Practice Essentials
Hyperthyroidism is a set of disorders that involve excess synthesis and secretion of thyroid hormones by the thyroid gland, which leads to the hypermetabolic condition of thyrotoxicosis. [1, 2] The most common forms of hyperthyroidism include diffuse toxic goiter (Graves disease), toxic multinodular goiter (Plummer disease), and toxic adenoma. In thyrotoxicosis, thyroid hormone levels are elevated with or without increased thyroid hormone synthesis. The most common forms of thyrotoxicosis are caused by excess intake of the thyroid hormone medication levothyroxine or result from a temporary excess release of thyroid hormone due to subacute thyroiditis. The most reliable screening measure of thyroid function is the thyroid-stimulating hormone (TSH) level. Treatment of hyperthyroidism includes symptom relief, as well as antithyroid pharmacotherapy, radioactive iodine-131 (131I) therapy (the preferred treatment of hyperthyroidism among US thyroid specialists), or thyroidectomy. Thyrotoxicosis from subacute thyroiditis is temporary and self-resolving, and the treatment is also symptom relief. See the image below.
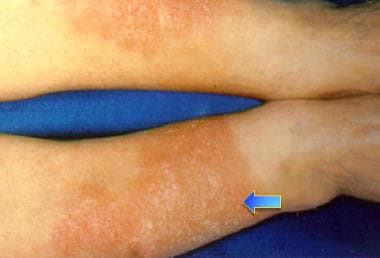 Bilateral erythematous infiltrative plaques on lower extremities in 42-year-old man with Graves disease are consistent with pretibial myxedema. Myxedematous changes of skin usually occur in pretibial areas and resemble orange peel in color and texture.
Bilateral erythematous infiltrative plaques on lower extremities in 42-year-old man with Graves disease are consistent with pretibial myxedema. Myxedematous changes of skin usually occur in pretibial areas and resemble orange peel in color and texture.
Signs and symptoms
Common symptoms of hyperthyroidism and thyrotoxicosis include the following:
-
Nervousness
-
Anxiety
-
Increased perspiration
-
Heat intolerance
-
Hyperactivity
-
Palpitations
Common signs of hyperthyroidism and thyrotoxicosis include the following:
-
Tachycardia or atrial arrhythmia
-
Systolic hypertension with wide pulse pressure
-
Warm, moist, smooth skin
-
Lid lag
-
Stare
-
Hand tremor
-
Muscle weakness
-
Weight loss despite increased appetite (although a few patients may gain weight, if excessive intake outstrips weight loss)
-
Reduction in menstrual flow or oligomenorrhea
Presentation of thyrotoxicosis varies, as follows [3] :
-
Younger patients tend to exhibit symptoms of sympathetic activation (eg, anxiety, hyperactivity, tremor)
-
Older patients have more cardiovascular symptoms (eg, dyspnea, atrial fibrillation) and unexplained weight loss
-
Patients with Graves disease often have more marked symptoms than patients with thyrotoxicosis from other causes
-
Ophthalmopathy (eg, periorbital edema, diplopia, or proptosis) and pretibial myxedema dermopathy specifically occur with Graves disease
-
Elevated thyroid hormone levels associated with subacute thyroiditis may occur as part of a postviral syndrome (subacute granulomatous thyroiditis) or within a year of the end of a pregnancy (postpartum subacute thyroiditis)
See Clinical Presentation for more detail.
Diagnosis
Thyroid function tests for hyperthyroidism and thyrotoxicosis are as follows:
-
Thyroid-stimulating hormone (TSH)
-
Free thyroxine (FT4) or free thyroxine index (FTI—total T4 multiplied by the correction for thyroid hormone binding)
-
Total triiodothyronine (T3)
Thyroid function study results in hyperthyroidism and thyrotoxicosis are as follows:
-
Hyperthyroidism and thyrotoxicosis are marked by suppressed TSH levels and elevated T3 and T4 levels
-
Patients with milder hyperthyroidism may have elevation of T3 levels only with a suppressed TSH level
-
Subclinical hyperthyroidism features decreased TSH and normal T3 and T4 levels
Autoantibody tests for hyperthyroidism are as follows:
-
Anti–thyroid peroxidase (anti-TPO) antibody - Elevation with autoimmune thyroid disease found in 85% of Graves patients
-
Thyroid-stimulating antibody (TSab) - Also known as thyroid-stimulating immunoglobulin (TSI), long-acting thyroid stimulator (LATS), or TSH-receptor antibody (TRab); found in 63-81% of Graves disease; a positive test is diagnostic and specific for Graves disease
Autoantibody titers in hyperthyroidism and thyrotoxicosis are as follows:
-
Graves disease - Significantly elevated anti-TPO, elevated TSI ab
-
Toxic multinodular goiter - Low or absent anti-TPO and negative TSI ab
-
Toxic adenoma - Low or absent anti-TPO and negative TSI ab
-
Patients without active thyroid disease may have mildly positive anti-TPO and TSI ab
-
Subacute thyroiditis - Low or absent anti-TPO and negative TSI ab
If the etiology of elevated thyroid hormone levels is not clear after physical examination and other laboratory tests, it can be confirmed by scintigraphy: the degree and pattern of isotope uptake indicate the type of thyroid disorder. Findings are as follows:
-
Graves disease – Diffuse enlargement of both thyroid lobes, with uniform uptake of isotope and elevated radioactive iodine uptake
-
Toxic multinodular goiter -- Irregular areas of relatively diminished and occasionally increased uptake; overall radioactive iodine uptake is mildly to moderately increased
-
Subacute thyroiditis – Very low radioactive iodine uptake, either with a painful thyroid (subacute granulomatous thyroiditis) or occurring within a year of pregnancy (postpartum subacute thyroiditis)
See Workup for more detail.
Management
Treatment of hyperthyroidism and thyrotoxicosis includes symptom relief, while hyperthyroidism also requires therapy with antithyroid medications, radioactive iodine-131 (131I), or thyroidectomy. Symptomatic treatment is as follows:
-
Oral rehydration for dehydrated patients
-
Beta-blockers for relief of neurologic and cardiovascular symptoms
-
For mild ophthalmopathy, saline eye drops as needed and tight-fitting sunglasses for outdoors
-
For vision-threatening ophthalmopathy, high-dose glucocorticoids, with consideration of orbital decompression surgery, ocular radiation therapy, or a recently approved treatment from the US Food and Drug Administration (FDA), teprotumumab-trbw, a monoclonal antibody that blocks the insulin-like growth factor-1 receptor (IGF-1R) and ameliorates proptosis by reducing inflammation and preventing muscle and fat-tissue remodeling in the orbit
Antithyroid drug treatment is as follows:
-
Used for long-term control of hyperthyroidism in children, adolescents, and pregnant women
-
In adult men and nonpregnant women, used to control hyperthyroidism before definitive therapy with radioactive iodine
-
Methimazole is more potent and longer-acting than propylthiouracil
-
Propylthiouracil is reserved for use in thyroid storm, first trimester of pregnancy, and methimazole allergy or intolerance
-
Antithyroid drug doses are titrated every 4 weeks until thyroid functions normalize
-
Patients with Graves disease may experience remission after treatment for 12-18 months, but recurrences are common within the following year
-
Toxic multinodular goiter and toxic adenoma will not go into remission
Radioactive iodine treatment is as follows:
-
Preferred therapy for hyperthyroidism
-
Administered orally as a single dose in capsule or liquid form
-
Causes fibrosis and destruction of the thyroid over weeks to many months
-
Hypothyroidism is expected
-
Pregnancy, breast feeding, and recent lactation are contraindications
-
Radioactive iodine should be avoided in children younger than 5 years [4]
-
Radioactive iodine is usually not given to patients with severe ophthalmopathy
-
Radioactive iodine is usually not given to patients who cannot comply with physician restrictions for avoidance of radiation exposure to others
Thyroidectomy is reserved for special circumstances, including the following:
-
Severe hyperthyroidism in children
-
Pregnant women who are noncompliant with or intolerant of antithyroid medication
-
Patients with very large goiters or severe ophthalmopathy
-
Patients who refuse radioactive iodine therapy
-
Refractory amiodarone-induced hyperthyroidism
-
Patients who require normalization of thyroid functions quickly, such as pregnant women, women who desire pregnancy in the next 6 months, or patients with unstable cardiac conditions
Guidelines for the management of hyperthyroidism and other causes of thyrotoxicosis were developed by the American Thyroid Association and the American Association of Clinical Endocrinologists; [4] these were updated in 2016. [5]
See Treatment and Medication for more detail.
Background
Hyperthyroidism is a set of disorders that involve excess synthesis and secretion of thyroid hormones by the thyroid gland. The resulting elevation in levels of free thyroxine (FT4), free triiodothyronine (FT3), or both leads to the hypermetabolic condition of thyrotoxicosis.
Thus, although many clinicians (endocrinologists excluded) use the terms hyperthyroidism and thyrotoxicosis interchangeably, the 2 words have distinct meanings. For example, both exogenous thyroid hormone intake and subacute thyroiditis can cause thyrotoxicosis, but neither constitutes hyperthyroidism, because the conditions are not associated with new hormone production.
The most common forms of hyperthyroidism include diffuse toxic goiter (Graves disease), toxic multinodular goiter (Plummer disease), and toxic adenoma (see Etiology). Together with subacute thyroiditis, these conditions constitute 85-90% of all causes of elevated thyroid hormone levels.
The most reliable screening measure of thyroid function in the healthy ambulatory adult population is the TSH level. The degree of thyrotoxicosis is determined by measurement of thyroid hormone levels. Autoantibody testing, and nuclear thyroid scintigraphy in some cases, can provide useful etiologic information. (See Workup.)
Treatment of hyperthyroidism includes symptom relief, as well as therapy with antithyroid medications, radioactive iodine, or thyroidectomy. However, antithyroid medications are not effective in thyrotoxicosis from subacute thyroiditis, because these cases result from release of preformed thyroid hormone. (See Treatment.)
For further information, see Pediatric Hyperthyroidism and Subacute Thyroiditis.
Pathophysiology
Normally, the secretion of thyroid hormone is controlled by a complex feedback mechanism involving the interaction of stimulatory and inhibitory factors (see the image below). Thyrotropin-releasing hormone (TRH) from the hypothalamus stimulates the pituitary to release TSH.
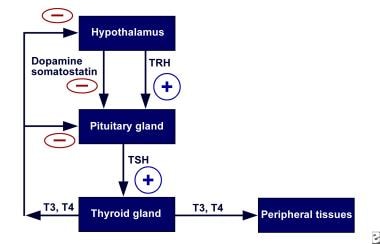 Hypothalamic-pituitary-thyroid axis feedback. Schematic representation of negative feedback system that regulates thyroid hormone levels. TRH = thyrotropin-releasing hormone; TSH = thyroid-stimulating hormone.
Hypothalamic-pituitary-thyroid axis feedback. Schematic representation of negative feedback system that regulates thyroid hormone levels. TRH = thyrotropin-releasing hormone; TSH = thyroid-stimulating hormone.
Binding of TSH to receptors on the thyroid gland leads to the release of thyroid hormones—primarily T4 and to a lesser extent T3. In turn, elevated levels of these hormones act on the hypothalamus to decrease TRH secretion and thus the synthesis of TSH.
Synthesis of thyroid hormone requires iodine. Dietary inorganic iodide is transported into the gland by an iodide transporter, converted to iodine, and bound to thyroglobulin by the enzyme thyroid peroxidase through a process called organification. This results in the formation of monoiodotyrosine (MIT) and diiodotyrosine (DIT), which are coupled to form T3 and T4; these are then stored with thyroglobulin in the thyroid’s follicular lumen. The thyroid contains a large supply of its preformed hormones.
Thyroid hormones diffuse into the peripheral circulation. More than 99.9% of T4 and T3 in the peripheral circulation is bound to plasma proteins and is inactive. Free T3 is 20-100 times more biologically active than free T4. Free T3 acts by binding to nuclear receptors (DNA-binding proteins in cell nuclei), regulating the transcription of various cellular proteins.
Any process that causes an increase in the peripheral circulation of unbound thyroid hormone can cause thyrotoxicosis. Disturbances of the normal homeostatic mechanism can occur at the level of the pituitary gland, the thyroid gland, or in the periphery. Regardless of etiology, the result is an increase in transcription in cellular proteins, causing an increase in the basal metabolic rate. In many ways, signs and symptoms of hyperthyroidism resemble a state of catecholamine excess, and adrenergic blockade can improve these symptoms.
In Graves disease, a circulating autoantibody against the thyrotropin receptor provides continuous stimulation of the thyroid gland. This stimulatory immunoglobulin is diagnostic for Graves disease and has been called long-acting thyroid stimulator (LATS), thyroid-stimulating immunoglobulin (TSI), thyroid-stimulating antibody (TSab), and TSH-receptor antibody (TRab). [6] These antibodies stimulate the production and release of thyroid hormones and thyroglobulin; they also stimulate iodine uptake, protein synthesis, and thyroid gland growth. Anti–thyroid peroxidase (anti-TPO) antibody is assessed in a nonspecific test for autoimmune thyroid disease. Although the anti-TPO antibody is not diagnostic for Graves disease, it is present in 85% of patients with the disorder and can be quickly measured in local laboratories. [7]
Ophthalmopathy
The underlying pathophysiology of Graves ophthalmopathy (also called thyroid-associated orbitopathy) is not completely characterized. It most likely involves an antibody reaction against the TSH receptor that results in activation of T cells against tissues in the retro-orbital space that share antigenic epitopes with thyroid follicular cells.
These immune processes lead to an active phase of inflammation, with lymphocyte infiltration of the orbital tissue and release of cytokines that stimulate orbital fibroblasts to multiply and produce mucopolysaccharides (glycosaminoglycans), which absorb water. In consequence, the extraocular muscles thicken and the adipose and connective tissue of the retro-orbit increase in volume.
Cigarette smoking and a high TSH-receptor autoantibody level are significant risk factors for ophthalmopathy. In addition, patients who smoke appear to be more likely to experience worsening of their ophthalmopathy if treated with radioactive iodine, as do patients who have high pretreatment T3 levels and posttherapy hypothyroidism.
Etiology
Genetic factors appear to influence the incidence of thyrotoxicosis. Autoimmune thyroid disease, including Hashimoto hypothyroidism and Graves disease, often occurs in multiple members of a family.
Several genetic syndromes have been associated with hyperthyroidism, especially autoimmune thyroid disease. McCune-Albright syndrome is caused by mutations in the GNAS gene. This gene encodes the stimulatory G-protein alpha subunit, which is a key component of many signal transduction pathways. Patients present with the classic triad of polyostotic fibrous dysplasia, irregular café-au-lait spots, and precocious puberty. The syndrome may also include facial asymmetry, Cushing syndrome, hyperthyroidism, and acromegaly. [8]
A number of disorders of thyroid function have been found to be caused by mutations in the TSHR gene, which encodes the TSH receptor protein. These disorders include the following:
-
Familial gestational hyperthyroidism
-
One type of nonimmune hyperthyroidism
-
Congenital nongoiterous thyrotoxicosis
-
Toxic thyroid adenoma with somatic mutation
Type II autoimmune polyendocrine syndrome is associated with hyperthyroidism and hypothyroidism, as well as type 1 diabetes mellitus and adrenal insufficiency. Patients may also have immune deficiency, as manifested by chronic mucosal candidiasis. [9]
Autoimmune thyroid disease has a higher prevalence in patients with human leukocyte antigen (HLA)-DRw3 and HLA-B89. Graves disease is felt to be an HLA-related, organ-specific defect in suppressor T-cell function. Similarly, subacute painful or granulomatous thyroiditis occurs more frequently in patients with HLA-Bw35. Like other immune diseases, these thyroid conditions occur more frequently in women than in men.
With the availability of genome-wide association studies, more than a dozen genes and gene regions have been found to be associated with an increased risk for development of thyrotoxicosis, particularly Graves disease. [10, 11, 12, 13, 14, 15] Unsurprisingly, these studies have shown associations between these same genes and the development of other endocrine autoimmune disorders, such as type 1 diabetes mellitus.
The loci for which specific function can be deduced appear to involve genes related to HLA, non-HLA immune function, and thyroid function. [14] However, the odds ratios that have been determined generally indicate only a mildly increased risk for Graves disease.
Most of the genome-wide association studies have focused on diffuse toxic goiter (ie, Graves disease). One study, however, found an association between development of toxic multinodular goiter (Plummer disease) and a single-nucleotide polymorphism (SNP) in the TSHR gene. [16] . This SNP was seen in 9.6% of normal patients, 16.3% of patients with Graves disease, and 33.3% of patients with toxic multinodular goiter.
Iodine intake
Iodine intake also appears to influence the occurrence of thyrotoxicosis. Clearly, patients in borderline iodine-deficient areas of the world develop nodular goiter, often with areas of thyroid autonomy. When members of this population move to areas of sufficient iodine intake, thyrotoxicosis occurs. Evidence exists that iodine can act as an immune stimulator, precipitating autoimmune thyroid disease and acting as a substrate for additional thyroid hormone synthesis.
Graves disease
The most common cause of thyrotoxicosis is Graves disease (50-60% of cases). Graves disease is an organ-specific autoimmune disorder characterized by a variety of circulating antibodies, including common autoimmune antibodies, as well as anti-TPO and anti-TG antibodies.
The most important autoantibody is thyroid-stimulating antibody (TSab; also called TSI, LATS, or TRab), which is directed toward epitopes of the TSH receptor and acts as a TSH-receptor agonist. Like TSH, TSab binds to the TSH receptor on the thyroid follicular cells to activate thyroid hormone synthesis and release and thyroid gland growth (hypertrophy). This results in the characteristic picture of Graves thyrotoxicosis, with a diffusely enlarged thyroid, very high radioactive iodine uptake, and excessive thyroid hormone levels compared with a healthy thyroid (see the images below).
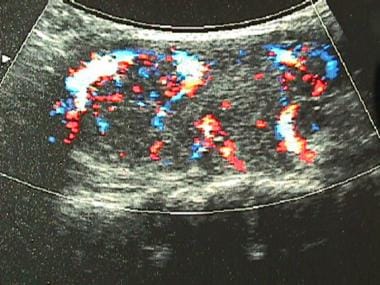 Color flow ultrasonogram in patient with Graves disease. Generalized hypervascularity is visible throughout gland (note red areas), which often can be heard as hum or bruit with stethoscope.
Color flow ultrasonogram in patient with Graves disease. Generalized hypervascularity is visible throughout gland (note red areas), which often can be heard as hum or bruit with stethoscope.
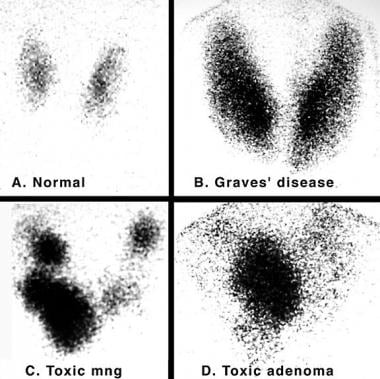 Iodine 123 (123I) nuclear scintigraphy: 123I scans of normal thyroid gland (A) and common hyperthyroid conditions with elevated radioiodine uptake, including Graves disease (B), toxic multinodular goiter (C), and toxic adenoma (D).
Iodine 123 (123I) nuclear scintigraphy: 123I scans of normal thyroid gland (A) and common hyperthyroid conditions with elevated radioiodine uptake, including Graves disease (B), toxic multinodular goiter (C), and toxic adenoma (D).
Thyroid hormone levels can be highly elevated in Graves disease. Clinical findings specific to Graves disease include thyroid ophthalmopathy (periorbital edema, chemosis [conjunctival edema], injection, or proptosis) and, rarely, dermopathy over the lower extremities. This autoimmune condition may be associated with other autoimmune diseases, such as pernicious anemia, myasthenia gravis, vitiligo, adrenal insufficiency, celiac disease, and type 1 diabetes mellitus.
In pregnant women with Graves disease, fetal or neonatal thyrotoxicosis can result from maternal TSH-receptor antibodies (TRabs) crossing the placenta. A literature review by van Dijk et al indicated that during pregnancy, neonatal thyrotoxicosis is a risk when the concentration of maternal TRabs reaches 4.4 U/L, a level 3.7 times the upper limit of normal. [17]
Subacute thyroiditis
The next most common cause of thyrotoxicosis is subacute thyroiditis (approximately 15-20% of cases), a destructive release of preformed thyroid hormone. A typical nuclear scintigraphy scan shows no radioactive iodine uptake (RAIU) in the thyrotoxic phase of the disease (see the images below). Thyroid hormone levels can be highly elevated in this condition.
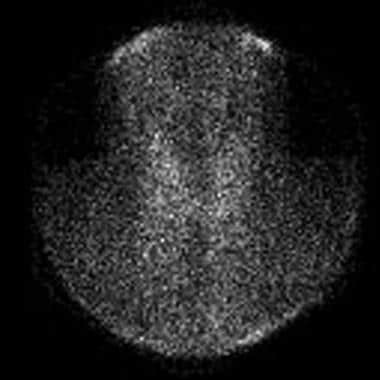 Absence of iodine 123 (123I) radioactive iodine uptake in patient with thyrotoxicosis and subacute painless or lymphocytic thyroiditis. Laboratory studies at time of scan demonstrated the following: thyroid-stimulating hormone (TSH), less than 0.06 mIU/mL; total thyroxine (T4), 21.2 µg/dL (reference range, 4.5-11); total triiodothyronine (T3), 213 ng/dL (reference range, 90-180); T3-to-T4 ratio, 10; and erythrocyte sedimentation rate (ESR), 10 mm/hr. Absence of thyroid uptake, low T3-to-T4 ratio, and low ESR confirm diagnosis of subacute painless thyroiditis.
Absence of iodine 123 (123I) radioactive iodine uptake in patient with thyrotoxicosis and subacute painless or lymphocytic thyroiditis. Laboratory studies at time of scan demonstrated the following: thyroid-stimulating hormone (TSH), less than 0.06 mIU/mL; total thyroxine (T4), 21.2 µg/dL (reference range, 4.5-11); total triiodothyronine (T3), 213 ng/dL (reference range, 90-180); T3-to-T4 ratio, 10; and erythrocyte sedimentation rate (ESR), 10 mm/hr. Absence of thyroid uptake, low T3-to-T4 ratio, and low ESR confirm diagnosis of subacute painless thyroiditis.
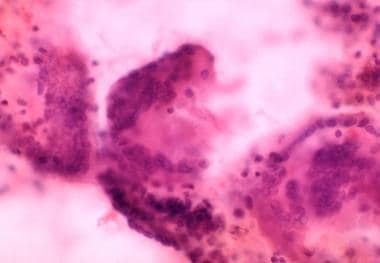 Three multinuclear giant cell granulomas observed in fine-needle aspiration biopsy of thyroid from patient with thyrotoxicosis from subacute painful or granulomatous thyroiditis.
Three multinuclear giant cell granulomas observed in fine-needle aspiration biopsy of thyroid from patient with thyrotoxicosis from subacute painful or granulomatous thyroiditis.
Toxic multinodular goiter
Toxic multinodular goiter (Plummer disease) accounts for 15-20% of thyrotoxicosis cases (see the image below). It occurs more commonly in elderly individuals, especially those with a long-standing goiter. Thyroid hormone excess develops very slowly over time and often is only mildly elevated at the time of diagnosis.
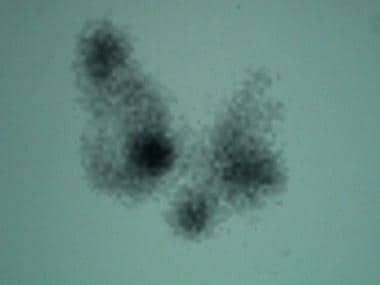 Scan in patient with toxic multinodular goiter. 5-Hour 123I-iodine uptake was elevated at 28% (normal 5-15%). Note multiple foci of variably increased tracer uptake.
Scan in patient with toxic multinodular goiter. 5-Hour 123I-iodine uptake was elevated at 28% (normal 5-15%). Note multiple foci of variably increased tracer uptake.
Symptoms of thyrotoxicosis are mild, often because only a slight elevation of thyroid hormone levels is present, and the signs and symptoms of thyrotoxicosis often are blunted (apathetic hyperthyroidism) in older patients. However, very high thyroid hormone levels may occur in this condition after high iodine intake (eg, with iodinated radiocontrast or amiodarone exposure).
Toxic adenoma
Toxic adenoma is caused by a single hyperfunctioning follicular thyroid adenoma. This disorder accounts for approximately 3-5% of thyrotoxicosis cases. The excess secretion of thyroid hormone occurs from a benign monoclonal tumor that usually is larger than 2.5 cm in diameter. The excess thyroid hormone suppresses TSH levels. RAIU usually is normal, and the radioactive iodine scan shows only the hot nodule, with the remainder of the normal thyroid gland suppressed because the TSH level is low.
Other causes of thyrotoxicosis
Several rare causes of thyrotoxicosis exist that deserve mention. Struma ovarii is ectopic thyroid tissue associated with dermoid tumors or ovarian teratomas that can secrete excessive amounts of thyroid hormone and produce thyrotoxicosis. [18]
Iodide-induced thyrotoxicosis (Jod-Basedow syndrome) occurs in patients with excessive iodine intake (eg, from an iodinated radiocontrast study). The antiarrhythmic drug amiodarone, which is rich in iodine and bears some structural similarity to T4, may cause thyrotoxicosis (see Thyroid Dysfunction Induced by Amiodarone Therapy). Iodide-induced thyrotoxicosis also occurs in patients with areas of thyroid autonomy, such as a multinodular goiter or autonomous nodule.
Iodide-induced thyrotoxicosis appears to result from loss of the normal adaptation of the thyroid to iodide excess. It is treated with cessation of the excess iodine intake and with administration of antithyroid medication. Usually, after depletion of the excess iodine, thyroid functions return to preexposure levels.
Patients with a molar hydatidiform pregnancy or choriocarcinoma have extremely high levels of beta human chorionic gonadotropin (β-hCG), which can weakly activate the TSH receptor. At very high levels of β-hCG, activation of the TSH receptors is sufficient to cause thyrotoxicosis.
Metastatic follicular thyroid carcinoma may also result in thyrotoxicosis. These lesions maintain the ability to make thyroid hormone, and in patients with bulky tumors, production may be high enough to cause thyrotoxicosis.
Epidemiology
Graves disease is the most common form of hyperthyroidism in the United States, causing approximately 60-80% of cases of thyrotoxicosis. The annual incidence of Graves disease was found to be 0.5 cases per 1000 population during a 20-year period, with the peak occurrence in people aged 20-40 years. [19]
Toxic multinodular goiter (15-20% of thyrotoxicosis) occurs more frequently in regions of iodine deficiency. Most persons in the United States receive sufficient iodine, and the incidence of toxic multinodular goiter in the US population is lower than that in areas of the world with iodine deficiency. Toxic adenoma is the cause of 3-5% of cases of thyrotoxicosis.
The incidences of Graves disease and toxic multinodular goiter change with iodine intake. Compared with regions of the world with less iodine intake, the United States has more cases of Graves disease and fewer cases of toxic multinodular goiters.
Race-, sex-, and age-related demographics
Autoimmune thyroid disease occurs with the same frequency in Caucasians, Hispanics, and Asians but at lower rates in African Americans.
All thyroid diseases occur more frequently in women than in men. Graves autoimmune disease has a male-to-female ratio of 1:5-10. The male-to-female ratio for toxic multinodular goiter and toxic adenoma is 1:2-4. Graves ophthalmopathy is more common in women than in men.
Autoimmune thyroid diseases have a peak incidence in people aged 20-40 years. Toxic multinodular goiters occur in patients who usually have a long history of nontoxic goiter and who therefore typically present when they are older than age 50 years. Patients with toxic adenomas present at a younger age than do patients with toxic multinodular goiter.
Mortality and morbidity
A literature review by Varadharajan and Choudhury indicated that the rate of thyroid cancer associated with hyperthyroidism is not insignificant. In patients who underwent surgery for Graves disease, toxic adenoma, or toxic multinodular goiter, the mean overall rate of thyroid cancer was found to be 8.5%. The mean rates, specifically, for malignancy in Graves disease, toxic adenoma, and toxic multinodular goiter were 5.9%, 6.5%, and 12%, respectively. Regarding cancer subtype, the mean rates for papillary thyroid cancer, micropapillary carcinoma, and follicular thyroid cancer were 3.1%, 5.1%, and 0.8%, respectively. [20]
A study by Kim et al reported hyperthyroidism to be a risk factor for myocardial infarction and ischemic stroke in females, persons aged 50 years or older, and nonobese individuals, independent of cardiovascular risk factors. However, hyperthyroidism was not found to significantly impact mortality secondary to cardiovascular events. [21]
A prospective observational study by Watanabe et al indicated that in about 20% of patients with thyrotoxicosis, high-sensitivity cardiac troponin I levels (hsTnI) are increased. It was also found that these troponin levels decreased as thyroid function improved and brain natriuretic peptide (BNP) concentrations fell. (Elevated BNP is associated with secondary cardiac complications in thyrotoxicosis.) None of the patients with elevated hsTNI were found on electrocardiogram to have definitive evidence of myocardial ischemia, indicating that levels rise in persons with thyrotoxicosis even in the absence of overt myocardial infarction. [22]
Prognosis
Hyperthyroidism from toxic multinodular goiter and toxic adenoma is permanent and usually occurs in adults. After normalization of thyroid function with antithyroid medications, radioactive iodine ablation usually is recommended as the definitive therapy. Long-term, high-dose antithyroid medication is not recommended. Toxic multinodular goiters and toxic adenomas probably will continue to grow slowly in size during antithyroid pharmacotherapy.
Generally, the thyrotoxic areas are ablated, and patients may remain euthyroid. Those who become hypothyroid after radioactive iodine therapy are easily maintained on thyroid hormone replacement therapy, with T4 taken once daily.
Patients with Graves disease may become hypothyroid in the natural course of their disease, regardless of whether treatment involves radioactive iodine or surgery. Eye disease may develop at a time distant from the initial diagnosis and therapy. Generally, after the diagnosis, the ophthalmopathy slowly improves over years.
Thyroid hormone excess causes left ventricular thickening, which is associated with an increased risk of heart failure and cardiac-related death. Thyrotoxicosis has been associated with dilated cardiomyopathy, [23] right heart failure with pulmonary hypertension, and diastolic dysfunction and atrial fibrillation. [24]
An increase in the rate of bone resorption occurs. Bone loss, measured by bone mineral densitometry, can be seen in severe hyperthyroidism at all ages and in both sexes. In mild subclinical disease, however, bone loss has been convincingly shown only in postmenopausal women.
A study by Zhyzhneuskaya et al of patients with subclinical hyperthyroidism due to Graves disease suggested that approximately one third will progress to overt hyperthyroidism, about one third will develop normalized thyroid function, and just under one third will remain in a state of subclinical hyperthyroidism. (One person in the study became hypothyroid.) Multivariate regression analysis indicated that risk of progression to overt hypothyroidism is greater in patients with older age or a positive anti–thyroid peroxidase antibody status. The study included 44 patients, with follow-up lasting at least 12 months. [25]
-
Severe proptosis, periorbital edema, and eyelid retraction from thyroid-related orbitopathy. This patient also had optic nerve dysfunction and chemosis (conjunctival edema) from thyroid-related orbitopathy.
-
Color flow ultrasonogram in patient with Graves disease. Generalized hypervascularity is visible throughout gland (note red areas), which often can be heard as hum or bruit with stethoscope.
-
Absence of iodine 123 (123I) radioactive iodine uptake in patient with thyrotoxicosis and subacute painless or lymphocytic thyroiditis. Laboratory studies at time of scan demonstrated the following: thyroid-stimulating hormone (TSH), less than 0.06 mIU/mL; total thyroxine (T4), 21.2 µg/dL (reference range, 4.5-11); total triiodothyronine (T3), 213 ng/dL (reference range, 90-180); T3-to-T4 ratio, 10; and erythrocyte sedimentation rate (ESR), 10 mm/hr. Absence of thyroid uptake, low T3-to-T4 ratio, and low ESR confirm diagnosis of subacute painless thyroiditis.
-
Three multinuclear giant cell granulomas observed in fine-needle aspiration biopsy of thyroid from patient with thyrotoxicosis from subacute painful or granulomatous thyroiditis.
-
Scan in patient with toxic multinodular goiter. 5-Hour 123I-iodine uptake was elevated at 28% (normal 5-15%). Note multiple foci of variably increased tracer uptake.
-
Iodine 123 (123I) nuclear scintigraphy: 123I scans of normal thyroid gland (A) and common hyperthyroid conditions with elevated radioiodine uptake, including Graves disease (B), toxic multinodular goiter (C), and toxic adenoma (D).
-
Gross photo of subtotal thyroidectomy for diffuse toxic goiter (Graves Disease) showing homogenous enlargement without nodules.
-
Low-power photomicrograph showing diffuse papillary hyperplasia (hallmark histologic feature of Graves disease).
-
High-power photomicrograph showing papillary hyperplasia of follicular cells with increased nuclear size and small nucleoli.
-
Bilateral erythematous infiltrative plaques on lower extremities in 42-year-old man with Graves disease are consistent with pretibial myxedema. Myxedematous changes of skin usually occur in pretibial areas and resemble orange peel in color and texture.
-
Hypothalamic-pituitary-thyroid axis feedback. Schematic representation of negative feedback system that regulates thyroid hormone levels. TRH = thyrotropin-releasing hormone; TSH = thyroid-stimulating hormone.









