diagnostic Evaluation
Patients with low-risk melanomas, less than 1 mm thick, do not routinely need laboratory studies. Dermoscopy is the first step that is essential for diagnosis of this lesion. The use of a dermatoscope, by a dermatologist or other physician trained in its use, can be very helpful in distinguishing lentigo maligna from other types of skin lesion. However, the dermoscopic appearance of early lentigo maligna can be difficult to distinguish from that of other pigmented lesions, particularly on the face.
Confocal microscopy can sometimes be helpful. Confocal fluorescence microscopy is a microscopic technique that provides true three-dimensional (3D) optical resolution. In microscopy, 3D resolution is generally realized by designing the instrument so that it is primarily sensitive to a specimen’s response coming from an in-focus plane, or by subsequently removing the contributions from out-of-focus planes. [13]
However, most centers order liver function tests and lactate dehydrogenase (LDH) levels as part of the initial laboratory evaluation. Serum LDH is used as part of metastasis staging (see Staging).
Imaging Studies
Most institutions obtain chest radiographs in patients with low-risk disease.
To determine the extent of disease, positron emission tomography (PET) scanning, staging computed tomography (CT) scanning, or magnetic resonance imaging (MRI) can be performed for patients with node-positive disease or those with symptoms suggestive of metastasis.
Dermoscopy
Dermoscopy is a tool that helps the dermatologist distinguish benign from malignant lesions. [14] It is a hand-held instrument that magnifies the skin lesion 10 times to enable a more accurate diagnosis.
Skin Biopsy
Ideally, biopsy of lesions should include full-thickness skin extending to the subcutaneous fat.
Avoid superficial skin biopsy by shaving, scissors excision, or curettage, because these techniques do not allow for assessment of tumor thickness, which is important for prognostication and treatment planning (see Staging).
A better biopsy choice would be excisional biopsy with a narrow margin of normal-appearing skin, which can usually be performed on lesions, unless the result would be disfiguring, in which case incisional biopsy is considered reasonable. Incisional biopsy is also indicated for lesions that are too large for complete excision.
The type of biopsy does not influence patient survival or rate of metastasis. Previous concerns that incision into a melanoma promotes its dissemination have been allayed. The excisional biopsy specimen may be obtained by elliptical excision, saucerization, or punch biopsy, if the lesion is small enough. The specimen should include a portion of subcutaneous fat to ensure that accurate microstaging can be determined.
Lymph Node Biopsy
Sentinel lymph node biopsy is done to assess regional lymph node involvement and to decide on adjuvant therapy. This technique is indicated in all melanoma patients except those with stage 0 or stage 1A disease—that is, patients with a lesion thinner than 1 mm (see Staging).
If metastasis is present, a full nodal dissection is done to fully stage the disease.
Staging
The American Joint Committee on Cancer (AJCC) Tumor, Node, Metastases (TNM) staging for melanoma uses tumor size, rather than invasion, and ulceration and number of mitoses per mm2 to stage the tumor. [15]
Tumor staging is as follows. For the stages below, the addition of “a” indicates without ulceration and of “b” indicates with ulceration, except for “T1a,” which indicates without ulceration and mitosis less than 1 per mm2 and “T1b,” which indicates with ulceration or mitoses 1 or more per mm [210] :
-
Tx: Primary tumor cannot be assessed (eg, diagnosis by curettage)
-
T0: No evidence of primary tumor (eg, unknown primary or completely regressed melanoma)
-
Tis: Melanoma in situ
-
T1: ≤1 mm or less in thickness; T1a, < 0.8 mm without ulceration; T1b, < 0.8 mm with ulceration or 0.8-1.0 mm with or without ulceration
-
T2: > 1.0-2.0 mm; T2a, > 1.0-2.0 mm without ulceration; T2b, > 1.0-2.0 mm with ulceration
-
T3: > 2.0-4.0 mm; T3a, > 2.0-4 mm without ulceration; T3b, > 2.0-4.0 mm with ulceration
-
T4: > 4.0 mm; T4a, > 4.0 mm without ulceration; T4b, > 4.0 mm with ulceration
Regional nodal staging is as follows. For the stages below, the addition of “a” indicates micrometastasis clinically occult (ie, detected by sentinel lymph node biopsy [SLNB]); of “b” indicates macrometastasis clinically apparent; and of “c” indicates in transit, satellite, or microsatellite metastasis without metastatic nodes [15] :
-
Nx: Nodes not assessed (eg, SLNB not performed, regional nodes previously removed for another reason); the exception is that a pT1 cM0 melanoma with no clinically detected regional metastases is designated as cN0 instead of pNX
-
N0: No regional metastases detected
-
N1: One tumor-involved node (N1a, N1b); or in-transit, satellite, and/or microsatellite metastases with no tumor-involved nodes (N1c)
-
N2: Two or three tumor-involved nodes; clinically occult (N2a) or at least one clinically detected (N2b); or one clinically occult or clinically detected with in-transit, satellite, and/or microsatellite metastases present
-
N3: Four or more metastatic nodes; in transit, satellite, or microsatellite metastasis with two or more tumor-involved nodes; or any number of matted nodes without or with in transit, satellite, or microsatellite metastases
-
N3a: Four or more clinically occult nodes
-
N3b: Four or more tumor-involved nodes, at least one of them clinically detected, or presence of any number of matted nodes
-
N3c: Two or more clinically occult or clinically detected tumor-involved nodes, or presence of any number of matted nodes, with in transit, satellite, or microsatellite metastases present
M indicates distant metastasis and is staged incorporating serum lactate dehydrogenase (LDH) levels as follows [15] :
-
M0: No detectable evidence of distant metastases
-
M1a: Metastases to skin, soft tissue (including muscle), or distant lymph nodes; M1a(0), LDH level not elevated; M1a(1), LDH level elevated
-
M1b: Metastases to lung, with or without M1a sites of disease; M1b(0), LDH level not elevated; M1b(1), LDH level elevated
-
M1c: Metastases to visceral sites other than the central nervous system (CNS), with or without M1a or M1b sites of disease; M1c(0), LDH level not elevated; M1c(1), LDH level elevated
-
M1d: Metastasis to CNS with or without M1a, M1b, or M1c sites of disease; M1d(0), LDH level normal; M1d(1), LDH level elevated
Management Overview
Melanoma should be managed by a multidisciplinary team that includes a dermatologist, surgeon, and medical oncologist, as well as other allied health professionals. Lentigo maligna is treated with surgery. The standard and preferred treatment is surgical excision.
Various nonsurgical modalities are available to patients in whom surgical therapy is not feasible, whether because the lesion is in a sensitive anatomic location (eg, face, neck) or the patient a poor candidate for surgery (typically, due to advanced age). Nonsurgical modalities include cryotherapy, immune response therapy with topical imiquimod, laser ablation, photodynamic therapy, and radiation therapy. [16, 17, 18]
Radiation therapy for lentigo maligna and lentigo maligna melanoma can be with Grenz rays or superficial x-rays. [19] A systematic review concluded that the available evidence, although low-level, suggests that radiotherapy may be safe and effective in this setting. [20] A primary risk of using radiation therapy for lentigo maligna is that it may miss focal lentigo maligna melanoma.
There is insufficient evidence to suggest nonsurgical modalities for widespread use. Nonsurgical therapies have a recurrence rate of 20-100% at 5 years.
In the presence of nodal disease, multiple chemotherapy and immunotherapy drugs have been tested. High-dose alpha-interferon remains the standard adjuvant option, especially with stage III cancer, in which it results in a 9-month increase in disease-free survival and a 1-year increase in overall survival.
With metastatic or unresectable disease, National Comprehensive Cancer Network (NCCN) guidelines recommend choosing therapy on the basis of the tumor's BRAF mutation status (wild type versus mutated). An additional consideration is whether the patient is expected to remain clinically stable for more than 12 weeks (in which case the therapeutic intent is long-term survival) or clinical deterioration is expected in 12 weeks or less. [21]
Vaccines have been tested in various studies, but none have led to any significant survival advantage. Granulocyte-macrophage colony-stimulating factor (GM-CSF) has been found to prolong time to progression compared to historical control in both node-positive and metastatic stage of the disease.
Nonsurgical procedures, such as laser treatment, topical imiquimod, cryosurgery, and radiation therapy are also used to treat lentigo maligna and lentigo maligna melanoma because of their sensitive anatomic location and common occurrence in the elderly population.
Special considerations
Potential medicolegal pitfalls include the following:
-
Failure to diagnose, which is often the case with skin lesions that were initially thought to be benign without a biopsy
-
Failure to provide adequate follow-up care
Cryotherapy
Cryotherapy is not standard for the management of lentigo maligna, but it is often used in patients who are not candidates for surgery. Temperatures of -4°C (24.8°F) to -7°C (19.4°F) selectively destroy melanocytes while preserving keratinocytes in skin and mucosal epithelia.
Most authors discourage using cryotherapy for lentigo maligna, because focal lentigo maligna melanoma cannot be detected clinically. In addition, cryotherapy can have complications that include hypopigmentation, infection, scarring, inability to detect invasive melanoma, and failure to treat periadnexal involvement.
Topical Imiquimod Therapy
Topical imiquimod 5% cream has been described in many studies to be effective in treating lentigo maligna, especially in patients who are not surgical candidates. Imiquimod is an immune response modifier that offers encouraging results, but further studies are needed to identify ideal treatment protocols.
A systematic review found that imiquimod monotherapy provides a 76% histologic and a 78% clinical clearance rate for lentigo maligna. The incidence of clinical recurrence was 2.3%. Both cumulative dose and treatment intensity affect tumor clearance; treatment with more than 60 total applications, or with more than five applications per week, was associated with a higher likelihood of histologic clearance. [22]
Surgical Intervention
Lentigo maligna is treated with definitive surgical therapy. The actual margins of atypia usually extend beyond the clinically apparent margin, so total removal may be difficult. National Comprehensive Cancer Network (NCCN) practice guidelines for melanoma in 2022 are as follows (tumor thickness, recommended peripheral surgical margin) [21] :
-
Tumor in situ – Margin size 0.5-1 cm
-
Tumor ≤1.0 mm – Margin size 1 cm
-
Tumor 1.01-2 mm – Margin size 1-2 cm
-
Tumor 2.01-4 mm – Margin size 2 cm
-
Tumor > 4 mm – Margin size 2 cm
The NCCN notes that for large melanoma in situ of the lentigo maligna type, surgical margins wider than 0.5 cm may be necessary. [21] A review by Kunishige and colleagues recommended a surgical margin of at least 9 mm of normal-appearing skin for all melanoma in situ (MIS) subtypes, including lentigo maligna, and that lesions on the head and neck, and those with a diameter greater than 1 cm, may require even wider margins and are best treated with Mohs micrographic surgery. [23]
In a retrospective study by Akhtar et al of 192 cases of MIS, 38% of which were lentigo maligna, MIS that was not lentigo maligna was unlikely to recur after adequate excision, even with narrow margins. With the lentigo maligna cases, however, the rate of incomplete excision was significantly higher than with other MIS, and 2.9% of lentigo maligna cases recurred after complete excision. These authors suggested more aggressive treatment of lentigo maligna, if possible, but noted that lentigo maligna occurs in much older patients who may have less tolerance of more extensive surgery. [24]
Surgical Outcomes
Standard excisions have a recurrence rate of 8-20%. Mohs surgery and staged excision may offer better control and lower recurrence rates (up to 5%). [25, 26, 27, 28] A study of Mohs surgery using immunostaining of frozen sections with melanoma antigen recognized by T cells 1 (MART-1) reported a recurrence rate of less than 1%. [29]
Surgical excision with 5-mm margin is often inadequate for lentigo maligna on sun-damaged skin where a background of melanocytic hyperplasia obscures the true borders of the lesion. The cure rate is 80-94%, but staged margin excisions using frozen, or permanent, sections consistently provided much higher cure rates. [30]
A review of staged excision as treatment of lentigo maligna concluded that this technique provides a long disease-free survival of 95% with follow-up of 57 months. [27] The technique included vertical excision with initial 2- to 3-mm margins and examination of permanent sections within 24 hours. Further excisions took place as guided by the histologic findings. [27]
Hazan et al studied staged excision of lentigo maligna and lentigo maligna melanoma in the head and neck region of 117 cases and reported that the mean total surgical margin for excision of lentigo maligna was 7.1 mm and 10.3 mm for lentigo maligna melanoma. [31]
Radiation Therapy
Radiation therapy (RT) may be an option for patients who are not surgical candidates (eg, due to comorbidities, impact on function, or cosmesis); in patients who refuse surgery; or in whom surgery has failed, with a positive margin. For lentigo maligna treated with definitive primary RT, a literature review by Fogarty et al reported a recurrence rate of 5% after a median of reported follow-up of 3 years. [32]
These authors describe their experience with definitive RT for lentigo maligna at their center, including decisions that need to be made in RT planning and treatment, and management of adverse effects. They note that effective treatment requires accurate mapping of the tumor volume, which in lentigo maligna extends to the bottom of the appendageal structures in the dermis and includes hair follicles and sweat glands. Underestimation of this area, which is usually larger than than the pigmented lesion visible to the naked eye, has led to field-edge recurrences. [32]
A systematic review of RT for lentigo maligna and lentigo maligna melanoma reported that for Grenz radiotherapy, studies have used 42–160 Gy in 3–13 fractions with single doses up to 20 Gy. For superficial radiotherapy, reports describe use of 5–23 fractions with a total dose of 35–57 Gy. [20]
The Radiotherapy or Imiquimod in Complex Lentigo Maligna (RADICAL) trial, currently in progress, is investigating the effectiveness of using either RT or imiquimod to treat lentigo maligna when surgery is not possible, is refused, or fails.
Systemic Therapy for Metastatic Disease
For patients with metastatic or unresectable disease whose tumor has wild-type BRAF V600, National Comprehensive Cancer Network (NCCN) preferred options for first-line systemic therapy include the following [21] :
-
Pembrolizumab
-
Nivolumab
-
Nivolumab/ipilimumab
For patients with BRAF V600 activating mutation (preferred if clinically needed for early response), NCCN recommends the following combination regimens as category 1 for first-line systemic therapy [21] :
-
Dabrafenib + trametinib
-
Vemurafenib + cobimetinib
-
Encorafeinb + binimetinib
For patients who experience disease progression or maximum clinical benefit from BRAF-targeted therapy, NCCN recommendations for second-line or subsequent therapy are as follows:
-
For patients who experience progression of melanoma during or shortly after first-line therapy, consider second-line agents if not used first line and not of same class. For patients who progressed on single-agent anti–PD-1 checkpoint immunotherapy, nivolumab/ipilimumab combination therapy, or ipilimumab monotherapy is a reasonable treatment option.
-
For patients who experience disease control with first-line therapy and have no residual toxicity, but experience disease progression or relapse > 3 months after treatment discontinuation, re-induction with the same agent or same class of agents may be considered.
-
Other single-agent regimens to consider are ipiliumab or high-dose interleukin-2
The NCCN considers the following regimens useful in certain circumstances:
vary by performance status. For those with performance status of 0-2, with wild-type BRAF, recommended agents include the following [21] :
-
Ipilimumab + intralesional talimogene laherparepvec
-
Cytotoxic agents
-
Imatinib (for tumors with activating mutations of C-KIT)
-
Larotrectinib or entrectinib (for tumors with NTRK gene fusion)
-
Binimetinib (for NRAS-mutated tumors that have progressed after prior immune checkpoint inhibitor therapy)
For patients with performance status 3-4, consideration of best supportive care is advised.
Outpatient Follow-up
Although early detection of a melanoma recurrence is a major concern, recommendations from different dermatological and oncological organizations vary with respect to who should provide follow-up, as well as duration and frequency of follow-up and the use of imaging and laboratory studies. However, all organizations agree that the physical examination is the cornerstone of follow-up care. [33]
The National Comprehensive Cancer Network (NCCN) recommends at least annual skin exams for life in all melanoma patients, along with patient education in self-examination of skin and lymph nodes. The follow-up schedule should be influenced by factors affecting risk of recurrence, such as family history and presence of atypical moles or dysplastic nevi. The NCCN does not recommend routine blood tests. [21]
For patients with stage 0 in situ through stage IIA melanoma with no evidence of disease, the NCCN recommends a history and physical examination with emphasis on nodes and skin every 6-12 months for 5 years, than annually as clinically indicated. The NCCN recommends against routine radiologic imaging to screen for asymptomatic recurrent metastatic disease. [21]
Patient education
Education plays an integral role in the follow-up care of patients with melanoma. Instruct patients on self-examination for new and locoregional recurrences. Teach them to recognize the classic characteristics of cutaneous melanoma in friends and family. In addition, during every follow-up visit, strongly counsel patients to avoid excess sun exposure, to use sunblock liberally, and to wear protective clothing.
For patient education information, see Melanoma.
Prognosis and Prognostic Factors
Melanoma is second only to adult leukemia in years of potential life lost in young adults. The disease is responsible for death in a disproportionately high number of young and middle-aged adults. [34] Lentigo maligna melanoma has a mortality rate similar to that of other melanoma types if depth of the tumor is taken into account. [35]
The overall prognosis is good for patients with localized melanoma and no nodal or distant metastases. An overall 5-year survival rate of 79% has been reported for patients with stage I or stage II lesions.
In patients with regional nodal disease (ie, stage III), the 3 dominant prognostic variables are the number of nodal metastases, the patient's age, and ulceration in the primary tumor (see Staging).
Numerous studies have shown that the number of nodes with metastases has significant prognostic value in patients with stage III disease. Patients with 1 positive node fared the best; 40% remained alive at 10 years. Those with 2-4 positive nodes had an intermediate 10-year survival rate of 26%. Patients with 5 or more positive nodes had the lowest 10-year survival rate, at 15%.
In both men and women with stage III melanoma, patients older than 50 years tend to do worse. In studies evaluating tumor ulceration as a prognostic factor in lentigo maligna melanoma, the 3-year survival rate for patients with an ulcerated primary tumor was 20%, compared with 35% for those with nonulcerated primary lesions.
Patients with stage IV melanoma generally have a poor prognosis. From the time the metastasis is diagnosed, the median survival is 6-7.5 months, with a 5-year survival rate of approximately 6%.
Patients with neck and scalp melanoma have shorter survival compared with melanoma at other sites (extremities, face, trunk). [36] In an analysis of the Surveillance, Epidemiology, and End Results (SEER) program data, patients with scalp and neck had a 1.84 risk of dying compared with patients affected at other sites such as extremity melanoma. The 5- and 10-year Kaplan Meier survival probabilities for scalp and neck melanoma were 83.1% and 76.2%, respectively, compared with melanoma of other sites at 92.1% and 88.7%, respectively. [36]
Lentigo Maligna Guidelines
Guidelines on the management of lentigo maligna from Cancer Council Australia and Melanoma Institute Australia include the following recommendations [1]
-
The diagnosis of lentigo maligna is made using the results from biopsy and histopathology studies. Because the risk of lentigo maligna progressing to invasive melanoma generally is low and can take many years, watchful waiting (monitoring the lesions over time) may be a more appropriate approach in very elderly patients or those with significant comorbidities. A biopsy of suggestive areas can be performed if significant dermatoscopic or clinical changes are noted.
-
Mohs micrographic surgery has been shown to be superior to conventional surgical removal, yielding improved complete clearance rates and reduced recurrence. When possible, complete surgical removal with clinical margins of 5-10 mm is recommended. Consideration should be given to using perioperative reflectance confocal microscopy for assessing clinical margins, if it is available.
-
An acceptable recommended alternative to surgical removal or as adjunctive treatment after surgical removal is ultrasoft x-ray/grenz ray therapy, particularly for large lesions.
-
If surgical excision and ultrasoft x-ray/grenz ray therapy are contraindicated or refused by the patient, imiquimod therapy is recommended. Note that radiotherapy has been shown to be superior to imiquimod in terms of complete clearance and recurrence. Tazarotene can be added to imiquimod therapy to increase the inflammatory response; however, whether this improves treatment efficacy has not been determined.
-
Neither cryotherapy nor laser therapy is recommended for treating lentigo maligna.
Questions & Answers
Overview
What is lentigo maligna melanoma?
What are the risk factors for lentigo maligna melanoma?
What is the pathophysiology of lentigo maligna melanoma?
What is the role of genetics in the etiology of lentigo maligna melanoma?
What is the US prevalence of lentigo maligna melanoma?
Which patient groups have the highest prevalence of lentigo maligna melanoma?
Which patient history findings are characteristic of lentigo maligna melanoma?
Which physical findings are characteristic of lentigo maligna melanoma?
Which conditions are included in the differential diagnoses of lentigo maligna melanoma?
How is lentigo maligna melanoma diagnosed?
What is the role of confocal microscopy in the workup of lentigo maligna melanoma?
What is the role of lab tests in the workup of lentigo maligna melanoma?
What is the role of imaging studies in the workup of lentigo maligna melanoma?
What is the role of dermoscopy in the diagnosis of lentigo maligna melanoma?
What is the role of skin biopsy in the diagnosis of lentigo maligna melanoma?
What is the role of lymph node biopsy in the diagnosis of lentigo maligna melanoma?
How is lentigo maligna melanoma staged?
How is lentigo maligna melanoma treated?
What are the potential medicolegal issues associated with lentigo maligna melanoma?
What is the role of cryotherapy in the treatment of lentigo maligna melanoma?
What is the role of topical medications in the treatment of lentigo maligna melanoma?
What is the role of surgery in the treatment of lentigo maligna melanoma?
How effective is surgery for lentigo maligna melanoma?
What is the role of radiation therapy (RT) in the treatment of lentigo maligna melanoma?
How is metastatic lentigo maligna melanoma treated?
What is included in the long-term monitoring of lentigo maligna melanoma?
What is included in patient education about lentigo maligna melanoma?
What is the prognosis of lentigo maligna melanoma?
What are the treatment guidelines for lentigo maligna melanoma?
-
Woman with solar lentigo. Common in sun-exposed skin, solar lentigines are benign lesions that must be distinguished from lentigo maligna melanoma.
-
Photograph shows lentigo maligna melanoma on a patient’s cheek. Lentigo maligna melanoma most commonly occurs on sun-exposed skin, especially of the face, in elderly persons. It is characterized by a highly irregular border, heterogeneous coloration, and—because of its long radial growth phase—a large diameter. Courtesy of CDC/Carl Washington, MD, Emory University School of Medicine, and Mona Saraiya, MD, MPH.
Tables
What would you like to print?
- Overview
- Etiology and Pathophysiology
- Epidemiology
- Clinical Evaluation
- Diagnostic Considerations
- Diagnostic Evaluation
- Imaging Studies
- Dermoscopy
- Skin Biopsy
- Lymph Node Biopsy
- Staging
- Management Overview
- Cryotherapy
- Topical Imiquimod Therapy
- Surgical Intervention
- Surgical Outcomes
- Radiation Therapy
- Systemic Therapy for Metastatic Disease
- Outpatient Follow-up
- Prognosis and Prognostic Factors
- Lentigo Maligna Guidelines
- Questions & Answers
- Show All
- Media Gallery
- References