Background
The immune system is an integral part of human protection against disease. The normally protective immune mechanisms can sometimes cause detrimental effects in the host called hypersensitivity reactions. A hypersensitivity reaction is an inappropriate or exaggerated response to an antigen or an allergen. The traditional classification for hypersensitivity reactions is that of Gell and Coombs and is currently the most commonly known classification system. [1] It divides the hypersensitivity reactions into the following 4 types:
-
Type I reactions (i.e., immediate hypersensitivity reactions) involve immunoglobulin E (IgE)–mediated release of histamine and other mediators from mast cells and basophils. [2] Examples include anaphylaxis and allergic rhinoconjunctivitis.
-
Type II reactions (i.e., cytotoxic hypersensitivity reactions) involve immunoglobulin G or immunoglobulin M antibodies bound to cell surface antigens, with subsequent complement fixation. An example is drug-induced hemolytic anemia.
-
Type III reactions (i.e., immune-complex reactions) involve circulating antigen-antibody immune complexes that deposit in postcapillary venules, with subsequent complement fixation. An example is serum sickness.
-
Type IV reactions (i.e., delayed hypersensitivity reactions, cell-mediated immunity) are mediated by T cells rather than by antibodies. An example is contact dermatitis from poison ivy or nickel allergy.
Some authors believe this classification system may be too general and favor a more recent classification system proposed by Sell et al. [3] This system divides immunopathologic responses into the following 7 categories:
-
Inactivation/activation antibody reactions
-
Cytotoxic or cytolytic antibody reactions
-
Immune-complex reactions
-
Allergic reactions
-
T-cell cytotoxic reactions
-
Delayed hypersensitivity reactions
-
Granulomatous reactions
This system accounts for the fact that multiple components of the immune system can be involved in various types of hypersensitivity reactions. For example, T cells play an important role in the pathophysiology of allergic reactions (see Pathophysiology). In addition, the term immediate hypersensitivity is somewhat of a misnomer because it does not account for the late-phase reaction or for the chronic allergic inflammation that often occurs with these types of reactions.
Allergic reactions manifest clinically as anaphylaxis, allergic asthma, urticaria, angioedema, allergic rhinoconjunctivitis, some types of drug reactions, and atopic dermatitis. These reactions tend to be mediated by IgE, which differentiates them from non-IgE-mediated (formerly called anaphylactoid) reactions that involve IgE-independent mast cell and basophil degranulation. Such reactions can be caused by iodinated radiocontrast media (RCM), opiates, or vancomycin and appear similar clinically to urticaria or even anaphylaxis. [4, 5, 79]
Patients prone to IgE-mediated allergic reactions are said to be atopic. Atopy is the genetic predisposition to make IgE antibodies in response to allergen exposure. [6]
The focus of this article is allergic reactions in general. Although some of the clinical manifestations listed previously are briefly mentioned, refer to the articles on these topics for more detail. For example, see Allergic and Environmental Asthma; Anaphylaxis; Food Allergies; Rhinitis, Allergic; and Urticaria.
Pathophysiology
Immediate hypersensitivity reactions are mediated by IgE, and T and B cells play important roles in the development of these antibodies. CD4+ T-cells are subdivided into classes: effector T-cells (TH1, TH2, TH17 cells), memory T-cells, and T-regulatory (Treg) cells. TH1 cells produce interferon-gamma and interleukin (IL)-2, and promote a cell-mediated immune response. TH2 cells produce IL-4 and IL-13, which then act on B-cells to promote the production of antigen-specific IgE. TH17 cells produce IL-17, IL-21, and IL-22 to help fight extracellular pathogens, to produce antimicrobial peptides, and to promote neutrophil inflammation essential for immunity at the skin and mucosal surfaces. [57] Memory T-cells rapidly differentiate into effector T-cells in secondary immune responses. CD4+CD25+FOXP3+ Treg cells are essential in peripheral tolerance and serve to suppress dysregulated immune responses. CD4+CD25+FOXP3+Tregs inhibit TH2 cytokine production through the secretion and action of IL-10 and TGF-β. Proper function of CD4+CD25+FOXP3+ Treg cells has been shown to be important in the tolerance of allergens. [8] Abnormalities in the CD4+CD25+FOXP3+ Treg population may play a role in the development of allergic disease.
The allergic reaction first requires sensitization to a specific allergen and it occurs in genetically predisposed individuals. The allergen is typically introduced through the respiratory tract (inhaled), through the gastrointestinal tract (ingested) or through contact of the integument (on the skin). The allergen/antigen is then processed by an antigen-presenting cell (APC), such as a dendritic cell, macrophage, or B-cell. [78] The antigen-presenting cell(s) then migrate to lymph nodes, where they prime naïve T-helper cells that bear receptors for the specific antigen.
After antigen priming, naïve T-helper cells differentiate into TH1, TH2, or TH17 cells based upon antigen and cytokine signaling. In the case of allergen sensitization, the differentiation of naïve T-helper cells is skewed toward a TH2 phenotype. These allergen-primed TH2 cells then release IL-4, IL-5, IL-9, and IL-13. IL-5 plays a role in eosinophil development, recruitment, and activation. IL-9 plays a regulatory role in mast cell activation. IL-4 and IL-13 act on B cells to promote production of antigen-specific IgE antibodies.
In order for B cells to produce antigen-specific IgE, the B cells must internalize the recognized antigen. The antigen is then processed via the major histocompatibility complex (MHC) class II antigen processing pathway where the MHC class II molecule presents the antigenic peptide on the B cell surface to the TH2 cell. The peptide:MHC class II complex is then recognized by the T-cell receptor (TCR) on the TH2 cell. This interaction requires co-stimulation where CD40 on the B cell interacts with CD40L on the TH2 cell surface to activate the signaling cascade in the TH2 cell to produce IL-4 and IL-13. The CD40:CD40L interaction is essential for the activation of the activation-induced cytosine deaminase (AICD) enzyme in B cells for isotype switching from IgM to IgE in the presence of IL-4 and IL-13 (see image below).
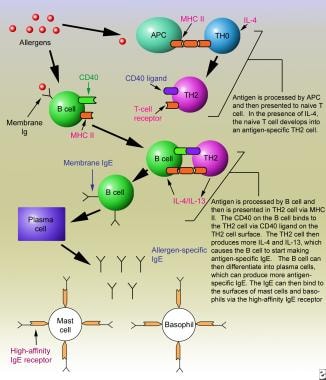 Immediate hypersensitivity reactions. Sensitization phase of an immunoglobulin E–mediated allergic reaction.
Immediate hypersensitivity reactions. Sensitization phase of an immunoglobulin E–mediated allergic reaction.
The antigen-specific IgE antibodies produced can then bind to high-affinity IgE receptors located on the surfaces of mast cells and basophils. Re-exposure to the antigen can then result in the antigen binding to and cross-linking the bound IgE antibodies on the mast cells and basophils. This causes the release and formation of chemical mediators from these cells. These mediators include preformed mediators, newly synthesized mediators, and cytokines. The major mediators and their functions are described as follows: [9, 10]
Preformed mediators
See the list below:
-
Histamine: This mediator acts on histamine 1 (H1) and histamine 2 (H2) receptors to cause contraction of smooth muscles of the airway and GI tract, increased vasopermeability and vasodilation, enhanced mucous production, pruritus, cutaneous vasodilation, and gastric acid secretion. [88]
-
Tryptase: Tryptase is a major protease released by mast cells. It has many roles where it degrades allergens and cross-linked IgE, potentiates histamine release, increases heart rate and airway smooth muscle contractility, activates TGF-β, generates C3a and bradykinin and plays a role in airway remodeling through the stimulation of collagen and fibroblast proliferation. [11, 8, 88] Tryptase is found in all human mast cells but in few other cells, thus is a good marker of mast cell activation.
-
Proteoglycans: Proteoglycans include heparin and chondroitin sulfate. The role of the latter is unknown; heparin seems to be important in storing the preformed proteases and may play a role in the production of α-tryptase.
-
Chymase: Chymase plays a role in mucous secretion; degradation of IL-4 and extracellular matrix; decrease T-cell adhesion to airway smooth muscle; and releasing stem cell factor. [88]
Newly formed mediators
Arachidonic acid metabolites
Leukotrienes - Produced via the lipoxygenase pathway:
-
Leukotriene B4 - Neutrophil chemotaxis and activation, augmentation of vascular permeability
-
Leukotrienes C4 and D4 - Potent bronchoconstrictors with airway smooth muscle proliferation, increase vascular permeability to cause tissue edema, enhance tissue fibrosis and arteriolar constriction
-
Leukotriene E4 - Enhances bronchial responsiveness and increases vascular permeability
-
Leukotrienes C4, D4, and E4 - Comprise what was previously known as the slow-reacting substance of anaphylaxis
Cyclooxygenase products:
-
Prostaglandin D2 - Produced mainly by mast cells; bronchoconstrictor, peripheral vasodilator to cause tissue edema, coronary and pulmonary artery vasoconstrictor, platelet aggregation inhibitor, neutrophil and eosinophil chemoattractant, and enhancer of histamine release from basophils
-
Prostaglandin F2-α - Bronchoconstrictor, peripheral vasodilator, coronary vasoconstrictor, and platelet aggregation inhibitor
-
Thromboxane A2 - Causes vasoconstriction, platelet aggregation, and bronchoconstriction
Platelet-activating factor (PAF): PAF is synthesized from membrane phospholipids via a different pathway from arachidonic acid. It aggregates platelets but is also a very potent mediator in allergic reactions. It increases vascular permeability, causes bronchoconstriction, and causes chemotaxis and degranulation of eosinophils and neutrophils.
Adenosine: This is a bronchoconstrictor that also potentiates IgE-induced mast cell mediator release.
Bradykinin: Kininogenase released from the mast cell can act on plasma kininogens to produce bradykinin. An additional (or alternative) route of kinin generation, involving activation of the contact system via factor XII by mast cell–released heparin, has been described. [12, 13] Bradykinin increases vasopermeability, vasodilation, hypotension, smooth muscle contraction, pain, and activation of arachidonic acid metabolites. However, its role in IgE-mediated allergic reactions has not been clearly demonstrated. [4]
Cytokines
See the list below:
-
IL-4: IL-4 stimulates and maintains TH2 cell differentiation and proliferation as well as switches B cells to IgE synthesis. [88]
-
IL-5: IL-5 is key in the maturation, chemotaxis, activation, and survival of eosinophils. IL-5 primes basophils for histamine and leukotriene release. [88]
-
IL-6: IL-6 promotes mucus production, increases IgE secretion, and enhances the survival of mast cells. [88]
-
IL-13: IL-13 has many of the same effects as IL-4. [88]
The collective biological activities of the aforementioned mediators can cause variable clinical responses depending on which organ systems are affected, as follows:
-
Urticaria/angioedema: Release of the above mediators in the superficial layers of the skin can cause pruritic wheals with surrounding erythema. If deeper layers of the dermis and subcutaneous tissues are involved, angioedema results. Angioedema is swelling of the affected area; it tends to be painful rather than pruritic.
-
Allergic rhinoconjunctivitis: Release of the above mediators in the upper respiratory tract can result in sneezing, itching, nasal congestion, rhinorrhea, and itchy or watery eyes.
-
Allergic asthma: Release of the above mediators in the lower respiratory tract can cause bronchoconstriction, mucus production, and inflammation of the airways, resulting in chest tightness, cough, shortness of breath, and wheezing.
-
Anaphylaxis: Systemic release of the above mediators, resulting in symptoms in two or more organ systems, is considered anaphylaxis. In addition to the foregoing symptoms, the GI system can also be affected with nausea, abdominal cramping, bloating, and diarrhea. Systemic vasodilation and vasopermeability can result in significant hypotension and is referred to as anaphylactic shock. Anaphylactic shock is one of the two most common causes for death in anaphylaxis; the other is throat swelling and asphyxiation. [4, 10]
Allergic reactions can occur as immediate reactions, late-phase reactions, and/or chronic allergic inflammation. Immediate or acute-phase reactions occur within seconds to minutes after allergen exposure. Some of the mediators released by mast cells and basophils cause eosinophil and neutrophil chemotaxis. Attracted eosinophils and resident lymphocytes are activated by mast cell mediators.
These and other cells (e.g., monocytes, T cells) are believed to cause the late-phase reactions that can occur hours after antigen exposure and after the signs or symptoms of the acute-phase reaction have resolved. The signs and symptoms of the late-phase reaction can include redness and swelling of the skin, nasal discharge, airway narrowing, sneezing, coughing, and wheezing. These effects can last a few hours and usually resolve within 24-72 hours.
Finally, continuous or repeated exposure to an allergen (e.g., a cat-allergic patient who owns a cat) can result in chronic allergic inflammation. Tissue from sites of chronic allergic inflammation contains eosinophils and T cells (particularly TH2 cells). Eosinophils can release many mediators (e.g., major basic protein), which can cause tissue damage and thus increase inflammation. Collectively, this results in structural and functional changes to the affected tissue. Furthermore, a repeated allergen challenge can result in increased levels of antigen-specific IgE, which ultimately can cause further release of IL-4 and IL-13, thus increasing the propensity for TH2 cell/IgE–mediated responses. [10]
Epidemiology
Frequency
The prevalence of atopic diseases (i.e., asthma, allergic rhinitis, food allergy, and atopic dermatitis) have increased since the year 2000. [8]
Food allergy prevalence in developed and developing countries is reportedly on the rise over the last 10–15 years. [92]
Allergic rhinitis is the most prevalent allergic disease; [6] it affects approximately 17-22% or more of the population. [15]
Asthma was estimated to affect approximately 25.7 million people in the United States in 2010. Asthma prevalence increased from 7.3% in 2001 to 8.4% in 2010. [16] The annual percentage increase in current asthma prevalence from 2001 to 2009 was 1.2%, while there are large variations in population subgroups. [93]
Atopic dermatitis has increased in prevalence in the US. [93]
The prevalence of severe anaphylaxis is estimated between 0.065% and 2% in the US. [94]
Approximately 300 million people worldwide are estimated to have asthma. [18] Prevalence rates vary around the world and are estimated to be from 3–38% in children [18] and 2–12% in adults. [19]
The International Study of Asthma and Allergies in Childhood (ISAAC) is an epidemiological research program that was established in 1991 to evaluate asthma, atopic dermatitis, and allergic rhinoconjunctivitis in children worldwide. The study is composed of three phases. Phase one used questionnaires designed to assess the prevalence and severity of asthma and allergic disease in defined populations in centers around the world. Phase two was designed to assess possible etiological factors based on information gathered from phase one. Phase three was a repetition of phase one to assess trends in prevalence. [20] Data from ISAAC showed prevalence variations in allergic diseases between countries but the overall mean prevalence did increase for all three disorders (asthma, atopic dermatitis and allergic rhinconjunctivitis) investigated. [21] ISAAC researchers found significant variability in the prevalence of allergic rhinoconjunctivitis in children from 56 countries. Rates varied from 1.4–45.1% and, although sites varied, a general trend of increasing prevalence of allergic rhinoconjunctivitis was found over the 7 years between phases one and three. [21] The prevalence in atopic dermatitis also varies widely between countries; 1.4% in China to 22.3% in Sweden, and prevalence is generally increasing. [21, 8] Lastly, asthma prevalence ranged greatly from 2.8% to 37.6%. [21] The ISAAC project is the only study of its kind to help establish prevalence trends of atopic disease in children in many areas of the world.
The trend of asthma prevalence is hard to truly attain and no study has demonstrated a trend over time for adults like the ISAAC project has for children. In 2010 a systematic review evaluated the trend of the prevalence of asthma, which did not show a decrease, but, again, an increase in many parts of the world. Moreover, according to the World Health Survey, the global prevalence of physician-diagnosed asthma is 4.3% with a wide range in prevalence amongst 70 countries included in this study. China had 0.2% prevalence where Australia had a prevalence of 21%. Contrary to China's low prevalence reported by the WHS, a 2019 cross-sectional study in China identified 45.7 million Chinese adults with asthma (4.2% prevalence). [69] Risk factors associated with asthma were cigarette smoking, allergic rhinitis, childhood pneumonia or bronchitis, parental history of respiratory disease, and low education attainment. [69] The European Community Health Respiratory Survey (ECHRS) assessed adult asthma prevalence in many, albeit more developed, countries. There was a six-fold variation in the prevalence with high rates in Australia, United States, and the UK with lower prevalence in Iceland, Italy, Germany, Algeria and India. [91] Again, these studies indicate the range of asthma prevalence in different parts of the world.
Mortality/Morbidity
Mortality from allergic diseases occurs primarily from anaphylaxis and asthma, although deaths from asthma are relatively rare. [10]
The rate of asthma deaths decreased from 15 per million in 2001 to 10 per million (n = 3518) in 2016. The death rate was higher in females and non-Hispanic blacks and nearly 5 times higher for adults than children. [95]
Approximately 1500 people die annually from anaphylaxis in the United States. [94]
Allergic diseases are a significant cause of morbidity. Asthma was the primary diagnosis for emergency room visits for 1.6 million individuals in 2017. [96] Asthma was also the primary diagnosis in 9.8 million office visits in 2016. [96] Furthermore allergic rhinitis affects 19.2 million adults and 5.2 million children where 12.0 million have office visits per year with allergic rhinitis as the primary diagnosis. [97]
Race
The reason for the differences in the prevalence of allergic diseases with respect to race are complex and not completely understood. In the US, Puerto Ricans have the highest prevalence at 16.6% followed by blacks (11.1%), whites (7.8%), Asians (5.3%), then Mexicans (4.9%). [89] Asthma prevalence also increases with increasing poverty. [89] Thus, it is likely that differences in allergic diseases among different racial or ethnic groups is multifactorial and includes genetic, environmental, and socioeconomic factors.
Geography
In the US, the prevalence of asthma is highest in the Northeast at 9.3% and the lowest in the South at 7.5%. [89]
Sex
Some unexplained differences exist in the prevalence of allergic diseases between the sexes. Asthma is more prevalent in boys from birth to age 17 years, but females have a higher prevalence overall. [89] Among asthmatic children, the rate of exacerbation over the last 12 months decreased from 2001 to 2016, but prevalence was higher in the birth to 4-year-old category than the 12- to 17-year-old category. [90]
Skin test reactivity in women can fluctuate with the menstrual cycle, but this is not clinically significant. [9]
Age
In general, allergic rhinitis symptoms (and skin test reactivity) tend to wane with increasing age. [15]
Food allergies and subsequent anaphylaxis are more prevalent in children; IgE-mediated food allergy is also on the rise. It is unclear the reason(s) for the increase in IgE-mediated food allergy, but a landmark article, the LEAP trial, published in 2015 showed sustained early introduction of peanut (first 11 months of life) into the diet of high risk infants significantly reduced risk of peanut allergy by 5 years of age. [85] Subsequently, the LEAP-on trial studied the same high-risk children from the LEAP trial. The children who tolerated peanut at age 5 avoided peanut protein consumption for 12 months without an increase in peanut allergy prevalence with reintroduction at age 6 years. [86] These studies have shifted the paradigm to early introduction and sustained consumption of peanut protein. Furthermore the results of these studies could possibly be extrapolated to all highly allergenic foods being introduced into the diet of children. When IgE-mediated food allergy develops some children may outgrow their allergies to certain foods, such as milk, soy, and egg allergy. However, anaphylaxis from food and other triggers is still a threat in adults. Some food allergies, such as allergy to shellfish, tree nuts, and finned fish, may last a lifetime.
Childhood asthma is more prevalent in boys and can often resolve by adulthood. However, females tend to develop asthma later in life and can also have asthma that is more severe. [9, 8]
-
Immediate hypersensitivity reactions. Sensitization phase of an immunoglobulin E–mediated allergic reaction.







