Background
In the late 19th century, serum was found to contain a nonspecific heat-labile complementary principle that interacted with antibodies to induce bacteriolysis. Ehrlich and Morgan termed this factor complement.
The complement system as understood today is a multimolecular system composed of more than 32 proteins and consisting of serum proteins, serosal proteins, and cell membrane receptors that bind to complement fragments. They constitute 10% of the globulin fraction of serum. Many of these proteins are designated by the letter C (C1, 4, 2, 3, 5, 6, 7, 8, and 9) and are assigned numbers in the order of their discovery. See tables 1-6 in Pathophysiology for more information.
Pathophysiology
The complement system consists of 7 serum and 9 membrane regulatory proteins, 1 serosal regulatory protein, and 8 cell membrane receptors that bind complement fragments. Most are synthesized mainly by the liver. Exceptions are C1, factor D, and properdin. These are probably synthesized by macrophages and even by T lymphocytes.
The tables in this section were adapted from Middleton's Textbook of Allergy and Immunology, 6th edition; data are chiefly from Morley BJ, Walport MJ, eds: The complement facts book, San Diego, 2000. Acute-phase levels are estimates based on limited data.
Activation
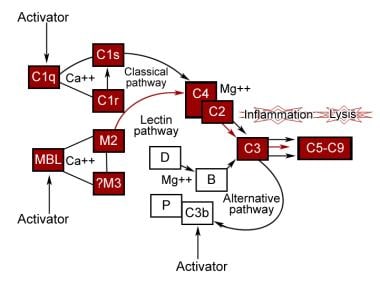
The complement system functions as an interactive sequence, with one reaction leading to another in the form of a cascade. It is initiated by a wide variety of substances and has 2 phases. In the first phase, a series of specific interactions leads to formation of intrinsic complement proteinase, termed C3 convertase. Depending on the nature of complement activators, the classic pathway, the alternative pathway, or the more recently discovered lectin pathway is activated predominantly to produce C3 convertase. Each of these pathways uses different proteins. The second phase for each involves cleavage of C3b, generating multiple biologically important fragments and large, potentially cytolytic complexes. See the image below.
Classic pathway
This pathway has 2 units. One, the recognition unit, consists of a trimolecular complex of C1q, 2 molecules of C1r, and 2 molecules of C1s held together by calcium. The other is an activation unit of C2, C3, and C4. The sequence starts with the binding of 2 or more C1q recognition units to the Fc nonantigen binding part of IgG and IgM molecules. This induces a conformational change, leading to autoactivation of C1r that then cleaves C1s to its active state. This then acts similarly to C1 esterase and cleaves C2 and C4 to form C2aC4b, which is the C3 esterase that cleaves C3 to form C3b. C1q can also be activated by mycoplasmal organisms, RNA viruses, bacterial endotoxins, and cell membranes of some organelles without the presence of antibody.
Table 1. Proteins of the Human Complement (C) System, Classical Pathway* (Open Table in a new window)
Component |
Mol Wt (~kD) |
Normal Serum levels (µg/mL) |
Acute Phase Serum levels (% Increase) |
Chromosomal Location |
C1q |
460 |
70 |
13% |
1p34-36.3 |
C1r |
83 |
34 |
|
12p13 |
C1s |
83 |
31 |
47% |
12p13 |
C4 |
200 |
600 |
34% |
6p21.3 |
C2 |
102 |
23 |
|
6p21.3 |
*C-reactive protein (CRP, not shown) leads to classic pathway activation analogous to lectin pathway activation by MBL and ficolins. |
||||
Alternate pathway
This was discovered by Pillemer and colleagues in 1954 but was recognized universally some years later. This pathway is activated by viruses, fungi, bacteria, parasites, cobra venom, immunoglobulin A, and polysaccharides and forms an important part of the defense mechanism independent of the immune response. Here, C3b binds to factor B that is cleaved by factor D to Bb. C3bBb complex then acts as the C3 convertase and generates more C3b through an amplification loop. Binding of factor H to C3b increases its inactivation by factor I. Properdin stabilizes it, preventing its inactivation by factors H and I. The alternate pathway does not result in a truly nonspecific activation of complement because it requires specific types of compounds for activation. It simply does not require specific antigen-antibody interactions for initiation.
Table 2. Proteins of the Human Complement (C) System, Alternative Pathway (Open Table in a new window)
Component |
Mol Wt (~kD) |
Normal Serum levels (µg/mL) |
Acute Phase Serum levels (% Increase) |
Chromosomal Location |
Factor D |
25 |
2 |
|
19 |
Factor B |
93 |
93 |
65% |
6p21.1-21.3 |
Lectin pathway
The lectin or mannan-binding pathway is activated similar to the classic pathway except that lectin replaces the antibody and mannan-binding lectin–associated proteases replace C1 enzymatic activity. Instead, mannan-binding lectin binds to sugar residues on the surface of a pathogen. Mannan-binding lectin is associated with serine proteases, similar to the C1r and C1s subcomponents of the classic pathway, that also activate C4 and C2, forming the classical pathway C3 convertase C4b2a.
Table 3. Proteins of the Human Complement (C) System, Lectin Pathway (Open Table in a new window)
Component |
Mol Wt (~kD) |
Normal Serum levels (µg/mL) |
Acute Phase Serum levels (% Increase) |
Chromosomal Location |
MBL |
288-576 |
2 |
Up to 1000% |
10q11.2-21.0 |
MASP-1 |
97 |
6 |
... |
3q27-28 |
MASP-2 |
80 |
... |
... |
1p36.23-36.31 |
MASP-3 |
105 |
... |
... |
3q27-28 |
Map19 |
19 |
... |
... |
1p36.23-36.31 |
L-ficolin/P35 |
630 |
13.7 |
... |
9 |
H-ficolin/Hakata antigen |
630 |
15 |
... |
... |
MBL = mannan-binding lectin; MASP = MBL-associated serine protease |
||||
Membrane attack complex
Only 5 proteins are involved in the direct killing of cells. C2a4b3b complex from the classic or MBL pathways or C3bBb from the alternative pathway cleaves C5. C5b activates the terminal complement pathway by associating with C6, C7, and C8 to form macromolecular complexes denoted as C5b-8, which can bind to cell membranes. C9 binds to this complex, inducing a conformational change that exposes a new antigenic site known as C9 neoantigen. Additional C9 molecules bind to membrane-bound C5b-9, forming ringlike pores, leading to transmembrane channels that cause cell lysis.
Table 4. Proteins of the Human Complement (C) System, C3 and Terminal Components (Open Table in a new window)
Component |
Mol Wt (~kD) |
Normal Serum levels (µg/mL) |
Acute Phase Serum levels (% Increase) |
Chromosomal Location |
C3 |
185 |
1200 |
30% |
19p13.2-13.3 |
C5 |
190 |
75 |
55% |
9q33 |
C6 |
128 |
45 |
|
5p12-14 |
C7 |
120 |
55 |
|
5p12-14 |
C8 |
163 |
68 |
|
1p32; 9q34.3 |
C9 |
79 |
60 |
49% |
5p13 |
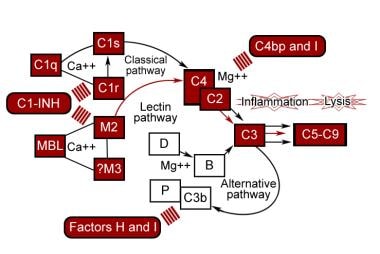
Regulation
The complement system serves a very important role in host defense, but if it is directed against self, it can lead to serious illness. Therefore, it is closely regulated at almost every step. See the image below.
Classic pathway
The classic pathway requires the identification of a target by the presence of an antibody. C1 inhibitor (C1-INH) inhibits C1r and C1s by binding covalently to them, causing disassembly of C1 macromolecular complex. The inhibitor is synthesized in the liver and blood monocytes; its gene is located on chromosome 11. C2a4b is very labile and undergoes spontaneous decay with release of C2a and loss of enzymatic activity. C4 binding protein binds C4, which accelerates its rate of dissociation from C2a and makes C4b more susceptible to proteolysis by factor I. Membrane-bound decay-accelerating factor (DAF) promotes release of C2a from C4b2a by physically interfering with C4b and C2a association.
Table 5. Proteins of the Human Complement (C) System, Control Proteins in Serum (Open Table in a new window)
Component |
Mol Wt (~kD) |
Normal Serum levels (µg/mL) |
Acute Phase Serum levels (% Increase) |
Chromosomal Location |
C1 inhibitor |
105 |
150 |
21% |
11q11-13.1 |
C4-binding protein |
550 |
225 |
... |
1q3.2 |
Factor H |
150 |
550 |
... |
1q3.2 |
Factor I |
88 |
35 |
... |
4q25 |
Properdin |
223 |
5 |
-14% |
Xp11.23-11.30 |
S protein |
75 |
340 |
... |
17q11 |
Clusterin |
80 |
340 |
... |
8p21 |
Anaphylotoxin inactivator |
290 |
35 |
... |
8p22-23, 10 |
Alternative pathway
Carbohydrate composition and its sialic acid content on the cell surface play an important role in the activation of the alternate pathway. Sialic acid blocks activation by favoring the binding of factor H to C3b, which is then inactivated by factor I. [1] Microorganisms lacking sialic acid are killed, whereas human cells covered with glycophorin A, a sialoglycoprotein, are protected.
C3bBb is relatively labile and undergoes spontaneous decay through dissociation of Bb. Properdin is synthesized by monocytes and T lymphocytes. Properdin binds to C3bBb and stabilizes it, preventing its decay. Factor H competes with factor B for binding to C3b and displaces Bb from C3bBb. It accelerates the inactivation of C3b by factor I. Factor I inactivates C3b to iC3b, a molecule that cannot function enzymatically. Complement receptor 1 (CR1) has factor H–like activity, permitting factor I to cleave C3b. Membrane cofactor protein also has factor H–like activity, mainly for alternative C3 convertase.
Membrane attack complex
Homologous restriction factor, C8 binding protein, is a cell membrane protein with significant sequence homology to both C8 and C9 and is widely distributed on peripheral blood cells. It prevents the interaction of C8 and C9. Membrane-bound CD59, also known as homologous restriction factor 20, prevents the binding of C5b-8 to C9 and inhibits the unfolding of C9 that is required for polymerization and formation of macroscopic pores in the cell membrane. S protein (vitronectin) binds to C5b-7 and abolishes its activity. SP-40,40 (clusterin) has effects similar to vitronectin.
Table 6. Proteins of the Human Complement (C) System, Membrane Receptor and Control Proteins (Open Table in a new window)
Component |
Mol Wt (~kD) |
Ligands |
Chromosomal Location |
DAF |
70 |
C4b2a |
1q3.2 |
MCFP |
60 |
C3b |
1q3.2 |
CD59 |
20 |
C8, C9 |
11p13-14 |
CR1 |
250 |
C3b, C4b |
1q3.2 |
CR2 |
145 |
C3dg, C3d, EBV |
1q3.2 |
CR3 |
250 |
iC3b, LPS, β-glucans |
16p11-13.1, 21q22.3 |
CR4 |
245 |
iC3b, LPS |
16p11.2, 21q22.3 |
C3aR |
100 |
C3a, C4a |
12p13 |
C5aR |
50 |
C5a |
19q13.3-13.4 |
DAF = decay-accelerating factor; MCFP = membrane cofactor protein; EBV = Epstein-Barr virus; LPS = lipopolysaccharides |
|||
Biologic Effects
The biologic effects of complement include promotion of chemotaxis and anaphylaxis, opsonization and phagocytosis of microorganisms, and removal of immune complexes from the circulation. Most complement components are acute phase reactants, and their concentration increases in states of infection, trauma, and injury.
C3a and C5a are anaphylatoxins and bind to mast cells, triggering the release of histamine and other mediators, leading to vasodilation, erythema, and swelling. When C3a or C5a is injected into the skin, it elicits an immediate wheal and flare response, similar to that found with allergen injection into the skin of individuals with allergies. C3a and C5a also produce bronchoconstriction with human tracheal or bronchial muscle strips in vitro. C5a is a major stimulus for influx of neutrophils, basophils, monocytes, and eosinophils.
C3b fixes to the antigen-antibody complex and permits its adherence to cells (eg, neutrophils, basophils, eosinophils, monocytes) that have receptors for C3b. This particular action of opsonization helps in phagocytosis. C3b-coated particles also bind to B lymphocytes and activate them to enhance the primary antibody response. Immune complexes formed in the circulation are coated with C3b and bind to erythrocytes, which then transport them to the liver and spleen for removal. This process maintains the solubility of the immune complexes. In the early phases of viral infection, when the amount of antibody is limited, the fixation of C3b to the viral antigen-antibody complex increases neutralization.
The terminal components of the complement system result in lysis of virus-infected cells, tumor cells, and some gram-negative microorganisms. They also have a role in neutralization of endotoxins in vitro and protection from their lethal effects in experimental animal models. C5b-9 neoantigen is found in the muscle in dermatomyositis, implying that the terminal complement system may have a role in the pathophysiology of that disease.
-
Activation of the complement pathways.
-
Control proteins of the complement pathways.