Practice Essentials
Kaposi sarcoma (KS) is an indolent angio-proliferative spindle-cell tumor derived from endothelial and immune cells infected with human herpes virus type 8 (HHV-8; also known as Kaposi sarcoma herpes virus [KSHV]). HHV-8 is identified as the causative agent of KS; it is present in 95-98% of all cases. [1] KS is categorized into the following 4 types [2] :
-
Epidemic (AIDS related)
-
Iatrogenic (immunosuppressant therapy related)
-
Classic, or sporadic
-
Endemic (African)
Increasing reports describe cases of KS in men who have sex with men but who have no evidence of HIV infection or immunodeficiency. These cases present in an indolent cutaneous form that resembles classic KS, and have been regarded as a fifth type of KS, termed nonepidemic KS. [3, 4, 5]
Although all types of KS have in common infection with HHV-8, each has a distinct clinical course. Therefore, it is likely that other factors, such as extent and type of immune suppression, influence the disease. [6] The presentation of KS ranges from minimal mucocutaneous disease to extensive organ involvement.
The epidemiology of KS has changed dramatically from 1872, when it was first described as a rare disease in Eastern European men. In the 1950s, an endemic form of KS was reported to be one of the most common neoplasms observed in central Africa, affecting men, women, and children. The cases in children are due to maternal-child transmission of HHV-8 through saliva. [6]
A surge in KS cases was noted just prior to the identification of the AIDS epidemic in the early 1980s. AIDS-related KS is the most common KS presentation in the United States. Estimates indicate that the risk of KS in people living with HIV from 2009-2012 was 500-fold higher than for the US general population. KS accounts for 12% of cancers in people living with HIV, with 765 to 910 new cases per year in the US. [7, 8, 9]
Notably, following the AIDS epidemic, the incidence of KS in Africa increased markedly. From 1968 to 1970, KS accounted for 6.6% of all cancers occurring in men; however, from 1989 to 1991, KS became the most commonly reported cancer in men. [10, 11]
Iatrogenic KS cases have also increased, due to greater use of immunosuppression in medical practice. These include the post-transplant setting and treatment of autoimmune disease. [9]
Signs and symptoms
Lesions in KS may involve the skin, oral mucosa, lymph nodes, and visceral organs. Most patients present with cutaneous disease (see the image below). Visceral disease may occasionally precede cutaneous manifestations.
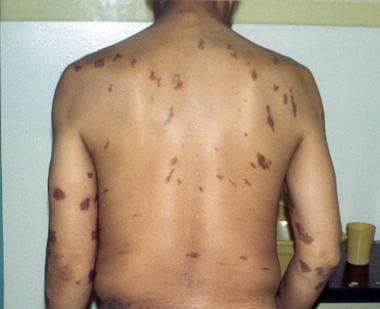 Epidemic Kaposi sarcoma (KS). Large violaceous truncal nodules with typical linear and symmetric distribution pattern.
Epidemic Kaposi sarcoma (KS). Large violaceous truncal nodules with typical linear and symmetric distribution pattern.
See Clues in the Oral Cavity: Are You Missing the Diagnosis?, a Critical Images slideshow, to help identify the causes of abnormalities of the oral cavity.
Cutaneous lesions in KS are characterized as follows:
-
Cutaneous lesions may occur at any location but typically are concentrated on the lower extremities and the head and neck region
-
Lesions may have macular, papular, nodular, or plaquelike appearances
-
Nearly all lesions are palpable and nonpruritic
-
Lesions may range in size from several millimeters to several centimeters in diameter
-
Lesions may assume a brown, pink, red, or violaceous color and may be difficult to distinguish in dark-skinned individuals
-
Lesions may be discrete or confluent and typically appear in a linear, symmetrical distribution, following Langer lines
-
Mucous membrane involvement is common (palate, gingiva, conjunctiva)
Gastrointestinal lesions can occur anywhere in the gastrointestinal tract. Lesions are often asymptomatic and clinically indolent, but signs and symptoms can include the following:
-
Odynophagia, dysphagia
-
Nausea, vomiting, abdominal pain
-
Hematemesis, hematochezia, melena
-
Bowel obstruction
Pulmonary lesions may be an asymptomatic radiographic finding, but signs and symptoms can include the following:
-
Cough
-
Dyspnea
-
Hemoptysis
-
Chest pain
Classic Kaposi sarcoma
This form of the disease has a more indolent course than AIDS-related KS, progressing over 10-15 years or more, with very gradual enlargement of cutaneous lesions and development over years of new ones.
See Presentation for more detail.
Diagnosis
Laboratory studies
CD4 lymphocyte counts and plasma HIV viral-load studies should be performed for patients with HIV infection.
Imaging studies
Chest radiographic findings in patients with KS are variable and nonspecific. They may include any of the following:
-
Diffuse reticulonodular infiltrates
-
Interstitial infiltrates
-
Pleural effusions
-
Hilar or mediastinal lymphadenopathy
-
An isolated pulmonary nodule
Thallium and gallium scans may help differentiate pulmonary KS from infection. Pulmonary KS lesions typically demonstrate intense thallium uptake and no gallium uptake, whereas infection is often gallium avid and thallium negative.
Procedures
-
Punch biopsy
-
Bronchoscopy
-
Esophagogastroduodenoscopy (EGD) or colonoscopy
Typical histologic findings in KS include the following:
-
Proliferation of spindle cells
-
Prominent, slitlike vascular spaces
-
Extravasated red blood cells.
See Workup for more detail.
Management
Antiretroviral therapy
Optimal control of HIV infection using highly active antiretroviral therapy (HAART) is an integral part of successful KS therapy. HAART may be tried as the sole modality in nonvisceral disease. For visceral disease, chemotherapy may be added.
Local therapy
The following local therapies can be used for palliation of locally advanced symptomatic disease or in individuals who have cosmetically unacceptable lesions:
-
Radiation therapy
-
Cryotherapy
-
Laser therapy
-
Surgical excision
-
Intralesional vinca alkaloid therapy
-
Topical retinoids
Immunomodulation
Immunomodulation with interferon-alfa has clinical activity in KS that may be mediated by its antiangiogenic, antiviral, and immunomodulatory properties.
Combination therapy
Combination therapy with regimens such as ABV (actinomycin D, bleomycin, vincristine) produces higher response rates than does single-agent therapy (such as doxorubicin), but time to progression and overall survival rates are similar.
Cytotoxic agents
Several single cytotoxic agents have been approved by the Food and Drug Administration (FDA) for AIDS-related KS; they include the following:
-
Liposomal doxorubicin (Doxil)
-
Liposomal daunorubicin (DaunoXome)
-
Paclitaxel (Taxol) or oral etoposide (VePesid)
Liposomal technology has resulted in higher response rates with less cardiac toxicity and myelotoxicity for liposomal doxorubicin and liposomal daunorubicin. [12, 13, 14]
See Treatment and Medication for more detail.
Background
Kaposi sarcoma (KS) was described initially in 1872 by a Hungarian dermatologist, Moritz Kaposi. The lesions are characterized by proliferation of spindle cells of endothelial origin, with varying degrees of abnormal vascularity, inflammatory infiltrates, and fibrosis. Red cells and hemosiderin deposits give lesions their characteristic purplish color. Spindle cells are infected with HHV-8. HHV-8 encodes a number of genes that induce proliferation, cytokine production, and angiogenesis and thereby contribute to pathogenesis.
Kaposi sarcoma follows a variable clinical course, ranging from minimal mucocutaneous disease to extensive organ involvement. See the table below. [1]
Types of Kaposi sarcoma (Open Table in a new window)
Classification |
Affected Population |
Clinical Course |
AIDS related |
HIV-infected persons (usually those with a low CD4+ count usually) |
Most aggressive (survival improved with antiretroviral therapy) |
Classical |
Eastern European or Mediterranean men |
Indolent (progression over years to decades) |
Endemic |
Subsaharan African children and adults |
Somewhat aggressive |
Iatrogenic |
Patients receiving immunosuppressive therapy (eg, following organ transplantation, for autoimmune disease) |
Somewhat aggressive |
Adapted from Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000 Apr 6. 342 (14):1027-38.[QxMD MEDLINE Link].
The common theme of immune dysregulation is associated with all 4 types of Kaposi sarcoma. Diminished responsiveness of cytotoxic T-lymphocytes is associated with Kaposi sarcoma pathogenesis. [15] Restoration of natural killer cell cytoxic effect may explain regression of Kaposi sarcoma in AIDS patients treated with antiretrovirla therapy. [16] Immune activation may also be a factor in Kaposi sarcoma, with a role for inflammatory cytokines such as gamma interferon and the initiation of HHV-8 infected cell proliferation by HIV-tat protein. [17, 18] This complex interaction of HIV, HHV-8, environmental factors, and the immune system requires further investigation to attempt to decipher the true pathogenesis of Kaposi sarcoma.
Epidemic (AIDS-related) Kaposi sarcoma
This entity occurs in patients with advanced HIV infection and is the most common presentation of Kaposi sarcoma. It is an AIDS-defining cancer, and is approximately 500 times more common in HIV-infected patients than the general US population. Kaposi sarcoma accounts for 12% of cancers in people living with HIV, with 765 to 910 new cases per year in the US. [7, 8, 9]
Decreased CD4 counts and increased HIV-1 viral loads are independent prognostic factors in the development of epidemic Kaposi sarcoma. The disease usually develops in HIV-infected patients with severe immunodeficiency; less than one sixth of HIV-infected patients with Kaposi sarcoma have CD4 counts of over 500 cells per microliter. Immune reconstitution during the first 3 months of HAART may contribute to the risk for AIDS-defining Kaposi sarcoma. [19]
Kaposi sarcoma is a frequent complication of AIDS in men who have sex with men (MSM). A cross-sectional analysis of factors affecting risk in 99 cases among 503 HHV-8 seropositive MSM with AIDS found that Kaposi sarcoma was [20] :
-
Significantly less common in blacks than in whites (risk ratio [RR] = 0.4; 95% confidence index [CI] = 0.2-0.8).
-
More common in subjects who had completed college (RR = 1.7; 95% CI = 1.1-2.7) or had annual income greater than $30,000 (RR = 1.5; 95% CI = 1.1-2.2).
-
Less common in cigarette smokers (RR = 0.6; 95% CI = 0.5-0.9) and users of crack cocaine (RR = 0.4; 95% CI = 0.1-0.8).
-
Less common in bisexual men than in men who were exclusively homosexual (estimated RR = 0.6; 95% CI = 0.4-0.9) and inversely associated with the number of female partners.
-
Less common in men who had received pay for sex (RR = 0.6; 95% CI = 0.4-1.0).
Cigarette smoking may be protective for Kaposi sarcoma risk in HHV-8–seropositive patients infected with HIV, and relative affluence may increase the risk of Kaposi sarcoma in HIV-positive patients.The incidence of HHV-8 infection is higher in homosexual men than in drug users. [21, 20]
The presence of HHV-8 antibodies in HIV-infected persons increases the risk of Kaposi sarcoma. Among HIV-infected persons, those who subsequently seroconvert for HHV-8 are at highest risk. A comparison of 69 men who became infected with HHV-8 after acquiring HIV-1 with 182 men who were HHV-8 seropositive before their HIV-1 infection found that the risk of developing Kaposi sarcoma was higher in those whose HHV-8 seroconversion followed acquisiiton of HIV-1 infection (risk ratio, 2.55; 95% confidence interval, 1.06–6.10). [21, 22]
Risk for Kaposi sarcoma in HHV-8-infected men increased by 60% (P< 0.001) for each year of HIV-1 infection. Faster CD4 cell loss and higher HIV-1 RNA levels significantly predicted Kaposi sarcoma. The quicker development of KS in men acquiring HHV-8 after HIV-1 and its association with CD4 slope argues that Kaposi sarcoma is more likely if HHV-8 infection occurs in an immunocompromised person. [21, 22]
Iatrogenic (immunosuppression-related) Kaposi sarcoma
This entity can occur following solid-organ transplantation or in patients receiving immunosuppressive therapy for other indications. The incidence of Kaposi sarcoma is increased 100-fold in transplant patients [23, 24] However, individuals with congenital immunodeficient states are not at increased risk for developing Kaposi sarcoma. Iatrogenic Kaposi sarcoma is rare, [1] but is more common in patients at risk for the classic form of the disease. [25]
The average time to development of Kaposi sarcoma following transplantation is 15-30 months. This subtype is usually aggressive and commonly presents as lymph node, mucosa, and visceral involvement. Disease regression is frequently seen with reduction or withdrawal of immunosuppressive therapy. [26] This is further evidence for the important role that immune suppression may play in the development of Kaposi sarcoma. Immune activation and suppression affect the natural history of HHV-8 in a very complex manner. [27]
One drug used for preventing transplant rejection, sirolimus (Rapamune), appears to have an anti–Kaposi sarcoma effect independent of its immunosuppressive action. [28, 29] In a study of 15 kidney transplant patients with biopsy-proven Kaposi sarcoma who were switched from cyclosporine to sirolimus, all cutaneous lesions in all patients resolved. [30]
Sirolimus may have a therapeutic effect in Kaposi sarcoma due to its antiangiogenesis effect, with reduction of vascular endothelial growth factor (VEGF) and the Flk-1/KDR receptor on tumor cells, as well as its inhibition of the mammalian target of rapamycin (mTOR) pathway by blocking of Akt. Notably, mTor can activate various mediators of proliferation and affect control points of translation in the cell cycle.
Classic (sporadic) Kaposi sarcoma
This entity typically occurs primarily in elderly men of Mediterranean and Eastern European background. It has a male predominance with a male-to-female ratio of 10-15:1. The age of onset is between 50 and 70 years.
Classic Kaposi sarcoma usually follows a protracted and indolent course. Common complications include venous stasis and lymphedema. This form of the disease rarely includes lymph node, mucous membrane, or visceral involvement. [31]
As many as 30% of patients with classic Kaposi sarcoma subsequently develop a second malignancy, typically a non-Hodgkin lymphoma. [31] These second malignancies may be due to immune suppression from age, host genetics, history of other neoplasm, and possible concurrent infections such as malaria. [32, 33, 34]
Contradictory evidence suggests that immune activation is requisite for the development of classic Kaposi sarcoma. Indeed both hypotheses may be correct, [17, 18] indicating a complicated mechanism of immune dysregulation.
Infrequent bathing; a history of asthma; and, in men, a history of allergy have been linked to classic Kaposi sarcoma in Italian patients. [35] Long-term use of topical steroids is associated with increased risk, either indicating an effect of chronic dermatitis or of the steroids themselves. As in epidemic AIDS-related Kaposi sarcoma, a protective effect of cigarette smoking has also been noted.
Endemic African Kaposi sarcoma
This entity occurs primarily in men but also in women and children who are HIV seronegative and may have an indolent or aggressive course. It was relatively common before the AIDS epidemic. Since the advent of AIDS, its incidence has increased about 20-fold in the African countries of Malawi, Swaziland, Uganda, Zambia, and Zimbabwe. [36, 1]
In central Africa, Kaposi sarcoma has become the most prevalent form of cancer in men and the second most prevalent in women. [37] It represents about 9% of all cancers seen in Ugandan males. This form of the disease involves lymph nodes more commonly than the classic variant. Factors associated with risk in HHV-8 seropositive patients are not as well characterized.
Rarely or never wearing shoes is associated with an increase of endemic Kaposi sarcoma in rural areas with volcanic clay soils, possibly related to chronic lymphatic obstruction in the feet and legs from fine soil particles. [38, 39] Relative affluence in Ugandan patients is associated with increased risk for endemic Kaposi sarcoma, a finding similar to that of Kaposi sarcoma in AIDS patients. [35]
A lymphadenopathic form of Kaposi sarcoma is also seen in Africa, chiefly in HIV-seronegative children before the age of puberty. Generalized lymphadenopathy is seen with visceral involvement and carries a dismal prognosis, with a 100% fatality rate at 3 years.
Pathophysiology
The discovery of Kaposi sarcoma human herpes virus (KSHV) in 1994 led to rapid progress in understanding the disease’s pathophysiology. Different epidemiologic and clinical presentations of the disease may be related to modifiable risk factors, such as uncontrolled HIV and immunosuppressive medications used in transplantation. This knowledge has helped spur individualized therapeutic approaches to the disease.
Kaposi sarcoma is caused by an excessive proliferation of spindle cells that are thought to have an endothelial cell origin. Despite their heterogeneity, the tumors are predominantly composed of KSHV genomic material with immunohistochemical markers of both lymphoid, spindle, and endothelial cells. [40]
Although the cell of origin is still unknown, increased endothelial factor VIIIa antigen, spindle cell markers such as smooth muscle alpha-actin, and macrophage markers such as PAM-1, CD68, and CD14 expressed by these spindlecells have been observed. [41] This suggests a pluripotent mesenchymal progenitor. The spindle cells proliferate in a background of reticular fibers, collagen, and mononuclear cells including macrophages, lymphocytes and plasma cells. They tend to be vascular, involving either the reticular dermis (patch stage) or the entire thickness of the dermis (plaque or nodular stage). [31]
KSHV contains a large genome with greater than 85 antigenically competent genes. Immunofluorescent assays in KSHV-infected primary effusion lymphoma and an enzyme-linked immunoassay (ELISA) to major antigens have been developed to measure antibodies to KSHV. [42, 43] Seropositivity varies and is more than 50% in sub-Saharan Africa, 20-30% in Mediterranean countries, and less than 10% in most of Europe, Asia, and the United States. Prevalence is higher in men who have sex with men, Amerindians in South America, and certain ethnic groups in China. [44, 45]
Molecular studies previously suggested that Kaposi sarcoma originates from a single cell clone rather than a multifocal origin. However, more recent data in a study of 98 patients with Kaposi sarcoma with primarily cutaneous disease analyzed by molecular diagnostic techniques comparing viral HHV8 DNA of the tumors showed that nearly 80% of the tumors arose independently from multiple cells. [46] The conclusion reached was that few Kaposi sarcoma tumors originate from a single cell and that Kaposi sarcoma may not be metastatic in its advanced form but multifocal and independently occurring at multiple sites. These data are primarily applicable to initially less aggressive cutaneous Kaposi sarcoma. They may not apply to de novo more aggressive visceral Kaposi sarcoma, which may be less likely to respond to therapy.
Human herpes virus 8 (HHV-8) genomic sequences have been identified by polymerase chain reaction in more than 90% of all types of Kaposi sarcoma lesions (including epidemic and endemic forms), suggesting a causative role for this DNA virus. The current working hypothesis is that HHV-8 must be present for the disease to develop. It is transmitted in saliva. Blood-borne transmission has yet to be proved. HIV significantly increases the risk of immune suppression.
These viral sequences additionally have been associated with body cavity–based lymphomas, Castleman disease, and leiomyosarcomas that occur in individuals infected with HIV. Other diseases may yet be found to be associated with KSHV, particularly in HIV-positive individuals. Factors that are thought to contribute to the development of Kaposi sarcoma in individuals infected with HHV-8 and HIV include an abnormal cytokine milieu associated with HIV infection, and involving the following angiogenic cytokines:
-
Interleukin-1 (IL-1) beta
-
Basic fibroblast growth factor (bFGF)
-
Acidic fibroblast growth factor
-
Endothelial growth factor
-
Vascular endothelial growth factor (VEGF)
Other cytokines include IL-6, granulocyte-monocyte colony stimulating factor (GM-CSF), transforming growth factor beta (TGF-beta), tumor necrosis factor (TNF), and platelet-derived growth factor alpha (PDGF-alpha) from interstitial and mononuclear cells.
Oncostatin M, IL-1, IL-6, fibroblast growth factor, tumor necrosis factor (TNF), and the HIV-tat protein—all of which originate from HIV-infected T cells—act as costimulants for Kaposi sarcoma cells. [31] Indeed, theTAT gene may be a key component responsible for conversion of the Kaposi sarcoma cell to a malignant phenotype. A specific viral gene, ORF74, encodes for a G-protein coupled receptor that causes production of VEGF and other angiogenic mediators. [47, 48]
In a comparison of plasma from 15 HIV-negative classic Kaposi sarcoma cases to plasma from 29 matched controls, Aka and collegues reported elevated plasma levels of CXCL10, sIL-1RII, sIL-2RA, and CCL3 in classic Kaposi sarcoma cases. However, the researchers caution that larger, prospective studies are needed to assess for possible diagnostic, prognostic, or etiologic importance for Kaposi sarcoma. in larger, prospective studies, including those involving HIV-infected patients with AIDS-related disease. [49]
Thus, Kaposi sarcoma may be caused by HHV-8 (KSHV) with stimulation by autocrine and paracrine growth factors secreted by the spindle cells themselves as well as the supporting network of mononuclear and endothelial cells. Coinfection with HIV may create a more aggressive course, which is mitigated by effective antiretroviral therapies. Indeed, the risk of Kaposi sarcoma development is amplified 500-10,000 times in patients coinfected with KSHV and HIV. The use of technologies such as comprehensive genetic profiling with gene expression arrays may further elucidate the very complicated viral gene-host interaction and facilitate identification of molecular targets for both prevention and treatment. [50]
In summary, complex immune dysregulation is the center theme for the pathogenesis of Kaposi sarcoma. This includes cellular immunity defects, [51, 52] humoral immunity defects [53, 54] and abnormalities of vascular endothelial growth factor. Apparent overlapping mechanisms for upregulation of multiple pathways produce the malignant phenotype.
Cofactors
The fact that Kaposi sarcoma develops in only a small percentage of HHV-8–seropositive individuals points to the importance of other factors. Some of these may be genetic: For example, individuals with certain polymorphisms in the MDM2 gene, which is involved in the function of the tumor suppressor protein p53, may be at increased risk for Kaposi sarcoma. [39]
It is possible that some agents may either stimulate or inhibit the development of Kaposi sarcoma, depending on the presence of influences such as genetic predisposition, environmental factors, drug intake, or lymphatic system disorders. The high prevalence of Kaposi sarcoma in areas where quinine and its derivatives are widely used for treatment of malaria may be partly attributable to the immunosuppressant effect of those drugs; however, quinolones also have several effects that could inhibit Kaposi sarcoma development. Likewise, angiotensin-converting enzyme (ACE) inhibitors have been described as inducing Kaposi sarcoma, in some reports, and inhibiting it, in others. [39]
Transmission
KSHV is now thought to be largely transmitted via saliva. Although associated with sexual risk factors, these may just be a surrogate for close contact. [55] Heterosexual risk factors largely do not play a role here. Transmission by blood or blood products can occur but use of leukopoor stored blood is likely to significant reduce this risk. [56] Transmission of KSHV may occur during solid organ donation, but it does not appear to affect clinical outcome in terms of survival or graft loss. In solid organ transplant recipients, the incidence of Kaposi sarcoma may be higher in those who are seropositive than in those who are seronegative. [57]
Etiology
Etiologic factors include the following:
-
Coinfection with HIV and HHV-8 (Kaposi sarcoma-associated herpesvirus [KSHV])
-
Iatrogenic immunosuppression (including corticosteroids)
-
Elevated expression of numerous cytokines and angiogenic growth factors, including tumor necrosis factor alpha, interleukin-6, basic fibroblast growth factor, HIV-tat protein, and oncostatin M
Epidemiology
Frequency
United States
Before the AIDS epidemic, Kaposi sarcoma was rare. Between 1975 and 1980, only 19 cases occurred in men aged 20-54 years, according to Surveillance, Epidemiology, and End Results (SEER) data (0.1 cases per 100,000). In 1981, an aggressive form of Kaposi sarcoma began to appear among men who have sex with men (MSM) as one of the harbingers of the AIDS epidemic. [37] At the beginning of the AIDS epidemic, just before 1980, 40-50% of MSM with AIDS developed Kaposi sarcoma. This phenomenon spurred research into the possibility of an infectious etiology. [58]
The rate in all SEER areas increased from in the late 1970s to 17.5 per 100,000 in the late 1980s and then decreased to 2.2 per 100,000 as of 1999-2000. [59] In the United States, the risk of Kaposi sarcoma in sexually active MSM is much greater than in others infected with HIV. [60] The incidence of Kaposi sarcoma reached its zenith in 1989 among white men aged 20-54 years when it was the most common AIDS-associated neoplasm. Its incidence has dramatically declined since then.
In the mid 1990s, approximately 1 in 4 MSM contracted the disease. This number has decreased precipitously with the advent of safer sexual practices in the early 1990s and accelerated with the introduction of highly active antiretroviral therapy (HAART) in the mid 1990s. [60, 61] The dramatic decrease supports the hypothesis of the need for severe immunosuppression for the presumed sexual transmission of an infective agent such as Kaposi sarcoma–associated herpesvirus/human herpes virus 8(KSHV/HHV-8). [31]
The decrease in HIV-related Kaposi sarcoma was most profound in men in the San Francisco area, from 7.9 to 1.6 cases per 100,000. [59] The incidence in other HIV risk groups initially was 10% in intravenous drug abusers, 4% in hemophiliacs, and 3% in children with AIDS. [62] It has decreased in these groups as well to a relatively steady rate of 2%, which is now the same rate for MSM. The disease may be contracted by other groups with HIV, such as women and heterosexual men, through unprotected sex. Overall, there has been a historic tendency to underreport Kaposi sarcoma in the AIDS population. [63]
The other major group in the United States in whom Kaposi sarcoma occurs is the posttransplant population, in whom the incidence is about 1 in 200. [64]
Currently, approximately 2,000 cases of Kaposi sarcoma, or about 6 cases per million population, occur yearly in the United States. [64] Historically, the incidence in African-American men peaked somewhat later than in white men, in 1991-1999. It was first noted in Hispanic men in 1992 when its incidence was transiently higher than in African Americans or whites. The dramatic drop in incidence has been seen in all major ethnic groups from 1992-2001, with stabilization since then. Currently, the highest rate is seen in African-American men, with a rate of 3 per 100,000, as opposed to rates less than 3 per 100,000 in decreasing incidence for Hispanics, whites, and Asians/Pacific Islanders, respectively.
As noted above, the incidence and severity of Kaposi sarcoma has lessened following the introduction of HAART. This reduction has been attributed to restoration of the immune system caused by these drugs. The regression occurs in parallel with increases in CD4 counts, usually within no more than 9-12 months. Conversely, progression occurs with increasing viral load, low CD4 counts, and opportunistic infection . [65]
Maurer et al, however, reported a cluster of cutaneous, refractory HIV-associated Kaposi sarcoma in patients in the San Francisco area with CD4 counts above 300 cells/μL and suppressed viral loads below 300 copies for at least 2 years. [66] All patients presented between November, 2004 and November, 2006 and were being treated with a protease inhibitor (PI) or non–nucleoside reverse transcriptase inhibitors (NNRTIs). None had a history of opportunistic infection. All the courses were indolent. These authors proposed that these cases are the result of aging in persons infected with both HIV and HHV8.
In a cohort study of 86,620 HIV-infected and 196,987 uninfected adults from 1996–2009, the cumulative incidence by age 75 for Kaposi sarcoma was 4.4% but dropped to 4.1% in the most recent period analyzed (2005–2009). [67]
The specific HAART regimen may be important, as the drugs may also act as antitumor or antiangiogenic agents; for example, PIs may inhibit Kaposi sarcoma. [68] However, a systematic review found that HAART significantly reduced the risk of incident Kaposi sarcoma regardless of the antiretroviral drug class used, even after adjusting for CD4 count. The CD4-adjusted incidence of Kaposi sarcoma decreased by approximately 50%, with either NNRTI-based- or PI-based HAART. [69]
International
Prior to the advent of HIV, Kaposi sarcoma was common in central Africa and prevalent in Mediterranean countries and the Middle East. It rarely occurred elsewhere. In Africa, the annual incidence of Kaposi sarcoma is very high at 37.7 per 100,000 in men and 20.5 per 100,000 in women. In Europe, the highest rates of classic Kaposi sarcoma are in Sicily (Ragusa, 30.1 cases per million in men/5.4 cases per million in women) and Sardinia (24.3 cases per million in men/7.7 cases per million in women).
A meta-analysis of the worldwide epidemiology of Kaposi sarcoma found the following incidence rates (per 100,000 person-years) [70] :
-
General population: 1.53 (95% confidence interval [CI] 0.33-7.08)
-
HIV-infected persons: 481.54 (95% CI 342.36-677.32)
-
HIV-infected MSM:1397.11 (95% CI 870.55-2242.18)
-
HIV-infected children: 52.94 (95% CI 39.90-70.20)
-
Transplant recipients: 68.59 (95% CI 31.39-149.86)
Race
In Africa and developing regions, epidemic AIDS-related Kaposi sarcoma is common in heterosexual adults and occurs less often in children. Classic Kaposi sarcoma typically occurs in elderly men of Mediterranean and Eastern European background. Endemic African Kaposi sarcoma occurs in HIV-seronegative men, women, and children in Africa.
The racial differences may be explained in part by the higher rates of seroprevalence of Kaposi’s sarcoma-associated herpesvirus (KSHV) in Central and Eastern Africa (>50%) and in Eastern Europe and the Mediterranean region (10%-30%) compared to North America, Western Europe and East Asia (< 10%). [71]
Sex
In the United States, AIDS-related Kaposi sarcoma occurs primarily in homosexual and bisexual men, and in the female sexual partners of bisexual men.
African Kaposi sarcoma occurs in heterosexual men and women with equal frequency.
Classic Kaposi sarcoma occurs primarily in men, with a male-to-female ratio of 10-15:1.
Age
AIDS-related Kaposi sarcoma generally occurs in young to middle-aged adults age 20-54 years. Classic Kaposi sarcoma typically occurs in patients age 50-70 years. African Kaposi sarcoma occurs in people of a younger age (35-40 y).
Prognosis
AIDS-related Kaposi sarcoma, unlike other forms of Kaposi sarcoma, tends to have an aggressive clinical course. Morbidity may occur from extensive cutaneous, mucosal, or visceral involvement. In patients receiving HAART, the disease often has a more indolent clinical course or may regress spontaneously. The most common causes of morbidity include cosmetically disfiguring cutaneous lesions, lymphedema, gastrointestinal involvement, or pulmonary involvement (see Presentation). Pulmonary involvement is the most common cause of mortality with uncontrolled pulmonary hemorrhage.
-
Endoscopic view of a Kaposi sarcoma lesion situated in the gastric antrum.
-
Epidemic Kaposi sarcoma (KS). Large violaceous truncal nodules with typical linear and symmetric distribution pattern.
-
Kaposi sarcoma (KS), facial. Confluent, violaceous nodules involving the chest wall; extensive facial involvement with accompanied edema.







