Background
The genus Klebsiella belongs to the tribe Klebsiellae, a member of the family Enterobacteriaceae.The organisms are named after Edwin Klebs, a 19th century German microbiologist. Klebsiellae are nonmotile, rod-shaped, gram-negative bacteria [1] with a prominent polysaccharide capsule. This capsule encases the entire cell surface, accounts for the large appearance of the organism on gram stain, and provides resistance against many host defense mechanisms.
Members of the Klebsiella genus typically express 2 types of antigens on their cell surface. The first is a lipopolysaccharide (O antigen); the other is a capsular polysaccharide (K antigen). Both of these antigens contribute to pathogenicity. About 77 K antigens and 9 O antigens exist. The structural variability of these antigens forms the basis for classification into various serotypes. The virulence of all serotypes appears to be similar.
Three species in the genus Klebsiella are associated with illness in humans: Klebsiella pneumoniae, Klebsiella oxytoca, and Klebsiella granulomatis. Organisms previously known as Klebsiella ozaenae and Klebsiella rhinoscleromatis are considered nonfermenting subspecies of K pneumoniae that have characteristic clinical manifestations. With those exceptions, strains within this genus ferment lactose, most produce highly mucoid colonies on plates because of the production of a luxuriant polysaccharide capsule, and all are nonmotile. [2] In recent years, klebsiellae have become important pathogens in nosocomial infections. [3] See the image below.
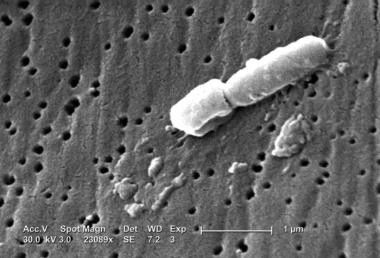 This scanning electron micrograph (SEM) reveals some of the ultrastructural morphologic features of Klebsiella pneumoniae. Courtesy of CDC/Janice Carr.
This scanning electron micrograph (SEM) reveals some of the ultrastructural morphologic features of Klebsiella pneumoniae. Courtesy of CDC/Janice Carr.
Pathophysiology
Host defense against bacterial invasion depends on phagocytosis by polymorphonuclear granulocytes and the bactericidal effect of serum, mediated in large part by complement proteins. Both classic-pathway and alternate-pathway complement activation have been described, but the latter, which does not require the presence of immunoglobulins directed against bacterial antigens, appears to be the more active pathway in K pneumoniae infections.
Recent data from preclinical studies suggest a role for neutrophil myeloperoxidase and lipopolysaccharide-binding protein in host defense against K pneumoniae infection. Neutrophil myeloperoxidase is thought to mediate oxidative inactivation of elastase, an enzyme implicated in the pathogenesis of various tissue-destroying diseases. Lipopolysaccharide-binding protein facilitates transfer of bacterial cell wall components to inflammatory cells. Investigators showed higher rates of infection in experimental mice deficient in the genes that control expression of these 2 agents.
The bacteria overcome innate host immunity through several means. They possess a polysaccharide capsule, which is the main determinant of their pathogenicity. The capsule is composed of complex acidic polysaccharides. Its massive layer protects the bacterium from phagocytosis by polymorphonuclear granulocytes. In addition, the capsule prevents bacterial death caused by bactericidal serum factors. This is accomplished mainly by inhibiting the activation or uptake of complement components, especially C3b. The bacteria also produce multiple adhesins. These may be fimbrial or nonfimbrial, each with distinct receptor specificity. These help the microorganism adhere to host cells, which is critical to the infectious process.
Lipopolysaccharides (LPS) are another bacterial pathogenicity factor. They are able to activate complement, which causes selective deposition of C3b onto LPS molecules at sites distant from the bacterial cell membrane. This inhibits the formation of the membrane attack complex (C5b-C9), which prevents membrane damage and bacterial cell death.
Availability of iron increases host susceptibility to K pneumoniae infection. Bacteria are able to compete effectively for iron bound to host proteins because of the secretion of high-affinity, low molecular weight iron chelators known as siderophores. This is necessary because most host iron is bound to intracellular and extracellular proteins. In order to deprive bacteria of iron, the host also secretes iron-binding proteins.
Epidemiology of Klebsiellae
Klebsiellae are ubiquitous in nature. In humans, they may colonize the skin, pharynx, or gastrointestinal tract. They may also colonize sterile wounds and urine. Carriage rates vary with different studies. Klebsiellae may be regarded as normal flora in many parts of the colon and intestinal tract and in the biliary tract. Oropharyngeal carriage has been associated with endotracheal intubation, impaired host defenses, and antimicrobial use.
K pneumoniae and K oxytoca are the 2 members of this genus responsible for most human infections. They are opportunistic pathogens found in the environment and in mammalian mucosal surfaces. The principal pathogenic reservoirs of infection are the gastrointestinal tract of patients and the hands of hospital personnel. Organisms can spread rapidly, often leading to nosocomial outbreaks.
Infection with Klebsiella organisms occurs in the lungs, where they cause destructive changes. Necrosis, inflammation, and hemorrhage occur within lung tissue, sometimes producing a thick, bloody, mucoid sputum described as currant jelly sputum. The illness typically affects middle-aged and older men with debilitating diseases such as alcoholism, diabetes, or chronic bronchopulmonary disease. This patient population is believed to have impaired respiratory host defenses. The organisms gain access after the host aspirates colonizing oropharyngeal microbes into the lower respiratory tract.
Klebsiellae have also been incriminated in nosocomial infections. Common sites include the urinary tract, lower respiratory tract, biliary tract, and surgical wound sites. The spectrum of clinical syndromes includes pneumonia, bacteremia, thrombophlebitis, urinary tract infection (UTI), cholecystitis, diarrhea, upper respiratory tract infection, wound infection, osteomyelitis, and meningitis. The presence of invasive devices, contamination of respiratory support equipment, use of urinary catheters, and use of antibiotics are factors that increase the likelihood of nosocomial infection with Klebsiella species. Sepsis and septic shock may follow entry of organisms into the blood from a focal source.
Klebsiella granulomatis (formerly Calymmatobacterium granulomatis) is a fastidious member of the genus that causes chronic genital ulcerative disease also known as granuloma inguinale or donovanosis. It is a relatively rare disease in the United States, with fewer than 100 cases reported annually. It has long been a recognized cause of genital ulceration in parts of India, Papua New Guinea, the Caribbean, and South America (particularly Brazil). Fortunately, the incidence of the disease has decreased in recent years.
Rhinoscleroma and ozena are 2 other infections caused by Klebsiella species. These diseases are rare. Rhinoscleroma is a chronic inflammatory process involving the nasopharynx, whereas ozena is a chronic atrophic rhinitis characterized by necrosis of nasal mucosa and mucopurulent nasal discharge.
K oxytoca has been implicated in neonatal bacteremia, especially among premature infants and in neonatal intensive care units. Increasingly, the organism is being isolated from patients with neonatal septicemia.
Extensive use of broad-spectrum antibiotics in hospitalized patients has led to both increased carriage of klebsiellae and, subsequently, the development of multidrug-resistant strains that produce extended-spectrum beta-lactamase (ESBL). These strains are highly virulent, show capsular type K55, and have an extraordinary ability to spread. Most outbreaks are due to a single clone or single gene; the bowel is the major site of colonization with infection of the urinary tract, respiratory tract, and wounds. Bacteremia and significant increased mortality have resulted from infection with these species.
In addition to prior antibiotic use, risk factors for infection include the presence of an indwelling catheter, feeding tube, or central venous catheter; poor health status; and treatment in an intensive care unit or nursing home. Acquisition of these species has become a major problem in most hospitals because of resistance to multiple antibiotics and potential transfer of plasmids to other organisms.
Carbapenem-resistant Enterobacteriaceae (CRE), which are sometimes known as K pneumoniae carbapenemase (KPC) and New Delhi metallo-beta-lactamase (NDM), are a family of bacteria that are difficult to treat because of their high levels of resistance to antibiotics. Some CRE bacteria have become resistant to most available antibiotics and cause mortality rates of up to 50%.
Hypervirulent K pneumoniae (hvKp), also termed as hypermucoviscous strains, have been reported globally with invasive infections and varying degrees of resistance and virulence patterns. [4, 5, 6, 7]
Epidemiology
Frequency
United States
In some parts of the world, K pneumoniae is an important cause of community-acquired pneumonia in elderly persons. Studies conducted in Malaysia and Japan estimate the incidence rate in elderly persons to be 15-40%, which is equal to, if not greater than, that of Haemophilus influenzae. However, in the United States, these figures are different. Persons with alcoholism are the main population at risk, and they constitute 66% of people affected by this disease. Mortality rates are as high as 50% and approach 100% in persons with alcoholism and bacteremia.
Klebsiellae are also important in nosocomial infections among adult and pediatric populations. Klebsiellae account for approximately 8% of all hospital-acquired infections. In the United States, depending on the study reviewed, they comprise 3-7% of all nosocomial bacterial infections, placing them among the top 8 pathogens in hospitals. Klebsiellae cause as many as 14% of cases of primary bacteremia, second only to Escherichia coli as a cause of gram-negative sepsis. They may affect any body site, but respiratory infections and UTIs predominate.
According to the 2019 Centers for Disease Control and Prevention (CDC) Antibiotic Resistance report, more than 2.8 million antibiotic-resistant infections occur in the United States, and more than 35,000 deaths annualy from 2012-2017 as a result. The US Centers for Disease Control and Prevention report that Klebsiella strains were responsible for 3% of all pathogenic epidemic outbreaks.
An investigation of Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae among patients of acute and long-term acute care hospitals was conducted in 2011. The investigation found extensive spread of KPC-producing Enterobacteriaceae throughout 4 adjacent counties in Indiana and Illinois over a 1-yr period. Long-term acute care hospitals played a central role in the outbreak, suggesting that guidelines for controlling KPC should be expanded to include long-term care facilities. Education of personnel and coordinated regional efforts among health care facilities are crucial for KPC control. [8]
Another multihospital study on transmission risk found that patients admitted to acute care hospitals from high-acuity long-term–care facilities were more likely to be colonized with KPC-producing Enterobacteriaceae than were patients admitted from the community. [9]
K oxytoca is among the top 4 pathogens that cause infection in patients in neonatal intensive care units. It is the second most frequent cause of gram-negative neonatal bacteremia.
International
Outbreaks of neonatal septicemia occur worldwide. Infection with K pneumoniae also has a worldwide distribution. Infection with K rhinoscleromatis is not common in the United States, although it has a worldwide distribution and is usually observed in areas of eastern Europe, southern Asia, central Africa, and Latin America. K granulomatis is more common outside the United States in countries such as India, Papa New Guinea, Caribbean, South America, Zambia, Zimbabwe, South Africa, Southeast Asia, and some parts of Australia.
Mortality/Morbidity
Klebsiella pneumonia is a necrotizing process with a predilection for debilitated people. It has a high mortality rate of approximately 50% even with antimicrobial therapy. The mortality rate approaches 100% for persons with alcoholism and bacteremia.
Klebsiella bacteremia and sepsis produce clinical manifestations similar to those caused by other gram-negative enteric organisms. Morbidity and mortality rates are comparable to those for other gram-negative organisms that cause sepsis and septic shock. In neonatal units, outbreaks caused by ESBL-producing strains present a more serious problem and may be associated with increased mortality.
Age
Community-acquired Klebsiella (Friedländer) pneumonia is a disease of debilitated middle-aged and older men with alcoholism.
Nosocomial infections may affect adults or children, and they occur more frequently in premature infants, patients in neonatal intensive care units, and hospitalized individuals who are immunocompromised.
-
This scanning electron micrograph (SEM) reveals some of the ultrastructural morphologic features of Klebsiella pneumoniae. Courtesy of CDC/Janice Carr.






