Background
Numerous trematodes cause disease in humans. Flukes that cause schistosomiasis, paragonimiasis, fascioliasis, clonorchiasis, and opisthorchiasis are included in the World Health Organization (WHO) list of neglected tropical diseases (NTD) to which interventions for poor and marginalized populations are prioritized given the significant health burden. [1, 2, 3] Although this article focuses on intestinal trematodes, a limited discussion on liver flukes (Clonorchis sinensis, Opisthorchis viverrini, Fasciola hepatica, Fasciola gigantica) is provided given the similarity in the mode of acquisition (foodborne) and the challenge in terms of diagnostic differentiation with the intestinal flukes.
Intestinal trematodes can be classified into at least 14 families (with their corresponding sources of infection found below), as follows [4] :
-
Brachylaimidae (from terrestrial snails)
-
Cathaemasiidae (from a yet unknown source)
-
Diplostomidae (from snakes, frogs, and tadpoles)
-
Echinostomatidae (from freshwater fish, frogs, mussels, snails, and tadpoles)
-
Fasciolidae (from aquatic vegetables and contaminated water)
-
Gastrodiscidae (from aquatic vegetables, crustaceans, molluscs, and amphibians)
-
Gymnophallidae (from oysters)
-
Heterophyidae (freshwater fishes)
-
Lecithodendriidae (from dragonflies)
-
Microphallidae (from shrimps and crabs)
-
Nanophyetidae (from salmonid fishes)
-
Paramphistomidae (from aquatic plants)
-
Plagiorchiidae (from insect larvae)
-
Strigeidae (from a yet unknown source)
Intestinal trematodes are flat hermaphroditic worms that vary in length from a few millimeters to many centimeters (see the image below). Of the approximately 70 species known to colonize the human intestine, only a few species are known to cause actual infection. Globally, it is likely that more than the estimated 40-50 million people are infected with intestinal trematodes, primarily infected via the foodborne route. Populations in Southeast Asia appear to be most vulnerable. An exhaustive 2009 review of these infections in this region provides detailed information on the large number of species infecting humans, their pathogenicity, diagnostic issues, and treatments. [5]
In 2012, the various manifestations, methods of diagnosis, and management of foodborne trematodiasis, which include the intestinal flukes, were detailed in the British Medical Journal [6] and the European Journal of Microbiology and Infectious Diseases. [7] In the former, the species of intestinal flukes considered to be of public importance include the following:
-
Echinostoma species
-
Fasciolopsis buski
-
Gymnophalloides seoi
-
Haplorchis species
-
Heterophyes species
-
Metagonimus species
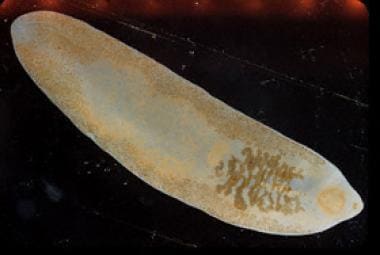 Adult fluke of Fasciolopsis buski. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Adult fluke of Fasciolopsis buski. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
The most common human intestinal trematode was said to be Fasciolopsis buski, which causes fasciolopsiasis, [8] and should be differentiated from Fasciola hepatica and Fasciola gigantica, which are liver flukes that cause fascioliasis. Conversely, the genus Echinostoma is the largest, with about 500 species of echinostomatid flukes. About 20 species belonging to 10 genera have been reported to cause human disease. [9] The genus Echinostoma is considered the largest, which includes Echinostomahortense, Echinostomaangustitestis, Echinostomacinetorchis, Echinostomaechinatum, Echinostoma ilocanum, Echinostomamacrorchis, and Echinostomarevolutum. E ilocanum was said to be the most common cause of infection in humans. [1, 10]
Heterophyes heterophyes, Metagonimus yokogawai, and Gymnophalloides species are less-common causes of human intestinal fluke infection. [1]
Other intestinal flukes that rarely cause human intestinal infection include Gastrodiscoides hominis, Phaneropsolus bonnei, and Prosthodendrium molenkampi. Intestinal flukes have likely infected humans for hundreds of years, if not longer. Evidence of G seoi infection has been traced back to the 17th century based on discovery of G seoi eggs in a Korean mummy. [1, 11]
See Common Intestinal Parasites, a Critical Images slideshow, to help make an accurate diagnosis.
Pathophysiology
Intestinal flukes cause inflammation, ulceration, and mucous secretion at the site of attachment. Severe infections may also cause intestinal obstruction or malabsorption, leading to hypoalbuminemia, protein-losing enteropathy, and impaired vitamin B-12 absorption. Foodborne illness has been associated with the ingestion of many different types of potentially infected foods, such as different types of both freshwater and brackish-water fish and snails, reptiles (amphibians and certain snakes), aquatic plants, and insects. [5]
Fasciolopsis buski
Fasciolopsis buski, which causes fasciolopsiasis, attaches to the duodenal and jejunal mucosa; however, in severe infections, it may attach to the ileum or colon.
In London, Busk first described Fasciolopsis buski in 1843 after finding it in the duodenum of a sailor. In 1925, Barlow first determined its life cycle in humans. A well-known illustrative life cycle schematic is shown below.
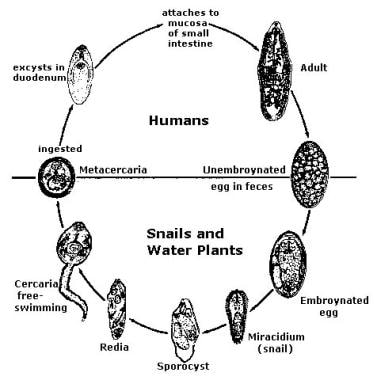 Life cycle of Fasciolopsis buski. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Life cycle of Fasciolopsis buski. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
The immature eggs (see the images below) are discharged from human feces and reach fresh water, hatching after 3-7 weeks and forming miracidia.
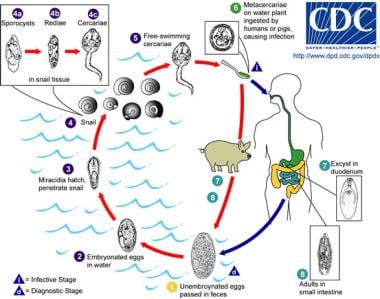 The life cycle of Fasciolopsis. Immature eggs are discharged into the intestine and stool and become embryonated in water. The eggs then release miracidia, which invade a suitable snail intermediate host, in which the parasites undergo several developmental stages (sporocysts, rediae, cercariae). The cercariae are released from the snail and encyst as metacercariae on aquatic plants, which are eaten by mammalian hosts (humans and pigs), who become infected. After ingestion, the metacercariae excyst in the duodenum and attach to the intestinal wall, where they develop into adult flukes (20-75 mm X 8-20 mm) in approximately 3 months and attach to the intestinal wall of the mammalian hosts. The adults have a life span of about one year. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
The life cycle of Fasciolopsis. Immature eggs are discharged into the intestine and stool and become embryonated in water. The eggs then release miracidia, which invade a suitable snail intermediate host, in which the parasites undergo several developmental stages (sporocysts, rediae, cercariae). The cercariae are released from the snail and encyst as metacercariae on aquatic plants, which are eaten by mammalian hosts (humans and pigs), who become infected. After ingestion, the metacercariae excyst in the duodenum and attach to the intestinal wall, where they develop into adult flukes (20-75 mm X 8-20 mm) in approximately 3 months and attach to the intestinal wall of the mammalian hosts. The adults have a life span of about one year. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
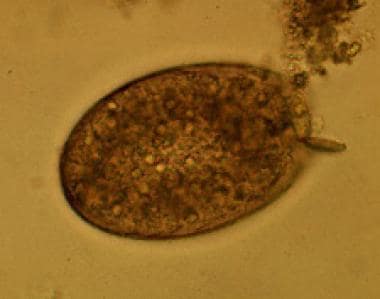 Egg of Fasciolopsis buski. Images reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Egg of Fasciolopsis buski. Images reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Upon contact with host snails, the miracidia penetrate the soft tissues and form sporocysts, first- and second-generation rediae, and, lastly, cercariae. The cercariae encyst on various plants such as water caltrop, water chestnut, lotus (on the roots), water bamboo, and other aquatic vegetables. Humans are infected by consuming these raw vegetables.
In the human duodenum, the metacercariae attach to the walls and become adult worms in approximately 3 months. The adult worm (see image below) causes traumatic, toxic, and obstructive damage to the intestinal mucosa. Deep inflammatory ulcerations develop at the site of attachment. Large numbers of worms provoke excess mucous discharge and can obstruct the lumen. The adult worm metabolites can also cause intoxication and sensitization when absorbed via the lumen. A recent case report provides evidence of heavy infestation as a risk factor for intestinal perforation. [12]
 Adult fluke of Fasciolopsis buski. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Adult fluke of Fasciolopsis buski. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Echinostoma species
In 1907, in Manila, Garrison first noted the genus Echinostoma, which is reported to have 12 species that may cause disease in humans. The most common species is E ilocanum, which has a characteristic horseshoe-shaped collar of 1-2 rows of straight spines that surround the dorsal and lateral sides of the oral sucker. E ilocanum flukes are small and elongated, measuring 5-15 mm in length and 1-2 mm in width. The most well-studied human-infecting species is E hortense. [13]
The adult worm, attached to the intestinal wall of humans, produces eggs that are passed in the feces. The eggs reach water, and miracidia develop and penetrate the first intermediate hosts—snails. During the course of 6-7 weeks inside the host snails, they develop into sporocysts, mother rediae, daughter rediae, and cercariae.
The cercariae leave the snails to encyst in the second intermediate hosts, which can be freshwater snails, fish, tadpoles, or vegetables. Humans are infected by ingesting raw or undercooked second intermediate hosts. Although not directly applicable to the human host, data in rodents suggest that, depending on trematode species, host species, and strain, primary expulsion of the fluke may occur. [14, 15] Inside human hosts, the flukes then attach to the small intestinal mucosa and, depending on the severity of infection, can produce shallow ulcers with mild inflammation and/or local necrosis. Mild infections do not cause symptoms, but heavy infections produce diarrhea, flatulence, and intestinal colic similar to fasciolopsiasis.
Disease manifestations in humans likely result from two pathogenetic mechanisms, including fluke-induced mechanical irritation and/or the related allergic reaction to various toxic metabolites. [13] Humoral responses to echinostomal infections may include elevated serum and gut-associated immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM) levels, as demonstrated by studies involving Echinostoma caproni. [16]
The intestinal epithelium appears to provide an important mechanism in mucosal defense. Its rapid epithelial cell turnover seems to facilitate the rejection of intestinal helminths. In rats, it has been shown that E caproni infection stimulates the augmented renewal of the intestinal epithelium, preventing the parasite from establishing itself in the host, resulting in clearance of the helminth. [17]
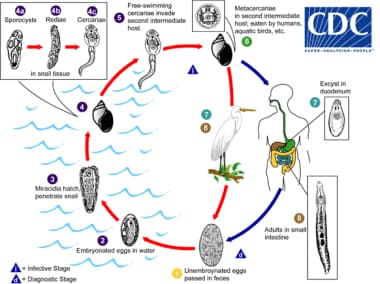 Various animals may be definitive hosts for different Echinostoma species, such as aquatic birds, carnivores, rodents, and humans. Unembryonated eggs are passed in stool (1), and development occurs in the water (2). The miracidium takes an average of 10 days to mature and then hatches (3), penetrating the first intermediate host, a snail (4). Snails, in general, serve as the first intermediate host. The intramolluscan stages are as follows: sporocyst (4a); rediae (4b); and cercariae (4c). Cercariae may then encyst as metacercariae in the same first intermediate host or leave to penetrate a new second intermediate host (5). Several animals may become the second intermediate host, such as other snails, bivalves, fish, and tadpoles. The definitive host gets infected after eating infected second intermediate hosts (6). The metacercariae excyst in the duodenum (7). Adults then live in the small intestine (8). Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Various animals may be definitive hosts for different Echinostoma species, such as aquatic birds, carnivores, rodents, and humans. Unembryonated eggs are passed in stool (1), and development occurs in the water (2). The miracidium takes an average of 10 days to mature and then hatches (3), penetrating the first intermediate host, a snail (4). Snails, in general, serve as the first intermediate host. The intramolluscan stages are as follows: sporocyst (4a); rediae (4b); and cercariae (4c). Cercariae may then encyst as metacercariae in the same first intermediate host or leave to penetrate a new second intermediate host (5). Several animals may become the second intermediate host, such as other snails, bivalves, fish, and tadpoles. The definitive host gets infected after eating infected second intermediate hosts (6). The metacercariae excyst in the duodenum (7). Adults then live in the small intestine (8). Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Heterophyes heterophyes
H heterophyes is the most common of the 10 species that compose the genus Heterophyes. H heterophyes is a small fluke, measuring 1-1.8 mm in length and 0.3-0.7 mm in width, with a broadly rounded posterior end. The oral sucker is subterminal and is one third the size of the ventral sucker.
H heterophyes are observed in the human intestine, jejunum, and ileum. The illustrative life cycle schematic for H heterophyes is shown below. These worms produce eggs, which are excreted in the feces and into the water. The first intermediate hosts, the snails, ingest the eggs. In the snails, the eggs hatch and undergo their developmental cycle, forming cercariae, which emerge from the snails and encyst on the second intermediate hosts—brackish or freshwater fish. In the second intermediate hosts, the cercariae are transformed into metacercariae, which infect humans upon ingestion of raw or undercooked fish. See the image below.
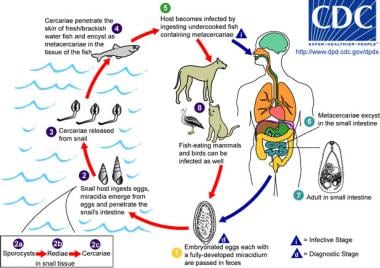 The life cycle of Heterophyes. The adult parasites release embryonated eggs (each with a fully developed miracidium), which are then passed in the host's feces. After ingestion by a suitable snail (first intermediate host), the eggs hatch and release miracidia, which penetrate the snail's intestine. Snails of the genera Cerithidea and Pirenella are important hosts in Asia and the Middle East, respectively. The miracidia undergo several developmental stages in the snail (sporocysts, rediae, cercariae). Many cercariae are produced from each redia. The cercariae are released from the snail and encyst as metacercariae in the tissues of a suitable freshwater or brackish-water fish (second intermediate host). The definitive host becomes infected by ingesting undercooked or salted fish that contains metacercariae. After ingestion, the metacercariae excyst, attach to the mucosa of the small intestine, and mature into adults (measuring 1-1.7 mm X 0.3-0.4 mm). Heterophyes heterophyes infects humans, various fish-eating mammals (eg, cats, dogs), and birds. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
The life cycle of Heterophyes. The adult parasites release embryonated eggs (each with a fully developed miracidium), which are then passed in the host's feces. After ingestion by a suitable snail (first intermediate host), the eggs hatch and release miracidia, which penetrate the snail's intestine. Snails of the genera Cerithidea and Pirenella are important hosts in Asia and the Middle East, respectively. The miracidia undergo several developmental stages in the snail (sporocysts, rediae, cercariae). Many cercariae are produced from each redia. The cercariae are released from the snail and encyst as metacercariae in the tissues of a suitable freshwater or brackish-water fish (second intermediate host). The definitive host becomes infected by ingesting undercooked or salted fish that contains metacercariae. After ingestion, the metacercariae excyst, attach to the mucosa of the small intestine, and mature into adults (measuring 1-1.7 mm X 0.3-0.4 mm). Heterophyes heterophyes infects humans, various fish-eating mammals (eg, cats, dogs), and birds. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
In humans, the flukes attach to the small bowel and cause shallow ulcers, mild inflammation, and/or superficial necrosis. Clinical presentation includes diarrhea, dyspepsia, and intestinal colic. Because of their small size, the eggs, and sometimes the adult flukes, enter blood vessels and embolize to the brain, producing symptoms similar to cerebral hemorrhage. Eggs may also enter the mesenteric lymphatics and travel to the heart, causing myocarditis, chronic congestive heart failure, and death.
Metagonimus yokogawai
M yokogawai, which is closely related to H heterophyes, is another important parasite. M yokogawai measures 1-2.5 mm in length and 0.4-0.75 mm in width. The ventral sucker is located to the right of the midline.
M yokogawai has a life cycle similar to that of H heterophyes, in which the adult worm in the human intestine produces eggs that are excreted in the feces. The illustrative life cycle schematic for M yokogawai is shown below. The eggs enter the water and infect the first intermediate hosts, the snails, where the eggs undergo their developmental cycle and become cercariae. Cercariae infect the second intermediate hosts, freshwater fish, and become metacercariae. Metacercariae infect humans after ingestion of raw or undercooked fish. The flukes then invade the mucosa of the small intestines, causing inflammation and ulcerations. Flukes eventually become encapsulated.
See the image below.
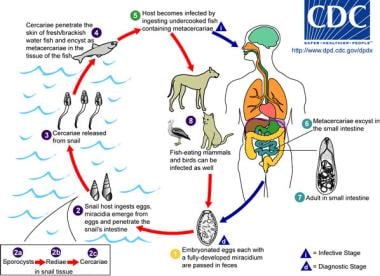 Life cycle of Metagonimus. The adult parasites release fully embryonated eggs (each with a fully developed miracidium), which are then passed in the host's feces. After ingestion by a suitable snail (first intermediate host), the eggs hatch and release miracidia, which penetrate the snail's intestine. Snails of the genus Semisulcospira are the most common intermediate host for Metagonimus yokogawai. The miracidia undergo several developmental stages in the snail (sporocysts, rediae, cercariae). Many cercariae are produced from each redia. The cercariae are released from the snail and encyst as metacercariae in the tissues of a suitable freshwater or brackish-water fish (second intermediate host). The definitive host becomes infected by ingesting undercooked or salted fish that contains metacercariae. After ingestion, the metacercariae excyst, attach to the mucosa of the small intestine, and mature into adults (measuring 1-2.5 mm X 0.4-0.75 mm). M yokogawai infects humans, fish-eating mammals (eg, cats, dogs), and birds. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Life cycle of Metagonimus. The adult parasites release fully embryonated eggs (each with a fully developed miracidium), which are then passed in the host's feces. After ingestion by a suitable snail (first intermediate host), the eggs hatch and release miracidia, which penetrate the snail's intestine. Snails of the genus Semisulcospira are the most common intermediate host for Metagonimus yokogawai. The miracidia undergo several developmental stages in the snail (sporocysts, rediae, cercariae). Many cercariae are produced from each redia. The cercariae are released from the snail and encyst as metacercariae in the tissues of a suitable freshwater or brackish-water fish (second intermediate host). The definitive host becomes infected by ingesting undercooked or salted fish that contains metacercariae. After ingestion, the metacercariae excyst, attach to the mucosa of the small intestine, and mature into adults (measuring 1-2.5 mm X 0.4-0.75 mm). M yokogawai infects humans, fish-eating mammals (eg, cats, dogs), and birds. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
As in infection with H heterophyes, M yokogawai occasionally embolize to other organs. Patients infected with M yokogawai present with mucous, diarrhea, and vague abdominal symptoms. Prognosis is usually good, except in cases of embolization. Fortunately, health studies performed in 2006 indicate that M yokogawai species and infections have become less endemic in certain regions. [18]
Epidemiology
Frequency
United States
Infection with intestinal flukes affects only immigrants from endemic areas.
International
Intestinal flukes are endemic in the Far East and Southeast Asia. Table 1 shows the geographic distribution and source of most commonly reported intestinal trematodes. The list of countries where human trematode infections have been reported is long, with several studies indicating variable incidence rates. [19, 20, 21, 5, 22, 23, 24, 10, 25, 26, 27]
Table 1. Common Intestinal Trematode Infections (Open Table in a new window)
Infection |
Source* |
Geographic Distribution** |
|---|---|---|
Fasciolopsiasis |
Freshwater plants (water caltrop, water chestnut) |
Asia and Indian subcontinent, especially in areas where humans raise pigs and consume freshwater plants (as of December 8, 2017) |
Echinostomiasis |
Tadpoles, freshwater snails, fish, frogs |
Worldwide, but human cases seen most frequently in Southeast Asia and in areas where undercooked or raw freshwater snails, clams, and fish are eaten (as of December 27, 2017) |
Heterophyiasis |
Fish |
Egypt, Middle East, Far East (as of January 3, 2018) |
Metagonimiasis |
Fish (cyprinid) |
Far East, Siberia, Manchuria, the Balkan states, Israel, Spain (as of December 29, 2017) |
* Adapted with permission from Tribble D, Wagner KF. Trematode infections. Infectious Disease Practice. 1996; 20:69-73. ** Updated data from the Centers for Disease Control and Prevention (DPDx Laboratory Identification of Parasites of Public Health Concern. Available: http://www.cdc.gov/dpdx/) |
||
Multiple families of trematodes cause intestinal infections, and most of them are found in Asia. The Brachylaimidae family can be found in Oceania. The families found in Africa include Echinostomatidae, Gastrodiscidae, Heterophyidae, and Paramphistomidae. Those that have been reported in Europe include Echinostomatidae, Gastrodiscidae, Heterophyidae, and Nanophyetidae. A minority are present in the Americas, particularly Heterophyidae and Paramphistomidae. [4]
The largest human outbreaks of Fasciola species infection have occurred in the Gilan Province of Iran, affecting more than 15,000 people. [28]
Bihar, East India, was recently found to be endemic for fasciolopsiasis, especially since this area is generally poor, with both villagers and local medical practitioners lacking awareness about the parasite. [29]
China, Japan, and South Korea are considered high prevalence areas for human E hortense infections. This is especially true in Koje-myon, Kochang-gun, Kyongsangnam-do Province of South Korea, where prevalence has been reported to be up to 9.5%. [30]
In Xieng Khouang Province, Lao PDR (a country close to Myanmar), Cambodia, China, Thailand, and Vietnam, the rate of positivity for small trematode eggs was 4.4%, including O viverrini, heterophyids, and lecithodendriids. [31]
In 2017, Tanzania reported their first case of E ilocanum infection after ingestion of raw fish (Tanganyika perch and Emperor Cichlid ”yellow belly”) caught from Lake Tanganyika by a group of travelers. [32]
Parasitism with heterophyids are mostly due to Heterophyes and Metagonimus. An extensive review by Hung et al showed a worldwide distribution of heterophyid species, with most human infections reported in Egypt and East Asia. [33]
In Boseong River, Gokseong-gun, South Korea, it has been shown that the eggs of M yokogawai and other Metagonimus species could be found in 24.3% of residents. [34]
In Tabiz, Iran, vegetable contamination (tarragon, mint) with heterophyid eggs has been reported, providing other potential sources of infection with these parasites. [35]
G seoi is endemic to Shinan-gun, Jeollanam-do, Korea, based on surveillance of oysters in 1996. [36] A 2017 report showed that 100% of oysters sampled from Shinan-gun were infected with G seoi metacercaria. [37]
Mortality/Morbidity
Death from infection is rare and usually occurs only in persons with a heavy worm burden who present with severe cachexia and prostration. Other intercurrent infection may also cause death. In cases of infection with H heterophyes or M yokogawai, death may occur after embolization of the eggs to the heart or brain. Embolization to the brain and spinal cord can also cause focal neurologic disease.
Race
Intestinal flukes are endemic in Asia and in some parts of North Africa, affecting groups who live in these areas.
Sex
Intestinal flukes have no predilection for either sex.
Age
Intestinal flukes can affect both children and adults, but children are affected more severely. These intestinal infections are important public health problems, particularly among school children in developing countries. [38]
Prognosis
Light infections may resolve spontaneously within one year, even without treatment. The prognosis may be grave in patients with heavy infection.
Immunocompromised hosts may be at an increased risk of complications. For example, G seoi worms were found to penetrate into colonic lymphoid tissue in a patient with colon cancer. [39]
Patient Education
Health education is essential. Instruct patients on proper food preparation to avoid infection with intestinal flukes. Proper washing and thorough cooking of vegetables or fish are essential to prevent egg ingestion. Thoroughly cooking fish and mollusks is always recommended.
Educational interventions that emphasize avoidance of fecal contamination of water where fish and aquatic plants thrive are recommended to prevent intestinal trematode infections.
-
Life cycle of Fasciolopsis buski. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
-
The life cycle of Fasciolopsis. Immature eggs are discharged into the intestine and stool and become embryonated in water. The eggs then release miracidia, which invade a suitable snail intermediate host, in which the parasites undergo several developmental stages (sporocysts, rediae, cercariae). The cercariae are released from the snail and encyst as metacercariae on aquatic plants, which are eaten by mammalian hosts (humans and pigs), who become infected. After ingestion, the metacercariae excyst in the duodenum and attach to the intestinal wall, where they develop into adult flukes (20-75 mm X 8-20 mm) in approximately 3 months and attach to the intestinal wall of the mammalian hosts. The adults have a life span of about one year. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
-
Egg of Fasciolopsis buski. Images reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
-
Adult fluke of Fasciolopsis buski. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
-
The life cycle of Heterophyes. The adult parasites release embryonated eggs (each with a fully developed miracidium), which are then passed in the host's feces. After ingestion by a suitable snail (first intermediate host), the eggs hatch and release miracidia, which penetrate the snail's intestine. Snails of the genera Cerithidea and Pirenella are important hosts in Asia and the Middle East, respectively. The miracidia undergo several developmental stages in the snail (sporocysts, rediae, cercariae). Many cercariae are produced from each redia. The cercariae are released from the snail and encyst as metacercariae in the tissues of a suitable freshwater or brackish-water fish (second intermediate host). The definitive host becomes infected by ingesting undercooked or salted fish that contains metacercariae. After ingestion, the metacercariae excyst, attach to the mucosa of the small intestine, and mature into adults (measuring 1-1.7 mm X 0.3-0.4 mm). Heterophyes heterophyes infects humans, various fish-eating mammals (eg, cats, dogs), and birds. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
-
Life cycle of Metagonimus. The adult parasites release fully embryonated eggs (each with a fully developed miracidium), which are then passed in the host's feces. After ingestion by a suitable snail (first intermediate host), the eggs hatch and release miracidia, which penetrate the snail's intestine. Snails of the genus Semisulcospira are the most common intermediate host for Metagonimus yokogawai. The miracidia undergo several developmental stages in the snail (sporocysts, rediae, cercariae). Many cercariae are produced from each redia. The cercariae are released from the snail and encyst as metacercariae in the tissues of a suitable freshwater or brackish-water fish (second intermediate host). The definitive host becomes infected by ingesting undercooked or salted fish that contains metacercariae. After ingestion, the metacercariae excyst, attach to the mucosa of the small intestine, and mature into adults (measuring 1-2.5 mm X 0.4-0.75 mm). M yokogawai infects humans, fish-eating mammals (eg, cats, dogs), and birds. Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
-
Various animals may be definitive hosts for different Echinostoma species, such as aquatic birds, carnivores, rodents, and humans. Unembryonated eggs are passed in stool (1), and development occurs in the water (2). The miracidium takes an average of 10 days to mature and then hatches (3), penetrating the first intermediate host, a snail (4). Snails, in general, serve as the first intermediate host. The intramolluscan stages are as follows: sporocyst (4a); rediae (4b); and cercariae (4c). Cercariae may then encyst as metacercariae in the same first intermediate host or leave to penetrate a new second intermediate host (5). Several animals may become the second intermediate host, such as other snails, bivalves, fish, and tadpoles. The definitive host gets infected after eating infected second intermediate hosts (6). The metacercariae excyst in the duodenum (7). Adults then live in the small intestine (8). Image reproduced from the Division of Parasitic Diseases, Centers for Disease Control and Prevention (CDC), Atlanta, GA.
-
Magnified image of Echinostoma spp. egg. Echinostoma eggs resemble F busk and Fasciola eggs, with the latter two only bigger in size. Specimen slides courtesy of the University of the Philippines - College of Public Health, Department of Parasitology.
-
Low power magnification (arrow) of a Heterophyid egg. Specimen slides courtesy of the University of the Philippines - College of Public Health, Department of Parasitology.
-
Magnified view of a Heterophyid egg. Specimen slides courtesy of the University of the Philippines - College of Public Health, Department of Parasitology.







