Practice Essentials
Hypothyroidism is a common endocrine disorder resulting from deficiency of thyroid hormone. In the United States and other areas of adequate iodine intake, autoimmune thyroid disease (Hashimoto disease) is the most common cause of hypothyroidism; worldwide, iodine deficiency remains the foremost cause.
The image below depicts the hypothalamic-pituitary-thyroid axis.
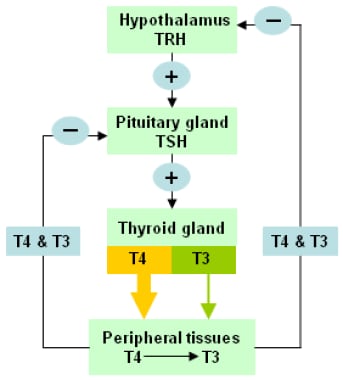 The hypothalamic-pituitary-thyroid axis. Levels of circulating thyroid hormones are regulated by a complex feedback system involving the hypothalamus and pituitary gland.
The hypothalamic-pituitary-thyroid axis. Levels of circulating thyroid hormones are regulated by a complex feedback system involving the hypothalamus and pituitary gland.
See 21 Hidden Clues to Diagnosing Nutritional Deficiencies, a Critical Images slideshow, to help identify clues to conditions associated with malnutrition.
ICD-10 codes
These include the following:
-
The International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code for “other hypothyroidism” is E03 [1]
-
The ICD-10-CM code for "hypothyroidism, unspecified," is E03.9 [2]
Signs and symptoms of hypothyroidism
Hypothyroidism commonly manifests as a slowing in physical and mental activity but may be asymptomatic. Symptoms and signs are often subtle and neither sensitive nor specific.
The following are symptoms of hypothyroidism:
· Fatigue, loss of energy, lethargy
· Weight gain
· Decreased appetite
· Cold intolerance
· Dry skin
· Hair loss
· Sleepiness
· Muscle pain, joint pain, weakness in the extremities
· Depression
· Emotional lability, mental impairment
· Forgetfulness, impaired memory, inability to concentrate
· Constipation
· Menstrual disturbances, impaired fertility
· Decreased perspiration
· Paresthesias and nerve entrapment syndromes
· Blurred vision
· Decreased hearing
· Fullness in the throat, hoarseness
The following are symptoms more specific to Hashimoto thyroiditis:
· Feeling of fullness in the throat
· Painless thyroid enlargement
· Exhaustion
· Transient neck pain, sore throat, or both
Physical signs of hypothyroidism include the following:
· Weight gain
· Slowed speech and movements
· Dry skin (or, rarely, yellow-hued skin from carotene)
· Jaundice
· Pallor
· Coarse, brittle, straw-like hair
· Loss of scalp hair, axillary hair, pubic hair, or a combination
· Dull facial expression
· Coarse facial features
· Periorbital puffiness
· Macroglossia
· Goiter (simple or nodular)
· Hoarseness
· Decreased systolic blood pressure and increased diastolic blood pressure
· Bradycardia
· Pericardial effusion
· Abdominal distention, ascites (uncommon)
· Hypothermia (only in severe hypothyroid states)
· Nonpitting edema (myxedema)
· Pitting edema of lower extremities
· Hyporeflexia with delayed relaxation (pseudomyotonia), ataxia, or both
Myxedema coma is a severe form of hypothyroidism that most commonly occurs in individuals with undiagnosed or untreated hypothyroidism who are subjected to an external stress. Features are as follows:
· Altered mental status
· Hypothermia
· Bradycardia
· Hypercapnia
· Hyponatremia
· Cardiomegaly, pericardial effusion, cardiogenic shock, and ascites may be present
See Clinical Presentation for more detail.
Diagnosis of hypothyroidism
Third-generation thyroid-stimulating hormone (TSH) assays are generally the most sensitive screening tool for primary hypothyroidism. [3] If TSH levels are above the reference range, the next step is to measure free thyroxine (T4) or the free thyroxine index (FTI), which serves as a surrogate of the free hormone level. Routine measurement of triiodothyronine (T3) is not recommended.
Biotin, a popular health supplement, may interfere with immunoassays of many hormones, resulting in values that are falsely elevated or suppressed, including for thyroid levels. To avoid misleading test results, the American Thyroid Association recommends cessation of biotin consumption at least 2 days prior to thyroid testing. [4]
Results in patients with hypothyroidism are as follows:
· Elevated TSH with decreased T4 or FTI
· Elevated TSH (usually 4.5-10.0 mIU/L) with normal free T4 or FTI is considered mild or subclinical hypothyroidism
Abnormalities in the complete blood count and metabolic profile that may be found in patients with hypothyroidism include the following [5] :
· Anemia [6]
· Dilutional hyponatremia (with increased antidiuretic hormone [ADH])
· Hyperlipidemia
· Reversible increases in creatinine [5]
· Elevations in transaminases and creatinine kinase
No universal screening recommendations exist for thyroid disease for adults. The American Thyroid Association recommends screening at age 35 years and every 5 years thereafter, with closer attention to patients who are at high risk, such as the following [7] :
· Pregnant women
· Women older than 60 years
· Patients with type 1 diabetes or other autoimmune disease
· Patients with a history of neck irradiation
However, the American College of Obstetricians and Gynecologists (ACOG) does not recommend universal screening for thyroid disease in pregnant women. However, those who are at increased risk warrant screening. This includes pregnant women with a personal or family history of thyroid disease, type 1 diabetes, or symptoms suggestive of thyroid disease. There is no proven benefit in screening pregnant women with a mildly enlarged thyroid gland, whereas those with a significant goiter or distinct thyroid nodules require screening. [8]
See Workup for more detail.
Management of hypothyroidism
The treatment goals for hypothyroidism are to reverse clinical progression and correct metabolic derangements, as evidenced by normal blood levels of thyroid-stimulating hormone (TSH) and free thyroxine (T4). Thyroid hormone is administered to supplement or replace endogenous production. In general, hypothyroidism can be adequately treated with a constant daily dose of levothyroxine (LT4).
Significant controversy persists regarding the treatment of patients with mild hypothyroidism. [9] Reviews by the US Preventive Services Task Force [10] and an independent expert panel [11] found inconclusive evidence to recommend aggressive treatment of patients with TSH levels of 4.5-10 mIU/L.
In patients with myxedema coma, an effective approach consists of the following:
-
Give 4 µg of LT4 per kilogram of lean body weight (approximately 200-250 µg) as an IV bolus in a single or divided dose, depending on the patient’s risk of cardiac disease and age
-
24 hours later, give 100 µg IV
-
Subsequently, give 50 µg/day IV, along with stress doses of IV glucocorticoids
-
Adjust the dosage on the basis of clinical and laboratory findings
-
Provide antibiotic coverage for sepsis
-
Avoid volume contraction
Background
Hypothyroidism is a common endocrine disorder resulting from deficiency of thyroid hormone. It usually is a primary process in which the thyroid gland is unable to produce sufficient amounts of thyroid hormone.
Hypothyroidism can also be secondary—that is, the thyroid gland itself is normal, but it receives insufficient stimulation because of low secretion of thyrotropin (ie, thyroid-stimulating hormone [TSH]) from the pituitary gland. This generally occurs in the presence of other pituitary hormone deficiencies. In tertiary hypothyroidism, inadequate secretion of thyrotropin-releasing hormone (TRH) from the hypothalamus leads to insufficient release of TSH, which in turn causes inadequate thyroid stimulation. However, this is rare.
Worldwide, iodine deficiency remains the foremost cause of hypothyroidism. In the United States and other areas of adequate iodine intake, autoimmune thyroid disease (Hashimoto disease) is the most common cause. Hypothyroidism may also be drug-induced or otherwise iatrogenic. (See Etiology.)
Some, but not all, studies have indicated that low vitamin D levels can be linked to autoimmune thyroid diseases, such as Hashimoto thyroiditis and Graves disease. However, intervention studies have not to date demonstrated a benefit of supplementation. No association has been found between vitamin D levels and thyroid cancer. This remains an area of investigation. [12]
The patient’s presentation may vary from asymptomatic to myxedema coma with multisystem organ failure. Because nearly all metabolically active cells require thyroid hormone, deficiency of the hormone has a wide range of effects. (See Presentation.)
Third-generation TSH assays are readily available and are generally the most sensitive screening tool for primary hypothyroidism. The generally accepted reference range for normal serum TSH is 0.40-4.2 mIU/L.
If TSH levels are above the reference range, the next step would be to measure free thyroxine (T4). Subclinical hypothyroidism, also referred to as mild hypothyroidism, is defined as normal serum levels of free T4 and triiodothyronine (T3) with a slightly high serum TSH concentration. As with clinical hypothyroidism, Hashimoto thyroiditis is the most common cause of subclinical hypothyroidism in the United States. [13, 14] (See Workup.)
For hypothyroidism, thyroid hormone is administered to supplement or replace endogenous production. In general, hypothyroidism can be adequately treated with a constant daily dose of levothyroxine (LT4). (See Treatment and Medication.)
Congenital hypothyroidism, which affects 1 of every 4000 newborns, is due to congenital maldevelopment of the thyroid (see Pediatric Hypothyroidism). This disorder is included in the newborn screening panel in the United States and many other countries, and it is readily treatable once detected. Cretinism refers to severe hypothyroidism in an infant or child. This is classically the result of maternal iodine deficiency, and thankfully is increasingly rare.
Pathophysiology
The hypothalamic-pituitary-thyroid axis governs thyroid hormone secretion (see the image below).
 The hypothalamic-pituitary-thyroid axis. Levels of circulating thyroid hormones are regulated by a complex feedback system involving the hypothalamus and pituitary gland.
The hypothalamic-pituitary-thyroid axis. Levels of circulating thyroid hormones are regulated by a complex feedback system involving the hypothalamus and pituitary gland.
Although hypothalamic or pituitary disorders can affect thyroid function, localized disease of the thyroid gland that results in decreased thyroid hormone production is the most common cause of hypothyroidism. Under normal circumstances, the thyroid releases 100-125 nmol of T4 daily and small amounts of T3. The ratio of T4:T3 production varies between about 14:1 and 4:1, depending on iodine sufficiency and TSH stimulation. The half-life of T4 is approximately 7-10 days, whereas the half-life of T3 is about 24 hours. T4, a prohormone, is converted via the action of deiodinases to T3, the active form of thyroid hormone.
Early in the disease process, compensatory mechanisms maintain T3 levels. Decreased production of T4 causes an increase in the secretion of TSH by the pituitary gland. TSH stimulates hypertrophy and hyperplasia of the thyroid gland and 5’-deiodinase activity, thereby increasing T3 production.
Deficiency of thyroid hormone has a wide range of effects. Systemic effects are the result of either derangements in metabolic processes or direct effects by myxedematous infiltration (ie, accumulation of glycosaminoglycans in the tissues).
The hypothyroid changes in the heart result in decreased contractility, cardiac enlargement, pericardial effusion, decreased pulse, and decreased cardiac output.
In the gastrointestinal (GI) tract, achlorhydria and prolonged intestinal transit time with gastric stasis can occur in hypothyroidism. Non-alcoholic fatty liver disease (NAFLD) may also be significantly associated with hypothyroidism, as shown in a meta-analysis of 44,140 individuals with diagnosed hypothyroidism. [15]
Delayed puberty, anovulation, menstrual irregularities, and infertility are common. TSH screening should be a routine part of any investigation into menstrual irregularities or infertility.
Decreased thyroid hormone effect can cause increased levels of total cholesterol and low-density lipoprotein (LDL) cholesterol and a possible change in high-density lipoprotein (HDL) cholesterol because of a change in metabolic clearance. In addition, hypothyroidism may result in an increase in insulin resistance.
A study by Wopereis et al looked at the increased risk for anemia arising in hypothyroidism, reporting that for overt hypothyroidism, the pooled hazard ratio (HR) for anemia development was 1.38, while for subclinical hypothyroidism, it was 1.18. Although it is not clear how hypothyroidism leads to anemia, there is evidence that reduced thyroid function may interfere with the production of healthy erythrocytes. The possibility exists that T3, T4, and TSH are directly involved in erythropoiesis. [6]
Etiology
In the United States and other areas of adequate iodine intake, autoimmune thyroid disease (Hashimoto disease) is the most common cause of hypothyroidism. The prevalence of antibodies is higher in women and increases with age. There is commonly a genetic predisposition for autoimmune thyroid disease occurring in 20-30% of the siblings of affected patients, with a greater prevalence seen in circulating thyroid antibodies (~50% of siblings of affected patients). [16] Additionally, higher concordance rates are seen in autoimmune thyroid disease in monozygotic twins (29-55%) compared with dizygotic twins (0-7%). [17] Congenital causes of thyroid dysfunction are less common (see below).
Primary hypothyroidism
Types of primary hypothyroidism include the following:
· Chronic lymphocytic (autoimmune) thyroiditis
· Postpartum thyroiditis
· Subacute (granulomatous) thyroiditis
· Drug-induced hypothyroidism
· Iatrogenic (postsurgical) hypothyroidism
Chronic lymphocytic (autoimmune) thyroiditis
The most frequent cause of acquired hypothyroidism is chronic lymphocytic (autoimmune) thyroiditis (Hashimoto thyroiditis). The body considers the thyroid antigens as foreign, and a chronic immune reaction ensues, resulting in lymphocytic infiltration of the gland and progressive destruction of functional thyroid tissue.
The majority of affected individuals will have circulating antibodies to thyroid tissue. Anti–thyroid peroxidase (anti-TPO) antibodies are the hallmark of this disease. It should be noted that antibody levels can vary over time, may not be present early in the disease process, and usually disappear over time. Given this change in antibody concentration, it should be understood that the absence of antibodies does not exclude the diagnosis of chronic lymphocytic (autoimmune) thyroiditis.
A study by Bothra et al reported that, compared with the general population, first-degree relatives of persons with Hashimoto thyroiditis have a nine-fold greater risk of developing it. [18]
The relationship between Hashimoto thyroiditis and thyroid cancer is under debate. The cellular changes of Hashimoto thyroiditis are often found surrounding thyroid cancers that have been removed, but it is not known whether the thyroid inflammation characterizing Hashimoto thyroiditis gives rise to the cancer or vice versa. A literature review by Lee et al indicated that pathologically confirmed Hashimoto thyroiditis has been identified in cases of papillary thyroid carcinoma more frequently than in benign thyroid disorders or other carcinomas, the occurrence rates being 2.8 and 2.4 times greater, respectively. [19, 20]
Postpartum thyroiditis
Up to 10% of postpartum women may develop lymphocytic thyroiditis (postpartum thyroiditis) in the 2-12 months after delivery. The frequency may be as high as 25% in women with type 1 diabetes mellitus. Although a short course of treatment with levothyroxine (LT4) may be necessary, the condition is frequently transient (2-4 months). Nonetheless, after initiation, hypothyroidism developing from postpartum thyroiditis can last as long as a year before resolving on its own, and patients with postpartum thyroiditis (anti-TPO–positive) are at increased risk for permanent hypothyroidism or recurrence of postpartum thyroiditis with future pregnancies. [21]
The hypothyroid state can be preceded by a short thyrotoxic state. High titers of anti-TPO antibodies during pregnancy have been reported to have high sensitivity and specificity for postpartum autoimmune thyroid disease.
In a 12-year longitudinal study, Stuckey et al found that hypothyroidism developed in 27 of 71 women (38%) who had a past history of postpartum thyroid dysfunction (PPTD). In comparison, only 14 of 338 women (4%) who had not had PPTD developed hypothyroidism. [22]
Subacute granulomatous thyroiditis
Also known as de Quervain, or painful, thyroiditis, subacute granulomatous thyroiditis is a relatively uncommon disease that occurs most frequently in women (5:1) and is rare in the elderly. Disease features include low grade fever, thyroid pain, dysphagia, and elevated erythrocyte sedimentation rate (ESR).
The disease is usually self-limited and does not normally result in longstanding thyroid dysfunction. It is important to note that inflammatory conditions or viral syndromes may be associated with transient hyperthyroidism followed by transient hypothyroidism (ie, de Quervain thyroiditis and subacute thyroiditis).
There have been several studies demonstrating an association between coronavirus disease 2019 (COVID-19) and the development of subacute thyroiditis. [23]
Riedel thyroiditis
This disease, characterized by dense fibrosis of the thyroid gland, typically occurs between the ages of 30-60 years and is more prevalent in women (3-4:1). It presents with a rock hard, fixed, and painless goiter. Symptoms are typically related to compressive effects on surrounding structures or hypoparathyroidism due to extension of the fibrosis.
The disease has been linked to immunoglobulin G4 (IgG4) and is associated with a systemic fibrotic process. Most patients initially present with euthyroidism but later develop hypothyroidism as normal thyroid tissue is replaced. ESR levels are often normal, but high concentrations of anti-TPO antibodies are frequently present (~67% of patients). Open biopsy provides definitive diagnosis, and treatment is often surgical, although some studies have shown that early treatment with glucocorticoids, methotrexate, or tamoxifen may be beneficial. [24, 25]
Systemic lupus erythematosus
Between 15% and 19% of patients with systemic lupus erythematosus (SLE) have primary hypothyroidism, with hypothyroidism being the most common thyroid disease in patients with SLE. Although all age groups of individuals with SLE have a greater frequency of hypothyroidism, this is especially true in patients under age 20 years, the odds ratio (OR) being 8.38. In addition, the tendency to develop clinical or subclinical hypothyroidism is greater in female patients with SLE than in males. [26]
Drug-induced and iatrogenic hypothyroidism
The following medications reportedly have the potential to cause hypothyroidism:
-
Iodinated contrast
-
Amiodarone
-
Interferon alfa
-
Thalidomide
-
Lithium
-
Stavudine
-
Oral tyrosine kinase inhibitors – Sunitinib, imatinib [27]
-
Bexarotene [28]
-
Perchlorate
-
Interleukin (IL)-2
-
Ethionamide
-
Rifampin
-
Phenytoin
-
Carbamazepine
-
Phenobarbital
-
Aminoglutethimide
-
Sulfisoxazole
-
p -Aminosalicylic acid
-
Immune checkpoint inhibitors – Ipilimumab, pembrolizumab, nivolumab
Several of these medications, such as the anticonvulsants, are cytochrome P450 hepatic enzyme inducers and may unmask a latent hypothyroid state due to their impact on thyroid hormone economy or binding.
The use of radioactive iodine (I-131) for the treatment of Graves disease generally results in permanent hypothyroidism within 3-6 months after therapy. The frequency of hypothyroidism after I-131 treatment is much lower in patients with toxic nodular goiters and those with autonomously functioning thyroid nodules. Patients treated with radioiodine should be monitored for clinical and biochemical evidence of hypothyroidism.
External neck irradiation (for head and neck neoplasms, breast cancer, or Hodgkin disease) of over 40 Gy commonly results in hypothyroidism. Patients who have received these treatments require monitoring of thyroid function.
Thyroidectomy results in hypothyroidism, although this depends on the extent of resection and the underlying disease. Patients who undergo a thyroid lobectomy, with or without isthmectomy, have an approximately 15-30% chance of developing thyroid insufficiency.
Amiodarone-induced thyroid dysfunction can manifest as thyrotoxicosis or hypothyroidism, with the latter being more common in iodine-sufficient populations such as the United States (~20% of patients treated with amiodarone). There may also be an association with underlying autoimmune thyroid disease, as a higher prevalence of amiodarone-induced hypothyroidism is seen in patients with preexisting thyroid autoantibodies. The mechanism of action is due in part to an excess of iodine release during the metabolism of amiodarone (with each 200 mg tablet containing 75 mg of iodine), as well as apoptosis of thyroid cells through an iodine-independent mechanism. [29]
The 24-hour uptake of I-123 is typically low, and findings on color flow Doppler ultrasonography are variable. Due to the long half-life of amiodarone (approximately 100 days), recovery of thyroid function is prolonged. Treatment of amiodarone-induced thyroid dysfunction includes supplementation with levothyroxine, typically at higher replacement doses due to decreased 5’-deiodinase activity in peripheral tissues, an effect mediated by amiodarone. [24]
Immune checkpoint inhibitors (ICIs) enhance T-cell activity via inhibition of the negative inhibitory effects of cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), and programmed death-ligand 1 (PD-L1). A variety of immune-related adverse effects have been associated with ICIs, with hypophysitis and thyroid dysfunction being the primary endocrine-related outcomes. The exact etiology of immune-related adverse thyroid effects is unknown, and while most cases are mild and self-limited, progression to permanent hypothyroidism can occur. Some cases suggest an underlying destructive thyroiditis that presents with an initial thyrotoxic phase (similar to tyrosine kinase inhibitor [TKI]–related thyroid dysfunction) and is followed by hypothyroidism. However, overt primary hypothyroidism as the initial event is also seen, with an incidence between 10-60%, and is typically irreversible. [30]
The reported incidence of hypophysitis associated with CTLA-4 inhibitor therapy is 0.4-17.0%; it is reported to occur more frequently in males and presents with central hypothyroidism and central hypoadrenalism. [31]
Primary thyroid dysfunction occurs with CTLA-4 and PD-1/PD-L1 inhibitors and can present more commonly as subclinical or overt hypothyroidism, transient thyrotoxicosis, or painless thyroiditis. Rarely, Graves disease and euthyroid orbitopathy can occur. The incidence and severity of thyroid dysfunction increases with combination CTLA-4 and PD-1 inhibitor therapy (6% incidence with ipilimumab alone vs 22% with a combination of ipilimumab and nivolumab, in a study reported by Ryder et al). [32]
Screening for thyroid dysfunction using TSH and free T4 levels is recommended before treatment initiation, at 4-6 weekly intervals, and should be repeated before each treatment cycle. For confirmed primary and central hypothyroidism, levothyroxine therapy is started, but hypocortisolism should be ruled out prior to treating central hypothyroidism. If cortisol is low, glucocorticoid therapy is initiated at least 3-5 days prior to thyroid hormone replacement to prevent an acute adrenal crisis. Subclinical hypothyroidism often resolves without treatment. [30, 31]
Tyrosine kinase inhibitors (TKIs) cause iatrogenic hypothyroidism via several different mechanisms, due to differences in their spectrum of targeted kinases. This in turn leads to varying rates of thyroid dysfunction. Destructive thyroiditis, postulated to be the primary process leading to thyroid dysfunction, causes an initial transient thyrotoxic phase that is followed by overt hypothyroidism. The anti-angiogenic effects of TKIs are mediated via anti-vascular endothelial growth factor receptor (anti-VEGFR) and platelet-derived growth factor receptor (PDGFR) signaling, which leads to decreased vascularization of the thyroid parenchyma, resulting in cellular hypoxia. In turn, thyroid hormone synthesis is also decreased by way of this process. If treatment is prolonged, permanent hypothyroidism can ensue.
TKIs may also play an inhibitory role in the secretion of TRH from the hypothalamus, via reduced nitric oxide production, leading to decreased TSH release. Independent of the thyroid gland, as seen in patient status post thyroidectomy, TKIs (particularly imatinib) increase levothyroxine requirements by increasing the activity of type 3 deiodinase and causing decreased tissue availability of T3. [33, 34]
Of the TKIs, sunitinib is the one most likely to cause new-onset hypothyroidism, with the disease occurring in 14-70% of patients who take the drug. The risk rises with prolonged therapy and an increased number of treatment cycles. It can reportedly take as little as 4 weeks and as long as 92 weeks for hypothyroidism to develop with sunitinib therapy. Of interest, iatrogenic hypothyroidism resulting from TKI use has been associated with prolonged survival rates of unknown etiology. [31]
TSH screening is recommended at TKI initiation, then monthly for the first 6 months. Thereafter, TSH can be checked every 2-3 months (or sooner if new symptoms or clinical signs of thyroid disease occur). In patients with established hypothyroidism, TSH should be checked every month for the first 3 months, and then every 3 months thereafter. If levothyroxine is prescribed during the course of treatment, a trial withdrawal can be considered at the conclusion of TKI treatment. [35]
Genetics
Genome-wide association studies have suggested that a single-nucleotide polymorphism located near the FOXE1 gene is associated with risk of developing thyroid disease and that the strongest association is with hypothyroidism. Persons found to have GG at the described location had an odds ratio (OR) of 1.35 for development of hypothyroidism, whereas persons found to have AG at the location had an OR of 1.00, and persons found to have AA at the location had an OR of 0.74. [36]
Approximately 10% of patients with congenital hypothyroidism have an error in thyroid hormone synthesis. [37] Mutations in the TPO gene appear to be the most common error of hormone synthesis, causing failure to produce adequate amounts of TPO. [38]
Mutations in the TSHR and PAX8 genes are known to cause congenital hypothyroidism without goiter. [39, 40] Mutations in the TSHR gene can cause hypothyroidism due to insensitivity to TSH, though most cases are notable for a clinically euthyroid state despite abnormal laboratory test results (elevated TSH with normal serum thyroid hormone concentrations). Mutations in the PAX8 gene cause hypothyroidism due to dysgenesis or agenesis of the gland .
Syndromic forms of hypothyroidism are also well described. Pendred syndrome is caused by a mutation in the SLC26A4 gene, which causes a defect in the organification of iodine (ie, incorporation into thyroid hormone), congenital sensorineural hearing loss, and, usually, an enlarged thyroid gland. It is inherited in an autosomal recessive manner. [41]
Autoimmune polyendocrinopathy type I is caused by a mutation in the AIRE gene and is characterized by the presence of Addison disease, hypoparathyroidism, and mucocutaneous candidiasis. A subset of patients with this disease also have a high prevalence of autoimmune thyroiditis and hypothyroidism and a novel mutation in the AIRE gene that is inherited in an autosomal dominant fashion. [42] Autoimmune polyendocrinopathy type 2 (Schmidt syndrome) is associated with adrenal insufficiency and hypothyroidism.
Iodine deficiency or excess
Worldwide, iodine deficiency is the most common cause of hypothyroidism. Excess iodine, as in radiocontrast dyes, amiodarone, health tonics (herbal and dietary supplements), and seaweed, can transiently inhibit iodide organification and thyroid hormone synthesis (the Wolff-Chaikoff effect). Most healthy individuals have a physiologic escape from this effect after 10-14 days. In patients with iodine overload, the sodium-iodide symporter shuts down, and this allows intracellular iodine levels to drop and hormone secretion to resume.
The Wolff-Chaikoff effect is short-lived because the sodium-iodide symporter is capable of rapid downregulation. However, exposure to excess iodine can produce more profound and sustained hypothyroidism in individuals with abnormal thyroid glands (eg, from autoimmune thyroiditis, subtotal thyroidectomy, or prior radioiodine therapy). [43]
Central hypothyroidism
Central hypothyroidism (secondary or tertiary) results when the hypothalamic-pituitary axis is damaged. The following potential causes should be considered [44, 45] :
· Pituitary adenoma
· Tumors impinging on the hypothalamus
· Lymphocytic hypophysitis
· Sheehan syndrome
· History of brain or pituitary irradiation
· Drugs (eg, dopamine, prednisone, or opioids)
· Congenital nongoitrous hypothyroidism type 4
· TRH resistance
· TRH deficiency
Tumors in or around the pituitary cause impaired pituitary function by exerting pressure on normal pituitary cells and thereby affect the secretion of TRH, TSH, or both. Radiation, hypophysitis, and Sheehan syndrome cause death of these cells. Drugs such as dopamine and corticosteroids result in decreased TSH secretion.
Congenital nongoitrous hypothyroidism type 4 is caused by a mutation in the TSHB gene and is inherited in an autosomal recessive pattern. Patients have hypothyroidism and a low TSH level that does not rise with administration of TRH. Many patients with this condition were the products of consanguineous unions. [46]
TRH resistance is a rare condition caused by a mutation in the TRHR gene and is inherited in an autosomal recessive manner. Patients with this condition have hypothyroidism and insensitivity to thyrotropin secretion. [47] .
TRH deficiency is caused by mutation in the TRH gene and is inherited in an autosomal recessive manner. [48] The index case was a girl evaluated for short stature who was found to have an isolated deficiency of TRH. [10]
Epidemiology
The National Health and Nutrition Examination Survey (NHANES 1999-2002) of 4392 individuals reflecting the US population reported hypothyroidism (defined as TSH levels exceeding 4.5 mIU/L) in 3.7% of the population. [49] Hypothyroidism is more common in women with small body size at birth and low body mass index during childhood. [50]
Iodine deficiency as a cause of hypothyroidism is more common in less-developed countries. Routine supplementation of salt, flour, and other food staples with iodine has decreased the rates of iodine deficiency.
World Health Organization (WHO) data from 130 countries taken from January 1994 through December 2006 found inadequate iodine nutrition in 30.6% of the population. The WHO recommends urinary iodine concentrations between 100 and 199 μg/L in the general population and a range of 150-249 μg/L in pregnant women. In developed countries, death caused by hypothyroidism is uncommon.
Age-related demographics
The frequency of hypothyroidism, goiters, and thyroid nodules increases with age. Hypothyroidism is most prevalent in elderly populations, with 2-20% of older age groups having some form of hypothyroidism. The Framingham study found hypothyroidism (TSH > 10 mIU/L) in 5.9% of women and 2.4% of men older than 60 years. [51] In NHANES 1999-2002, the odds of having hypothyroidism were 5 times greater in persons aged 80 years and older than in individuals aged 12-49 years. [49]
Sex-related demographics
Community studies use slightly different criteria for determining hypothyroidism; therefore, female-to-male ratios vary. Generally, the prevalence of thyroid disease is reportedly 2-8 times higher in females.
Race-related demographics
NHANES 1999-2002 reported that the prevalence of hypothyroidism (including the subclinical form) was higher in whites (5.1%) and Mexican Americans than in African Americans (1.7%). African Americans tend to have lower median TSH values. [49]
Prognosis
Undertreatment of hypothyroidism leads to disease progression, with gradual worsening of symptoms and further metabolic derangements. Ultimately, untreated hypothyroidism can result in profound coma or even death, and in infants it can cause irreversible intellectual disability.
Thyroid hormone therapy reverses the signs and symptoms of hypothyroidism. With treatment, other secondarily affected laboratory values (eg, circulating lipid levels and elevated prolactin levels) should improve.
Using disease-specific (ThyPRO questionnaire) and generic (36-item Short Form Health Survey [SF-36]) measures of health-related quality of life (HRQL), Winther et al discovered that levothyroxine treatment resulted in improvement in some, but not all, aspects of HRQL in patients with hypothyroidism resulting from autoimmune thyroiditis. This included significant improvements in nine of 13 ThyPRO scales after 6 weeks of therapy. [52]
Nonetheless, a study by Sohn et al found that in individuals with hypothyroidism (defined in this study as overt hypothyroidism in patients undergoing long-term levothyroxine treatment), there was significantly higher all-cause mortality than in persons without hypothyroidism, with the adjusted hazard ratio (HR) being 1.14. Over a mean 6-year follow-up, the death rate for patients with hypothyroidism was 5.2%, compared with 3.9% for the controls. [53]
A study by Chang et al suggested that subclinical and overt hypothyroidism are linked to reduced renal function, with subclinical hypothyroidism raising the risk of chronic kidney disease (estimated glomerular filtration rate of below 60 mL/min/1.73m2) by 2.03-fold, and overt hypothyroidism increasing the risk by 7.68-fold. The increased risk remained significant even after other potential risk factors for chronic kidney disease were taken into account. The study also indicated, however, that subclinical and overt hypothyroidism have a lesser effect on proteinuria risk. [54]
Similarly, a prospective observational study by Tsuda et al indicated that in patients with chronic kidney disease, subclinical hypothyroidism is an independent risk factor for poor outcome. The report found, for example, that in chronic kidney disease patients with subclinical hypothyroidism, the hazard ratio for a composite endpoint of doubling of serum creatinine, end-stage renal disease, or death was 1.61, compared with euthyroid patients. [55]
Research indicates that hypothyroidism may be an independent risk factor for NAFLD. A study by Almomani et al did not find that thyroid hormone replacement reduced the risk by a statistically significant amount, although other reports have suggested that prevention or reversal of NAFLD is potentially possible with such replacement. [56]
A study by Sato et al suggested that in patients with heart failure, those with subclinical hypothyroidism have a worse prognosis, finding a significant increase in the rates of cardiac events and all-cause mortality in heart failure patients in the study with subclinical hypothyroidism compared with those who were euthyroid. [57]
In a meta-analysis by Tsai et al, overt hypothyroidism was significantly associated with increased all-cause mortality, but not cardiovascular mortality, among the elderly. [58]
A study by Thvilum et al indicated that hypothyroidism increases the risk of dementia, with the risk rising by 12% for every 6 months of elevated TSH. [59]
Patient Education
Emphasize proper compliance at each visit. Clearly discuss the lifelong nature of hypothyroidism, the need for lifelong levothyroxine therapy, the proper way to take medicine, and the need for TSH testing at least annually.
Patients should take thyroid hormone as a single daily dose. Thyroid hormone is better absorbed in the small bowel; therefore, absorption can be affected by malabsorptive states, small bowel disease (eg, celiac sprue), and the patient’s age. Many drugs (eg, iron, calcium carbonate, calcium acetate aluminum hydroxide, sucralfate, raloxifene, and proton pump inhibitors) can interfere with absorption and therefore should not be taken within 2-4 hours of LT4 administration. [60] Continuous tube feedings interfere with thyroid hormone absorption; the tube feedings should be interrupted for at least 30-60 minutes before and after hormone administration.
For patients with malabsorption issues, such as those with celiac disease, Helicobacter pylori infection, lactose intolerance, inflammatory bowel disease, atrophic gastritis, or status post bariatric surgery, liquid LT4 formulations may be more efficient than tablet form for replacement and suppressive therapy. For those without malabsorption, either form is sufficient. [61]
The effects of using softgel LT4 may also prove beneficial in malabsorptive states, and its effects have been found to be consistent with the liquid formulation. [62] For both liquid and softgel LT4 formulations, cost is often a limiting factor for use.
Although it has generally been recommended that thyroid hormone be administered in the morning before breakfast, studies of bedtime dosing have demonstrated acceptable absorption if the hormone is taken 3 or more hours after the evening meal. [63, 64]
Estrogen/progestin oral contraceptives and pregnancy are associated with changes in thyroid-binding globulin. These changes may impact thyroid hormone dosing.
For patient education information, see the Thyroid & Metabolism Center as well as Thyroid Problems and Chronic Fatigue Syndrome.
-
The hypothalamic-pituitary-thyroid axis. Levels of circulating thyroid hormones are regulated by a complex feedback system involving the hypothalamus and pituitary gland.









