Practice Essentials
Infertility in men can result from deficiencies in sperm formation, concentration, or transportation. This general division allows an appropriate workup of potential underlying causes of infertility and helps define a course of action for treatment.
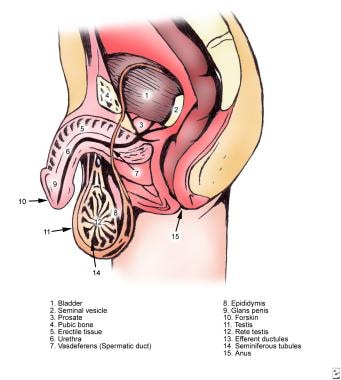
The image below depicts male ductal anatomy.
Signs and symptoms
The initial step in the evaluation of an infertile male is to obtain a thorough medical and urologic history. Such a history should include consideration of the following:
-
Duration of infertility
-
Previous fertility in the patient and the partner
-
Timing of puberty (early, normal, or delayed)
-
Childhood urologic disorders or surgical procedures
-
Current or recent acute or chronic medical illnesses
-
Sexual history
-
Testicular cancer and its treatment
-
Social history (eg, smoking and alcohol use)
-
Medications
-
Family history
-
Respiratory disease
-
Environmental or occupational exposure
-
Spinal cord injury
The physical examination should include a thorough inspection of the following:
-
Testicles (for bilateral presence, size, consistency, symmetry)
-
Epididymis (for presence bilaterally, as well as any induration, cystic changes, enlargement, tenderness)
-
Vas deferens (for presence bilaterally, defects, segmental dysplasia, induration, nodularity, swelling)
-
Spermatic cord (for varicocele)
-
Penis (for anatomic abnormalities, strictures, or plaques)
-
Rectum (for abnormalities of the prostate or seminal vesicles)
-
Body habitus
Depending on the findings from the history, detailed examination of other body functions may also be warranted.
See Presentation for more detail.
Diagnosis
The semen analysis is the cornerstone of the male infertility workup and includes assessment of the following:
-
Semen volume (normal, 1.5-5 mL)
-
Semen quality
-
Sperm density (normal, > 15 million sperm/mL)
-
Total sperm motility (normal, > 40% of sperm having normal movement)
-
Sperm morphology (sample lower limit for percentage of normal sperm is 4%)
-
Signs of infection – An increased number of white blood cells (WBCs) in the semen may be observed in patients with infectious or inflammatory processes
-
Other variables (eg, levels of zinc, citric acid, acid phosphatase, or alpha-glucosidase)
Other laboratory tests that may be helpful include the following:
-
Antisperm antibody test
-
Hormonal analysis (FSH, LH, TSH, testosterone, prolactin)
-
Genetic testing (karyotype, CFTR, AZF deletions if severe oligospermia (< 5 million sperm/mL)
Imaging studies employed in this setting may include the following:
-
Transrectal ultrasonography
-
Scrotal ultrasonography
-
Vasography
An abnormal postcoital test result is observed in 10% of infertile couples. Indications for performing a postcoital test include semen hyperviscosity, increased or decreased semen volume with good sperm density, or unexplained infertility.
If the test result is normal, consider sperm function tests, such as the following:
-
Capacitation assay
-
Acrosome reaction assay
-
Sperm penetration assay
-
Hypoosmotic swelling test
-
Inhibin B level
-
Vitality stains
Testicular biopsy is indicated in azoospermic men with a normal-sized testis and normal findings on hormonal studies to evaluate for ductal obstruction, to further evaluate idiopathic infertility, and to retrieve sperm.
See Workup for more detail.
Management
The following causes of infertility, if identified, can often be treated by medical means:
-
Endocrinopathies
-
Antisperm antibodies
-
Retrograde ejaculation
-
Poor semen quality or number
-
Lifestyle issues
-
Infections
Surgical interventions to be considered include the following:
-
Varicocelectomy
-
Vasovasostomy or vasoepididymostomy
-
Transurethral resection of the ejaculatory ducts
-
Sperm retrieval techniques
-
Electroejaculation
-
Artificial insemination
-
Assisted reproduction techniques
-
In vitro fertilization
-
Gamete intrafallopian transfer (GIFT) and zygote intrafallopian transfer (ZIFT)
-
Intracytoplasmic sperm injection
See Treatment and Medication for more detail.
Background
Infertility is defined as the inability to achieve pregnancy after one year of unprotected intercourse. An estimated 15% of couples meet this criterion and are considered infertile, with approximately 35% due to female factors alone, 30% due to male factors alone, 20% due to a combination of female and male factors, and 15% unexplained. Conditions of the male that affect fertility are still generally underdiagnosed and undertreated.
Infertile men may have deficiencies in sperm formation, concentration (eg, oligospermia [too few sperm], azoospermia [no sperm in the ejaculate]), or transportation. The causes can be categorized as obstructive or nonobstructive. This general division allows an appropriate workup of potential underlying causes of infertility and helps define a course of action for treatment.
The initial evaluation of the male patient should be rapid, noninvasive, and cost-effective, as nearly 70% of conditions that cause infertility in men can be diagnosed with history, physical examination, and hormonal and semen analysis alone. More detailed, expensive, and invasive studies can then be ordered if necessary.
Treatment options are based on the underlying etiology and range from optimizing semen production and transportation with medical therapy or surgical procedures to complex assisted reproduction techniques. Technological advancements have made conceiving a child possible with as little as one viable sperm and one egg. [1] Although the workup was traditionally delayed until a couple was unable to conceive for 12 months, evaluation may be initiated at the first visit in slightly older couples.
Pathophysiology
Gonadal and sexual functions are mediated by the hypothalamic-pituitary-gonadal axis, a closed-loop system with feedback control from the testicles. The hypothalamus, the primary integration center, responds to various signals from the central nervous system (CNS), pituitary gland, and testicles to secrete gonadotropin-releasing hormone (GnRH) in a pulsatile pattern approximately every 70-90 minutes. The half-life of GnRH is 2-5 minutes.
Release of GnRH is stimulated by melatonin from the pineal gland and inhibited by testosterone, inhibin, corticotropin-releasing hormone, opiates, illness, and stress. GnRH travels down the portal system to the anterior pituitary, located on a stalk in the sella turcica, to stimulate the release of the gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone (FSH). See the image below.
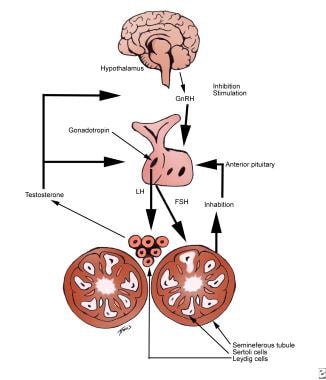 Male infertility. Hypothalamic-pituitary-gonadal axis stimulatory and inhibitory signals. Gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary. FSH stimulates the Sertoli cells to facilitate sperm production, while LH stimulates testosterone release from the Leydig cells. Feedback inhibition is from testosterone and inhibin.
Male infertility. Hypothalamic-pituitary-gonadal axis stimulatory and inhibitory signals. Gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary. FSH stimulates the Sertoli cells to facilitate sperm production, while LH stimulates testosterone release from the Leydig cells. Feedback inhibition is from testosterone and inhibin.
FSH and LH, glycopeptides with a molecular weight of 10,000 Daltons, are each composed of an alpha chain that is identical to that of human chorionic gonadotropin (hCG) and thyroid-stimulating hormone (TSH), but with a beta chain that is unique for each. FSH has a lower plasma concentration and longer half-life than LH, and it has less obvious pulsatile changes. The pulsatile nature of GnRH is essential to normal gonadotropin release; a continuous stimulation inhibits their secretion.
The hypothalamus also produces thyrotropin-releasing hormone (TRH) and vasoactive intestinal peptide (VIP), both of which stimulate prolactin release from the anterior pituitary, and dopamine, which inhibits prolactin release. Men with elevated prolactin levels present with gynecomastia, diminished libido, erectile dysfunction, and occasionally galactorrhea. Prolactin inhibits the production of GnRH from the hypothalamus and LH and FSH from the pituitary. Gonadotropin release is modulated by various other signals, such as estradiol (a potent inhibitor of both LH and FSH release), and inhibin from the Sertoli cell, which causes a selective decrease in FSH release.
FSH and LH are released into the systemic circulation and exert their effect by binding to plasma membrane receptors of the target cells. LH mainly functions to stimulate testosterone secretion from the Leydig cells of the testicle, while FSH stimulates Sertoli cells to facilitate germ cell differentiation.
Testosterone is secreted in a diurnal pattern, peaking a few hours after the man awakens from sleep. In the body, testosterone circulates 2% in the free form, 44% bound to sex hormone–binding globulin (SHBG), and 54% bound to albumin. Testosterone is converted to dihydrotestosterone (DHT) by the action of 5-alpha reductase, both locally and in the periphery, and to estrogen in the periphery. Testosterone and estradiol function as feedback inhibitors of gonadotropin release.
The testicle contains the Leydig cells and the Sertoli cells and is covered by the tunica albuginea, which also provides septae that divide it into approximately 200-350 pyramids (see image below). These pyramids are filled with the seminiferous tubules. A normal testicle contains 600-1200 seminiferous tubules with a total length of approximately 250 meters. The interstitium between the seminiferous tubules contains the Leydig cells, fibroblasts, lymphatics, blood vessels, and macrophages. Histologically, Leydig cells are polygonal with eosinophilic cytoplasm. Occasionally, the cytoplasm contains crystalloids of Reinke after puberty.
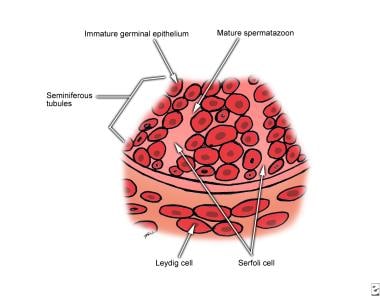 Male infertility. Testicular histology magnified 500 times. Leydig cells reside in the interstitium. Spermatogonia and Sertoli cells lie on the basement membrane of the seminiferous tubules. Germ cells interdigitate with the Sertoli cells and undergo ordered maturation, migrating toward the lumen as they mature.
Male infertility. Testicular histology magnified 500 times. Leydig cells reside in the interstitium. Spermatogonia and Sertoli cells lie on the basement membrane of the seminiferous tubules. Germ cells interdigitate with the Sertoli cells and undergo ordered maturation, migrating toward the lumen as they mature.
Seminiferous tubules are made up of Sertoli cells and germ cells and are surrounded by peritubular and myoid cells.
Sertoli cells are columnar, with irregular basal nuclei that have prominent nucleoli and fine chromatin. They rest on the basement membrane and serve mainly to support, nourish, and protect the developing germ cells and to provide a blood-testis barrier to provide a microenvironment that facilitates spermatogenesis and maintains the germ cells in an immunologically privileged location. Sertoli cells also secrete inhibin, which provides negative feedback on the hypothalamus, and androgen-binding protein, which helps modulate androgen activity in the seminiferous tubules. In addition to stimulation by FSH, Sertoli cell function is modulated by intratesticular testosterone and signals from peritubular myoid cells.
Germ cells (precursors to spermatozoa) are derived from the gonadal ridge and migrate to the testicle before testicular descent. In response to FSH stimulation at puberty, germ cells become spermatogonia and undergo an ordered maturation to become spermatozoa. The entire process of development from spermatogonium to spermatid takes 74 days and is described in 14 steps; as they mature, the developing spermatids progress closer to the lumen of the seminiferous tubule.
Spermatogonia rest on the basement membrane and contain dense nuclei and prominent nucleoli. Three types are described: A dark (Ad), A pale (Ap), and B cells. Ad cells (stem cells) divide to create more Ad cells (stem cell renewal) or differentiate into daughter Ap cells every 16 days. Ap cells mature into B spermatogonia, which then undergo mitotic division to become primary spermatocytes, which are recognized by their large centrally located nuclei and beaded chromatin. The mitotic division does not result in complete separation; rather, daughter cells maintain intracellular bridges, which have functional significance in cell signaling and maturation.
Primary spermatocytes undergo meiosis as the cells successively pass through the preleptotene, leptotene, zygotene, and pachytene stages to become secondary spermatocytes. During this time, the cells cross from the basal to the adluminal compartments. Secondary spermatocytes contain smaller nuclei with fine chromatin. The secondary spermatocytes undergo a second meiosis and become spermatids. This reduction division (ie, meiosis) results in a haploid chromosome number. Therefore, a total of 4 spermatids are made from each spermatocyte.
Next, the spermatids undergo the process of spermiogenesis (through stages named Sb1, Sb2, Sc, Sd1, and Sd2), which involves the casting of excess cytoplasm away as a residual body, the formation of the acrosome and flagella, and the migration of cytoplasmic organelles to their final cellular location. The acrosome, a derivative of the Golgi process, surrounds the nucleus anteriorly and contains enzymes necessary to penetrate the ovum. The mature spermatid is then located adjacent to the tubule lumen and contains dark chromatin with an oval-shaped nucleus.
After their release from the Sertoli cells into the lumen of the seminiferous tubules, the spermatids successively pass through the tubuli recti, rete testis, ductuli efferentes, and, finally, the epididymis (see image below). The epididymis is a 3- to 4-cm long structure with a tubular length of 4-5 m. As sperm move from the head to the tail, they mature and acquire fertilization capacity. Sperm from the head move with immature wide arcs and are generally unable to penetrate the egg, while those from the tail propel forward and have better penetration capacity. The transit time varies with age and sexual activity but is usually from 1-12 days. The epididymis additionally secretes substances for sperm nutrition and protection such as glycerophosphorylcholine, carnitine, and sialic acid.
Sperm next enter the vas deferens, a 30- to 35-cm muscular conduit of Wolffian duct origin. The vas is divided into the convoluted, scrotal, inguinal, retroperitoneal, and ampullary regions and receives its blood supply from the inferior vesical artery. In addition to functioning as a conduit, the vas also has absorptive and secretory properties.
During emission, sperm are propelled forward by peristalsis. After reaching its ampullary portion behind the bladder, the vas joins with the seminal vesicles, at the ejaculatory duct, which empties next to the verumontanum of the prostate.
During ejaculation, the ejaculate is propelled forward by the rhythmic contractions of the smooth muscle that surrounds the ducts and by the bulbourethral muscles and other pelvic muscles. Bladder neck closure during ejaculation is vital to ensure antegrade ejaculation.
Normal ejaculate volume ranges from 1.5 to 5 mL and has a pH level of 7.05-7.8. The seminal vesicles provide 40-80% of the semen volume, which includes fructose for sperm nutrition, prostaglandins and other coagulating substances, and bicarbonate to buffer the acidic vaginal vault. Normal seminal fructose concentration is 120-450 mg/dL, with lower levels suggesting ejaculatory duct obstruction or absence of the seminal vesicles.
The prostate gland contributes approximately 10-30% (0.5 mL) of the ejaculate. Products include enzymes and proteases to liquefy the seminal coagulum. This usually occurs within 20-25 minutes. The prostate also secretes zinc, phospholipids, phosphatase, and spermine. The testicular-epididymal component includes sperm and comprises about 5% of the ejaculate volume.
In addition to the components already listed, semen is also composed of secretions from the bulbourethral (Cowper) glands and the (periurethral) glands of Litre, each producing 2-5% of the ejaculate volume, serving mainly to lubricate the urethra and to buffer the acidity of the residual urine. The ordered sequence of release is important for appropriate functioning.
For conception, sperm must reach the cervix, penetrate the cervical mucus, migrate up the uterus to the fallopian tube, undergo capacitation and the acrosome reaction to digest the zona pellucida of the oocyte, attach to the inner membrane, and release its genetic contents within the egg. The cervical mucus changes consistency during the ovulatory cycle, being most hospitable and easily penetrated at mid cycle. After fertilization, implantation may then take place in the uterus. Problems with any of these steps may lead to infertility.
Etiology
Causes of male infertility generally can be divided into pretesticular, testicular, and post-testicular. Despite a thorough workup, nearly 25% of infertile men have no discernible cause for their infertility.
Pretesticular causes of infertility
Pretesticular causes of infertility include congenital or acquired disorders of the hypothalamus, pituitary, or peripheral organs that alter the hypothalamic-pituitary axis.
Disorders of the hypothalamus lead to hypogonadotropic hypogonadism. If gonadotropin-releasing hormone (GnRH) is not secreted, the pituitary does not release luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Ideally, such patients respond to replacement with exogenous GnRH or human chorionic gonadotropin (hCG), an LH analogue, although this does not always occur.
Idiopathic hypogonadotropic hypogonadism
A failure of GnRH secretion without any discernible underlying cause may be observed alone (isolated) or as part of Kallmann syndrome, which is associated with midline defects such as anosmia, cleft lip and cleft palate, deafness, cryptorchidism, and color blindness. Kallmann syndrome has been described in both familial (X-linked and autosomal) and sporadic forms, and its incidence is estimated as 1 case per 30,000 male births and 1 per 120,000 female births.
A failure of GnRH neurons to migrate to the proper location in the hypothalamus has been implicated. Patients generally have long arms and legs due to a delayed closure of the epiphyseal plates, delayed puberty, and atrophic testis. Testosterone therapy may allow patients to achieve normal height but does not improve spermatogenesis. Exogenous testosterone should never be administered in an attempt to boost sperm production because it actually decreases intratesticular testosterone levels owing to feedback inhibition of GnRH release.
Pulsatile GnRH and hCG have been used but result in only 20% of patients achieving complete spermatogenesis.
Adding recombinant human FSH to hCG has been shown to be effective in achieving spermatogenesis in most patients, leading to natural conception in most cases. [2]
Select patients with adult-onset idiopathic hypogonadotropic hypogonadism may respond to clomiphene citrate therapy. [3]
Prader-Willi syndrome
Patients have characteristic obesity, developmental delay, small hands and feet, and hypogonadotropic hypogonadism due to a GnRH deficiency. Prader-Willi syndrome is caused by a disorder of genomic imprinting with deletions of paternally derived chromosome arm 15q11-13.
Laurence-Moon-Biedl syndrome
Patients with this syndrome have retinitis pigmentosa and polydactyly. Infertility is due to hypogonadotropic hypogonadism.
Other conditions
Various other lesions and diseases, such as central nervous system tumors, temporal lobe seizures, and many drugs (eg, dopamine antagonists) may interrupt the hypothalamic-pituitary axis at the hypothalamus.
Both pituitary insufficiency and pituitary excess cause infertility. Pituitary failure may be congenital or acquired. Acquired causes include tumor, infarction, radiation, infection, or granulomatous disease. Nonfunctional pituitary tumors may compress the pituitary stalk or the gonadotropic cells, interrupting the proper chain of signals leading to pituitary failure. In contrast, functional pituitary tumors may lead to unregulated gonadotropin release or prolactin excess, interrupting the proper signaling.
Prolactinoma
A prolactin-secreting adenoma is the most common functional pituitary tumor. Prolactin stimulates breast development and lactation; therefore, patients with infertility due to a prolactinoma may have gynecomastia and galactorrhea. In addition, loss of peripheral visual fields bilaterally may be due to compression of the optic chiasm by the growing pituitary tumor.
A prolactin level of more than 150 mcg/L suggests a pituitary adenoma, while levels greater than 300 mcg/L are nearly diagnostic. Patients should undergo an MRI or CT scan of the sella turcica for diagnostic purposes to determine whether a microprolactinoma or a macroprolactinoma is present.
Bromocriptine and cabergoline are dopamine agonists used to suppress prolactin levels. These are both first-line treatment options for microprolactinoma. Some men respond with an increase in testosterone levels; many also recover normal sperm counts. Transsphenoidal resection of a microprolactinoma is 80-90% successful, but as many as 17% recur. Surgical therapy of a macroprolactinoma is rarely curative, although this should be considered in patients with any of the following:
-
Visual field defects
-
Failure of dopamine agonists to decrease prolactin levels or tumor size
-
Intolerance of bromocriptine
Isolated LH deficiency (fertile eunuch)
In these patients, LH levels are decreased while FSH levels are within the reference range. Patients have eunuchoidal body habitus, large testis, and low ejaculatory volume. The treatment of choice is exogenous hCG.
Isolated FSH deficiency
This is a very rare cause of infertility. Patients present with oligospermia but have LH levels within the reference range. Treatment is with human menopausal gonadotropin (HMG) or exogenous FSH.
Thalassemia
Patients with thalassemia have ineffective erythropoiesis and may require multiple blood transfusions. Excess iron from multiple transfusions may be deposited in the pituitary gland and the testis, causing parenchymal damage and both pituitary and testicular insufficiency. Treatment is with exogenous gonadotropins and iron-chelating therapy.
Cushing disease
Increased cortisol levels cause a negative feedback on the hypothalamus, decreasing GnRH release.
Other disorders
The hypothalamus-pituitary axis may be interrupted by hormonally active peripheral tumors or other exogenous factors, due to cortical excess, cortical deficiency, or estrogen excess.
Excess cortisol may be produced by adrenal hyperplasia, adenomas, carcinoma, or lung tumors. High cortisol levels may also be seen with exogenous steroid use, such as that administered to patients with ulcerative colitis, asthma, arthritis, or organ transplant. For example, high cortisol levels are seen in patients with Cushing syndrome, which causes negative feedback on the pituitary to decrease LH release.
Cortical deficiency may be seen in patients with adrenal failure due to infection, infarction, or congenital adrenal hyperplasia (CAH). CAH may involve deficiency of one of several adrenal enzymes, most commonly 21-hydroxylase. Because cortisol is not secreted, a lack of feedback inhibition on the pituitary gland occurs, leading to adrenocorticotropic hormone (ACTH) hypersecretion. This leads to increased androgen secretion from the adrenal gland, causing feedback inhibition of GnRH release from the hypothalamus. Patients present with short stature, precocious puberty, small testis, and occasionally bilateral testicular rests. Screening tests include assays for increased plasma 17-hydroxylase and urine 17-ketosteroids.
Estrogen excess may be seen in patients with Sertoli cell tumors, Leydig tumors, liver failure, or severe obesity. Estrogen causes negative feedback on the pituitary gland, inhibiting LH and FSH release.
Primary testicular causes of infertility
Primary testicular problems may be chromosomal or nonchromosomal in nature. While chromosomal failure is usually caused by abnormalities of the sex chromosomes, autosomal disorders are also observed.
Chromosomal abnormalities
An estimated 6-13% of infertile men have chromosomal abnormalities (compared with 0.6% of the general population). Patients with azoospermia or severe oligospermia are more likely to have a chromosomal abnormality (10-15%) than infertile men with sperm density within the reference range (1%). A karyotype test and a Y chromosome test for microdeletions are indicated in patients with nonobstructive azoospermia or severe oligospermia (< 5 million sperm/mL), although indications are expanding. [4, 5, 6]
Klinefelter syndrome is the most common chromosomal cause of male infertility, estimated to be present in 1 per 500-1000 male births. Classic Klinefelter syndrome has a 47, XXY karyotype and is caused by a nondisjunction during the first meiotic division, more commonly of maternal origin; mosaic forms are due to nondisjunction following fertilization. The only known risk factor for Klinefelter syndrome is advanced maternal age.
Infertility is caused by primary testicular failure, and most patients are azoospermic. Hormonal analysis reveals increased gonadotropin levels, while 60% have decreased testosterone levels. Surprisingly, most patients have normal libido, erections, and orgasms, so testosterone therapy has only a limited role; exogenous testosterone may also suppress any underlying sperm production.
Physical examination reveals gynecomastia, small testis, and eunuchoid body habitus due to delayed puberty. Other genital abnormalities such as hypospadias, undescended testicles (cryptorchidism) or an unusually small penis (micropenis) can also sometimes be present. In some patients, secondary sex characteristics develop normally, but they are usually completed late. These men are at a higher risk for breast cancer, leukemia, diabetes, empty sella syndrome, and pituitary tumors. Testicular histology reveals hyalinization of seminiferous tubules. [7]
Some men with Klinefelter syndrome may be able to conceive with the help of assisted reproductive techniques. Of azoospermic patients with Klinefelter syndrome, 20% show the presence of residual foci of spermatogenesis. Although the XXY pattern is observed in the spermatogonia and primary spermatocytes, many of the secondary spermatocytes and spermatids have normal patterns. The chromosomal pattern of the resultant embryos can be assessed with preimplantation genetic diagnosis.
Regular medical follow-up is required for patients with Klinefelter syndrome and androgen placement therapy initiation is recommended when testosterone levels are in the hypogonadotropic range after fertility issues have been addressed. [8, 9]
XX male (sex reversal syndrome)
An XX karyotype is due to a crossover of the sex-determining region (SRY) of the Y chromosome (with the testis determining factor) to either the X chromosome or an autosome. Patients are often short, with small firm testis and gynecomastia, but they have a normal-sized penis. Seminiferous tubules show sclerosis.
XYY male
An XYY karyotype is observed in 0.1-0.4% of newborn males. These patients are often tall and severely oligospermic or azoospermic. This pattern has been linked with aggressive behavior. Biopsy reveals maturation arrest or germ cell aplasia. Functional sperm that are present may have a normal karyotype.
Noonan syndrome (46, XY)
Patients with Noonan syndrome, also known as male Turner syndrome, have physical characteristics similar to that of women with Turner syndrome (45, X). Features include a webbed neck, short stature, low-set ears, ptosis, shield-like chest, lymphedema of hands and feet, cardiovascular abnormalities, and cubitus valgus. Leydig cell function is impaired, and most patients are infertile due to primary testicular failure.
Mixed gonadal dysgenesis (45, X/46, XY)
Patients usually have ambiguous genitalia; a testis on one side and a streaked gonad on the other.
Androgen receptor dysfunction
Because the androgen receptor is essential for the process of spermatogenesis, dysfunctions in this receptor can cause infertility. Reifenstein syndrome in males involves partial androgen insensitivity in males and presents as a spectrum of abnormal external genitalia and infertility. [10] Because cells respond inadequately to androgen stimulation, spermatogenesis is impaired. This results in negative feedback stimulation of the hypothalamic-pituitary axis, causing an increased release of gonadotropins and testosterone.
These receptor dysfunctions may be explained by defects in specific chromosomal areas. A specific portion of the androgen receptor gene, exon 1, has been studied in infertile males and a meta-analysis that involved males with idiopathic infertility and fertile controls found that infertility was directly correlated with the length of CAG repeats in this exon. [11]
Y chromosome microdeletion syndrome
The long arm of the Y chromosome (Yq) is considered critical for fertility, especially Yq11.23 (interval 6). Macroscopic deletions of Yq11 are often observed in patients with azoospermia, although many newly identified microdeletions have been implicated as a significant cause of infertility. These microdeletions are not observed on regular karyotype testing; rather, their identification requires polymerase chain reaction (PCR)–based sequence-tagged site mapping or Southern blot analysis. Three regions have been described, called azoospermic factors a, b, and c (AZFa, AZFb, AZFc). [12, 9]
These deletions are observed in 8-12% of azoospermic males and 3-7% of patients with oligospermia. AZFc deletions represent the most common type of microdeletion (65-70%), followed by Y-deletions of the AZFb and AZFb+c or AZFa+b+c regions (25-30%). AZFa region deletions are rare (5%). According to European Association of Urology (EAU) and the European Academy of Andrology (EAA) guidelines, AZF deletion screening is indicated for azoospermic and severely oligospermic patients (< 5 million/mL). [13, 9]
For patients with azoospermia or severe oligospermia seeking assisted reproductive techniques, microdeletion screening is particularly important, as when there are complete AZFa and AZFb microdeletions, the likelihood of sperm retrieval is virtually zero. Therefore testicular sperm extraction (TESE) procedures are contraindicated. Furthermore, genetic counseling is mandatory in patients found to have AZF deletions, as any Y-deletions will be transmitted to male offspring, putting them at risk for spermatogenic failure, Turner syndrome (45, XO), and other phenotypic abnormalities. [13, 14, 15]
Bilateral anorchia (vanishing testes syndrome)
Patients have a normal male karyotype (46, XY) but are born without testes bilaterally. The male phenotype proves that androgen was present in utero. Potential causes are unknown, but the syndrome may be related to infection, vascular disease, or bilateral testicular torsion. Karyotype shows a normal SRY gene. Patients may achieve normal virilization and adult phenotype through the administration of exogenous testosterone, but they are infertile.
Down syndrome
These patients have mild testicular dysfunction with varying degrees of reduction in germ cell number. LH and FSH levels are usually elevated.
Myotonic dystrophy
This is an autosomal dominant defect in the dystrophin gene that causes a delay in muscle relaxation after contraction. Seventy-five percent of patients have testicular atrophy and primary testicular failure due to degeneration of the seminiferous tubules. Leydig cells are normal. Histology reveals severe tubular sclerosis. No effective therapy exists.
Nonchromosomal testicular failure
Testicular failure that is nonchromosomal in origin may be idiopathic or acquired by gonadotoxic drugs, radiation, orchitis, trauma, or torsion.
Varicocele
A varicocele is a dilation of the veins of the pampiniform plexus of the scrotum. Although varicoceles are present in 15% of the male population, a varicocele is considered the most common correctable cause of infertility (30-35%) and the most common cause of secondary (acquired) infertility (75-85%). Varicoceles are observed more commonly on the left side than the right. Patients with isolated right-sided varicoceles should be evaluated for retroperitoneal pathology.
Varicoceles are generally asymptomatic, and most men with varicoceles do not have infertility or testicular atrophy. However, varicoceles may lead to impaired testicular spermatogenesis and steroidogenesis, potentially due to an increased intratesticular temperature, reflux of toxic metabolites, and/or germ cell hypoxia; this appears to be progressive over time.
Additionally, because insulin-like growth factor (IGF) has been shown to have an effect on semen quality, its role in varicocele pathology has been studied. [16] One study showed that IGF levels significantly increased after a varicocelectomy to levels that were no different than fertile controls, suggesting that varicocele-related infertility may involve IGF. [17]
Varicoceles lead to an increased incidence of sperm immaturity, apoptosis, and necrosis with severe disturbances in meiotic segregation compared with fertile men without varicoceles. These parameters generally improve after repair.
Patients with a grade 1-3 varicocele (visible or palpable) associated with infertility should consider having the varicocele repaired. After repair, 40-70% of patients have improved semen parameters, while 40% are able to impregnate their partner without other interventions. Those with a varicocele diagnosable only on scrotal ultrasonography have subclinical varicoceles and will likely not benefit from repair. [18] Adolescents with a varicocele and testicular atrophy or lack of growth should similarly consider repair.
Controversy exists regarding whether to routinely repair an adolescent varicocele not associated with testicular atrophy. According to the EAU guidelines, prophylactic varicolecetomy is currently advised only in cases of documented testicular atrophy or abnormal semen quality, as most patients with a varicocele will have no problem achieving pregnancy later as adults. [19, 9]
In those with azoospermia and a varicocele, sperm may appear after repair in up to one third, but most of these men return to an azoospermic state within a few months. If sperm appears, these men should be offered cryopreservation.
An estimated 3% of full-term males are born with an undescended testicle, but in many cases the testicle descends spontaneously in the first 6 months of life; by age 1 year, less than 1% continue to have cryptorchidism. Undescended testicle may be an isolated finding or may be observed as part of a syndrome such as prune belly syndrome.
Patients are at increased risk of infertility, even if the testicle is brought down into the scrotum, as the testicle itself may be inherently abnormal. The farther from its normal anatomic location in the scrotum and the longer the time that the testicle resides out scrotum, the greater the likelihood of infertility. Testicular histology typically reveals a decreased number of Leydig cells and decreased spermatogenesis. Even men with unilateral cryptorchidism have lower than expected sperm counts, which suggests that cryptorchidism may involve inherent defects in both testes.
Trauma
Testicular trauma is the second most common acquired cause of infertility. The testes are at risk for both thermal and physical trauma because of their exposed position.
Sertoli-cell-only syndrome (germinal cell aplasia)
Patients with germinal cell aplasia have LH and testosterone levels within the reference range but have an increased FSH level. The etiology is unknown but is probably multifactorial. Patients have with small- to normal-sized testes and azoospermia, but normal secondary sex characteristics. Histology reveals seminiferous tubules lined by Sertoli cells and a normal interstitium, although no germ cells are present.
Chemotherapy
Chemotherapy is toxic to actively dividing cells. In the testicle, germ cells (especially up to the preleptotene stage) are especially at risk. The agents most often associated with infertility are the alkylating agents such as cyclophosphamide. For example, treatment for Hodgkin lymphoma has been estimated to lead to infertility in as many as 80-100% of patients.
Radiation therapy
While Leydig cells are relatively radioresistant because of their low rate of cell division, the Sertoli and germ cells are extremely radiosensitive. If stem cells remain viable after radiation therapy, patients may regain fertility within several years. However, some experts have suggested that patients should avoid conception for 6 months to 2 years after completion of radiation therapy because of the possibility of chromosomal aberrations in their sperm caused by the mutagenic properties of radiation therapy. Even with the testis shielded, radiation therapy below the diaphragm may lead to infertility due to the release of reactive oxygen free radicals.
Orchitis
The most common cause of acquired testicular failure in adults is viral orchitis, such as that caused by the mumps virus, echovirus, or group B arbovirus. Of adults who are infected with mumps, 25% develop orchitis; two thirds of cases are unilateral, and one third are bilateral. While orchitis typically develops a few days after the onset of parotid gland inflammation, it may also precede it.
The virus may either directly damage the seminiferous tubules or indirectly cause ischemic damage as the intense swelling leads to compression against the tough tunica albuginea. After recovery, the testicle may return to normal or may atrophy. Atrophy is observed within 1-6 months, and the degree of atrophy does not correlate with the severity of orchitis or infertility. Normal fertility is observed in three fourths of patients with unilateral mumps orchitis and in one third of patients in bilateral orchitis.
Human-beta defensin abnormalities
Epididymis human-beta defensin is a protein that has been shown to have an important role in sperm maturation, and defects in it have been associated with decreased egg-penetrating ability. [20] One specific subtype, human-beta defensin–1 (HBD1), which has a wide distribution in various epithelia throughout the body and plays a role in antimicrobial activities against viruses, bacteria, and fungi, has also been investigated.
HBD1 is expressed in the seminal plasma and ejaculated sperm, more specifically in the lower head and midpiece of the sperm from fertile individuals. Expression of HBD1 is reduced in individuals with asthenozoospermia and leukocytospermia. In one study, treatment with recombinant HBD1 in asthenozoospermic and leukocytospermic patients who were deficient in HBD1 resulted in improved bactericidal activity and sperm quality, which supports this protein’s role in fertility and its potential role in managing infertility. [21]
Other causes
Causes of testicular failure also include the following:
-
Granulomatous disease – Leprosy and sarcoidosis may infiltrate the testicle
-
Sickle cell disease – Sickling of cells within the testis leads to microinfarcts
-
Excessive use of alcohol, cigarettes, caffeine, or marijuana
Sexually transmitted infections, such as with Chlamydia trachomatis, Neisseria gonorrhoeae, genital mycoplasma (ie, Mycoplasma genitalium, M hominis, Ureaplasma urealyticum [22] ), Trichomonas vaginalis, and human papillomavirus (HPV) have been reported to reduce sperm count and motility. Chronic prostatitis and epididymitis caused by bacterial or viral infection can also lead to male infertility. [23]
Post-testicular causes of infertility
Post-testicular causes of infertility include problems with sperm transportation through the ductal system, either congenital or acquired. Genital duct obstruction is a potentially curable cause of infertility and is observed in 7% of infertile patients. Additionally, the sperm may be unable to cross the cervical mucus or may have ultrastructural abnormalities.
Congenital blockage of the ductal system
An increased rate of duct obstruction is observed in children of mothers who were exposed to diethylstilbestrol (DES) during pregnancy. Segmental dysplasia is defined as a vas deferens with at least 2 distinct sites of vasal obstruction.
Acquired blockage of the ductal system
Complete and partial ejaculatory duct obstruction has been implicated as a cause of 1-5% of patients with male infertility. Patients may have a normal palpable vas deferens bilaterally but show decreased ejaculate volume and hematospermia and may experience pain upon ejaculation. Etiologies include the following:
-
Cysts (midline and eccentric)
-
Ductal calcification and stones
-
Infection (eg, chlamydia, gonorrhea, tuberculosis)
-
Young syndrome, which leads to inspissation of material and subsequent blockage of the epididymis
-
Trauma
-
Previous attempts at sperm aspiration
-
Inguinal surgery
-
Scrotal surgery, including vasectomy, hydrocelectomy (5-6%), and spermatocelectomy (up to 17%), which may lead to epididymal injury and subsequent obstruction [24]
Transrectal ultrasonography (TRUS) may reveal enlarged seminal vesicles, but this is not universal. The American Urologic Association (AUA) recommends transrectal ultrasound for patients with palpable vasa and low ejaculate volumes. [5, 25]
Cystic fibrosis (CF)
CF is the most common genetic disorder in whites. Patients with CF nearly uniformly have congenital bilateral absence of the vas deferens (CBAVD). The cystic fibrosis transmembrane regulator (CFTR) protein plays a role in mesonephric duct development during early fetal life, so these patients may also have urinary tract abnormalities. Patients may be candidates for assisted reproduction techniques after appropriate genetic screening in the partner. [26]
Antisperm antibodies
Antisperm antibodies bind to sperm, impair motility, and lead to clumping, impairing movement through the female reproductive tract and interaction with the oocyte.
Defects in cilia
Immotile cilia syndrome may occur as an isolated disorder or as part of Kartagener syndrome with situs inversus. Because of a defect in the dynein arms, spokes, or microtubule doublet, cilia in the respiratory tract and in sperm do not function properly. In addition to sperm immobility, patients experience sinusitis, bronchiectasis, and respiratory infections.
Ejaculation issues
Anejaculation/retrograde ejaculation may be due to an open bladder neck or a lack of rhythmic contractions during ejaculation. Etiologies include the following:
-
Diabetic neuropathy
-
Bladder neck surgery
-
Retroperitoneal lymph node dissection
-
Transurethral resection of the prostate or prostatectomy
-
Colon or rectal surgery
-
Multiple sclerosis
-
Spinal cord injury
-
Use of medicines such as alpha-antagonists
The diagnosis of anejaculation or retrograde ejaculation is suggested by the following:
-
Compatible medical or surgical history
-
Low ejaculate volume
-
Presence of 10-15 sperm per high-power field (HPF) in the postejaculatory urine
Epidemiology
United States
An estimated 10-15% of couples are considered infertile, defined by the World Health Organization (WHO) as the absence of conception after at least 12 months of unprotected intercourse. In US men, the risk correlates to approximately 1 in 25. Low sperm counts, poor semen quality, or both account for 90% of cases; however, studies of infertile couples without treatment reveal that 23% of these couples conceive within 2 years, and 10% more conceive within 4 years. Even patients with severe oligospermia (< 2 million sperm/mL) have a 7.6% chance of conception within 2 years. [27]
International
Patterns of male infertility vary greatly among regions and even within regions. The highest reported fertility rates are in Finland, while Great Britain has a low fertility rate. A combination of social habits, environmental conditions, and genetics is suspected to contribute to this variation.
Debate has occurred in the literature regarding a poorer semen quality, decreased sperm counts (113 million/mL in 1940 compared with 66 million/mL in the 1990s), and decreased fertility in men today compared with fertility 50 years ago. [28] Investigators hypothesize that environmental conditions and toxins have led to this decline; however, others argue that this is solely because of differences in counting methods, laboratory techniques, and geographic variation.
Sex
Isolated conditions of the female are responsible for infertility in 35% of cases, isolated conditions of the male in 30%, conditions of both the male and female in 20%, and unexplained causes in 15%. Even if one partner has an obvious cause for the infertility, a thorough evaluation of both partners for completeness is prudent. In addition, both partners may be aided by evaluation of their sexual practices.
Age
The effect of aging on fertility is unclear. As men age, their testosterone levels decrease, while estradiol and estrone levels increase. Studies have shown that, as men age, their sperm density decreases. Young men have spermatids present in 90% of seminiferous tubules, which decreases to 50% by age 50-70 years and to 10% by age 80 years. Additionally, 50% of Sertoli cells are lost by age 50 years, and 50% of Leydig cells are lost by age 60 years. Despite this, aging men may achieve fertility rates similar to those in younger men, although conception often takes longer.
Prognosis
The prognosis of a patient with infertility depends on its underlying cause. The appropriate workup must be performed, and then the appropriate intervention may be used. Prognosis is individualized depending on these results.
Many patients who present with infertility as their primary complaint have a serious underlying medical disease, such as pituitary adenomas, hormonally active tumors, testicular cancer, liver and renal failure, and cystic fibrosis (CF). Evaluating patients for such life-threatening or life-altering conditions during the workup is important.
In addition, the risk of cancer appears to be increased in infertile men. In a study of 2238 infertile men, 451 with azoospermia and 1787 without, male infertility was associated with an increased risk of developing cancer in comparison with the general population. [29, 30] Median age at initial evaluation was 35.7 years, and median follow-up was 6.7 years.
Overall, 29 men developed some type of cancer, including 10 (2.2%) with azoospermia and 19 (1.1%) without azoospermia. [30] Compared with the general population of Texas, infertile men had a higher risk of overall cancer (standardized incidence ratio [SIR], 1.7; 95% confidence interval [CI], 1.2-2.5).
The risk was significantly higher in azoospermic men than in nonazoospermic men (SIR, 2.9; 95% CI, 1.4-5.4). [30] The risk of cancer in nonazoospermic infertile men was similar to that in the general population (SIR, 1.4; 95% CI, 0.9-2.2), although there was a trend toward an elevated risk.
The men who developed cancer in the study developed a variety of malignancies, including prostate cancer, testicular cancer, CNS cancer, melanoma, and stomach cancer. [30]
Patient Education
Couples should be counseled that the most effective regimen is to perform coitus every 48 hours at mid cycle. For patient education information, see the Male Infertility Directory.
-
Male infertility. Hypothalamic-pituitary-gonadal axis stimulatory and inhibitory signals. Gonadotropin-releasing hormone (GnRH) from the hypothalamus stimulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary. FSH stimulates the Sertoli cells to facilitate sperm production, while LH stimulates testosterone release from the Leydig cells. Feedback inhibition is from testosterone and inhibin.
-
Male infertility. Testicular histology magnified 500 times. Leydig cells reside in the interstitium. Spermatogonia and Sertoli cells lie on the basement membrane of the seminiferous tubules. Germ cells interdigitate with the Sertoli cells and undergo ordered maturation, migrating toward the lumen as they mature.
-
Male infertility. Normal male ductal anatomy.
-
Male infertility. Varicocele. A - Physical examination revealing the characteristic "bag of worms." B - Anatomy of the dilated pampiniform plexus of veins.
-
Male infertility. Technique of open vasography: The vas distal to the site of incision is determined to be patent if saline is injected without resistance. Alternatively, radiographic contrast dye is injected through the vas deferens and radiography is performed, or blue dye may be injected and visualized in the urine to confirm patency. A vasovasostomy or vasoepididymostomy may then be performed at this level.
-
Male infertility. Technique of microscopic varicocelectomy. The individual veins of the pampiniform plexus are isolated (top) and ligated, taking care to preserve the testicular artery (bottom) isolated using the intraoperative Doppler.
-
Male infertility. Technique of vasovasostomy: Upper left is confirmation of sperm from the proximal vas deferens, proving proximal patency. Upper right is the inner layer anastomosis using interrupted #10-0 Prolene. Lower left is the inner layer anastomosis completed. Lower right is the outer layer anastomosis using #9-0 Prolene completed.
-
Male infertility. Technique of vasoepididymostomy. Left upper is confirmation of mature sperm in epididymis. Right upper is the inner layer anastomosis of the end of the vas to the side of the epididymal tubule using interrupted #10-0 Prolene. Left lower is the inner layer completed. Right lower is the outer layer anastomosis using interrupted #9-0 Prolene completed.
-
Male infertility. Technique of intracytoplasmic sperm injection (ICSI). A micropipette is used to inject a single sperm directly into an egg.