Practice Essentials
The spinal cord transmits information between the brain and spinal cord to the nerves and muscles. The distal or terminal portion of the spinal cord is referred to as the conus medullaris. In adults, the spinal cord typically terminates at approximately the level of L1. This space is created by differential growth of the vertebral column as compared to the spinal cord, which causes the spinal cord to ascend with growth. The nerve roots then descend through this fluid sac containing cerebrospinal fluid and are referred to as the cauda equina ("tail of a horse"). This is the collection of lumbar and sacral spinal nerve roots that course in a caudal direction to emerge from their respective foramina.
Compression of the spinal cord and nerve roots by tumors of the cauda equina and the conus medullaris typically produces pain and possibly progressive deterioration of neurologic function, including motor weakness, sensory deficits, and bowel and bladder dysfunction. These symptoms are collectively known as the cauda equina syndrome when the compression occurs on the cauda equina.
Tumors of the cauda equina and the conus medullaris are categorized according to the tissue compartment in which the tumors are located. This classification is based on their relationship to the meninges (lining around the spinal cord) that enclose the central nervous system. [1, 2]
-
Extradural tumors - Arise outside the spinal cord and meninges and epidural tissue
-
Intradural-extramedullary tumors - Arise inside the dural sac, from leptomeninges or the nerve root, outside the substance of the spinal cord parenchyma
-
Intradural-intramedullary tumors - Arise within the substance of the spinal cord
Tumor, disk herniation, fracture, and infection (eg, epidural abscess) are all possible causes of cauda equina syndrome. Determining the precise nature of the lesion (eg, intradural-extramedullary vs intradural-intramedullary) and the exact type of tumor (eg, ependymoma vs astrocytoma) based on clinical findings can be difficult.
The ability to noninvasively image neural elements with magnetic resonance imaging (MRI) to assess evolving neurologic deficits in addition to chronic conditions such as low back pain has facilitated diagnosis of this disorder.
(See the image below.)
Treatment for tumors of the cauda equina or the conus medullaris is primarily surgical resection. Radiation therapy and intrathecal chemotherapy are reasonable adjuvants for treatment of these tumors in patients with contraindications for surgical treatment.
Historical background
In 1887, Sir Victor Horsley performed the first successful removal of a spinal cord tumor—an extramedullary-intradural fibromyxoma that was compressing the spinal cord. The patient subsequently regained gait function.
In 1907, Eiselsberg-Renzi was the first to successfully remove an intradural intramedullary tumor. In 1905, Cushing reported the first attempted surgical resection of an intramedullary spinal neoplasm. In 1925, Charles Elsberg reported on the first large series of patients who underwent resection of a spinal cord neoplasm. Unfortunately, during this period, patients endured significant associated morbidity and mortality related to operative techniques.
In 1963, Greenwood and associates presented a modern series on successful removal of intramedullary tumors. [3] These authors concluded that because of the relatively direct surgical approach to the lumbar spinal canal, tumors in that area are amenable to successful surgical resection.
Anatomy
Conus and cauda equina tumors represent a unique group of tumors due to their specific location in the spinal canal. The spinal cord is within the vertebral canal and measures approximately 45 cm in the adult male and 42 cm in the adult female. It is a caudal extension of the medulla oblongata after passing through the foramen magnum. The most caudal part of the spinal cord is the conus medullaris, and it is usually located at the rostral edge of the second lumbar vertebra. [1]
The conus medullaris is where the axons of the distal nerve roots originate and where the spinal bowel and bladder centers are located. The cauda equina is the conglomeration of nerve roots of the lumbar and sacral spinal nerves distal to the conus area. These 2 areas form a transition between the central and peripheral nervous systems. [1] The distal spinal cord terminates at the conus medullaris and contains the sacral and vestigial coccygeal cord. The collection of nerve roots distal to the conus that supply the lower part of the body is known as the cauda equina.
Extending distally from the apex of the conus medullaris is a delicate filament known as the filum terminale. The first 15 cm is contained within the dural sac—the filum terminale internum, which consists of fibrous tissue that is continuous with the pia mater.
The central canal of the spinal cord continues down into the filum terminale for the first 5-6 cm to form an area that also contains neural and ependymal cells. Extending beyond the apex of the dural sac is the filum terminale externum, which anchors the spinal cord and the fluid-filled dural sac to the caudal end of the vertebral canal — the coccygeal vertebra. [4, 5]
Epidemiology
Tumors of the spinal cord are rare and are reported to represent approximately 10-15% of all central nervous system tumors. Overall, they represent an estimated incidence of 0.5-2.5 cases per 100,000 population. Extradural spinal cord tumors constitute by far the greatest majority of spinal tumors and include metastatic tumors. Intradural-extramedullary tumors account for approximately 20% of all spinal tumors, less than 5% of which are intradural-intramedullary tumors.
Ependymomas
In the adult population, ependymomas (intradural-intramedullary) are the most common intra-axial tumors of the conus medullaris and filum terminale. They account for more than one third of tumors in the region. Ependymomas represent approximately 60% of all glial neoplasms of the entire spinal cord and are the most common glial neoplasms below the midthoracic region.
Conus medullaris tumors are diagnosed in the third to fourth decade of life, with a slight male predominance. Myxopapillary ependymomas, a variant of ependymomas, tend to affect the distal conus medullaris and the filum terminale exclusively; cystic degeneration is reported in approximately 50% of cases. Tumors of the conus medullaris are also observed in the pediatric population.
In the cranium, ependymomas are relatively rare intracranial gliomas that account for only 5-6% of these tumors. They are typically more common in children.
Spinal schwannomas and myxopapillary ependymomas may have similar imaging characteristics; a detailed examination of magnetic resonance T2W imaging and postcontrast T1W imaging may facilitate their differentiation. [6]
Myxopapillary ependymoma is a rare tumor of the CNS; this subtype of ependymoma occurs most frequently in cauda equina, conus medullaris, or filum terminale. Treatment consists of complete removal of the tumor, including its capsule when possible, because it is usually a solitary lesion. Non-Hodgkin lymphoma of the CNS is found in only 1.3% of cauda equina tumors. Although rare, this phenomenon (coexisting tumors within the same lesion) should be known by neurosurgeons because the occurrence of collision tumors affects treatment and patient prognosis. [7, 8]
Astrocytomas
Astrocytomas, another type of primary glial neoplasm, occur less frequently in the region of the conus medullaris and the filum terminale (in just less than one third of cases) compared with ependymomas. Astrocytomas also have a slight male predominance and tend to occur in the third to fifth decade of life. In contrast to ependymoma, astrocytoma is the most common intra-axial brain tumor.
Other tumors
Other tumors of the spinal cord account for less than one third of intramedullary spinal cord tumors. These include dermoid and epidermoid tumors, vascular tumors, hemangioblastomas, and, rarely, lymphomas or oligodendrogliomas. [9] Other rare tumors have been reported periodically.
Intradural-extramedullary tumors are predominantly (more than two thirds) schwannomas, neurofibromas, and meningiomas. Reported incidences of these are approximately equal. Lipomas have also been described. Exophytic components of intramedullary ependymoma and astrocytoma can extend into the intradural-extramedullary compartment. [10]
Cauda equina paragangliomas are rare, benign, slow-growing tumors, which are often diagnosed at surgery. They are well encapsulated and are cured by surgery, with radiotherapy reserved for tumors that are incompletely resected. Tumor relapse may occur up to 30 years after surgery. [11, 12]
Etiology
Tumors of the nervous system and the spinal cord are classified according to their cells of origin. Following is a modified classification put forth by the World Health Organization. [13]
Astrocytic tumors
-
Pilocytic astrocytoma - Grade I
-
Diffuse (fibrillary) astrocytoma (low grade) - Grade II
-
Anaplastic astrocytoma - Grade III
-
Glioblastoma- Grade IV
Oligodendroglial tumors
-
Oligodendroglioma
-
Anaplastic oligodendroglioma
Mixed gliomas
-
Oligoastrocytoma
-
Anaplastic oligoastrocytoma
Ependymal tumors
-
Ependymoma - Cellular, papillary, clear cell. tanycytic
-
Anaplastic ependymoma
-
Myxopapillary ependymoma
Neuronal and mixed neuronal-glial tumors
-
Gangliocytoma
-
Desmoplastic infantile astrocytoma/ganglioglioma
-
Dysembryoplastic neuroepithelial tumor
-
Ganglioglioma
-
Paraganglioma of the filum terminale [12]
Tumors of meninges
-
Meningioma
-
Meningeal hemangiopericytoma
Tumors of nerve sheath cells
-
Schwannoma (ie, neurilemmoma)
-
Neurofibroma
Other tumors
-
Tumor of primitive undifferentiated cells (medulloblastoma)
-
Lymphoma [9] – Primary and secondary
-
Metastatic tumor
Histologic findings of the major tumor types are presented in Ependymoma; Low-Grade Astrocytoma; Meningioma; Dermoid, Limbal; Neurofibromatosis; and Lipomas.
Pathophysiology
The distal or terminal region of the spinal cord—the conus medullaris and the cauda equina—is a complex region of spinal anatomy and transition from the central to the peripheral nervous system. Motor nerve roots of the cauda equina exit and sensory nerves enter through the conus and are considered peripheral nerves. The pathophysiology remains unclear but may be related to damage to the nerve roots composing the cauda equina from direct mechanical compression and venous congestion or ischemia. [14]
Conus lesions primarily affect central functions, and cauda lesions affect peripheral functions. As these 2 areas are in close proximity, lesions in one area can affect the function of the other area. Lesions in each area give rise to specific deficits and are appropriately called cauda equina syndrome (CES) and conus medullary syndrome (CMS). [1]
An isolated lesion at the conus may cause symptoms of a lower motor neuron lesion with or without spinal cord symptoms. Therefore, the patient may present with various symptoms, from flaccid paralysis or paresis of the lower extremities to spasticity.
Early on, symptoms may be unilateral and may be localized to a specific muscle group. The sensory deficit, at least initially, may be localized to a unilateral dermatomal distribution. As lesions become larger, symptoms may progress and may become bilateral. Numbness and paresthesia progress to a saddle distribution and extend into the lower extremity.
Lesions of the conus medullaris may manifest as sensory dysfunction of the perineum in a saddle distribution and as bowel and bladder dysfunction. Patients may present with back pain that is primarily midline and less radicular in nature. Lesions that are truly isolated to the conus medullaris may demonstrate sparing of the lower extremities and may affect only the bladder and the perineum.
If the lesion is large enough to include some lumbar cord segments, symptoms extend into the lower extremities. If the lumbar cord is affected, lesions have the characteristics of upper motor neuron lesions with hyperreflexic motor weakness.
Genitourinary (GU) dysfunction is seen in persons with conus medullaris lesions or cauda equina lesions. No distinct signs or symptoms differentiate one type of lesion from the other. Because the conus medullaris includes most of the sacral cord that controls GU function, a lesion frequently results in GU deficits. By comparison, the cauda equina has roots of both lumbar and sacral origin, and GU sparing may occur.
Tumors arising within the lumbar spine may involve the vertebrae, the distal end of the spinal cord (conus medullaris), or the nerve roots (cauda equina). These tumors may be primary or metastatic, benign or malignant, and may have a broad range of presentations. The most common tumors involving the vertebrae are metastatic lesions, and the most commonly involved area is the vertebral body. The most common tumors arising from the conus medullaris are ependymoma and astrocytoma. A metastatic lesion within the conus medullaris is possible but is very uncommon. The most common tumor involving the cauda equina is a schwannoma. [1]
Both conus medullaris and cauda equina lesions are associated with urinary retention or incontinence and fecal incontinence or constipation. Both types of lesions may be associated with sexual dysfunction, including erectile dysfunction and impotence.
Clinical Presentation
Manifestations of spinal cord tumors may include myriad neurologic symptoms. Findings obtained from a careful history and physical examination can help guide the clinician to the diagnosis of a spinal cord tumor. The evolution of symptoms may be slow and progressive, or it may be abrupt with rapid progression.
Neurologic symptoms affecting the distal nerve roots are known as the cauda equina syndrome or the conus medullaris syndrome (see Cauda Equina and Conus Medullaris Syndromes). Following are the main elements of the neurologic presentation. [15]
Pain
Pain is the most common symptom. Pain is increased with movement or with the Valsalva maneuver (if radicular). Pain that increases during recumbency, particularly at night, may suggest a spinal cord tumor.
Pain may be described as radicular (usually of dermatomal distribution), localized (near the midline of the spine), or medullary and nonradicular in distribution (possibly bilateral, and possibly described as burning or dysesthetic pain).
Motor disturbance
Motor disturbance is the next most common symptom. Patients may present with weakness, ataxia, clumsiness, atrophy, twitching and fasciculation, or gait disturbance.
Other disturbances
Patients may present with nonpainful sensory disturbances such as paresthesia, dysesthesia, dissociative syndrome (ie, decreased pain and temperature sensation with touch preserved), or radicular or medulllary distribution.
Bowel and bladder dysfunction may be present. Patients may present with sphincter problems; possible incontinence, retention, and incomplete bowel or bladder evacuation; or possible erectile dysfunction and impotence.
Physical deformity (visible mass over the area) may indicate a coronal deformity (scoliosis).
In one study of patients with cauda equina syndrome, findings included decreased anal tone (7.6%), fecal incontinence (3.8%), urinary retention (7.6%), bladder incontinence (8.9%), constipation (2.5%), and saddle anesthesia (8.9%). [16]
Differential Diagnosis
The differential diagnosis for lumbosacral cord dysfunction includes nonneoplastic causes of myelopathy. An expanded differential diagnosis is presented in the Medscape Reference article Cauda Equina and Conus Medullaris Syndromes. Following are some of the major groupings. [15]
Congenital
Syringomyelia is a cystic cavitation of the spinal cord that may communicate with the central canal or the subarachnoid space. It can be congenital or posttraumatic.
Acquired
A herniated lumbar disk can cause nerve root impingement, usually in the setting of congenital stenosis. It often manifests as sciatica or radiculopathy. A herniated disk may be associated with the following:
-
Pain radiating to the lower extremity
-
Motor weakness in a specific distribution
-
Sensory loss or paresthesia in a specific dermatomal pattern
-
Diminished reflexes
Spinal stenosis can be the result of a congenitally shallow canal and may manifest as the following:
-
Arthropathy of the facet
-
Hypertrophied ligamentum flavum
-
Bulging annulus or herniated disc
With neurogenic claudication (compared with vascular claudication), the pain is dermatomal and worsens with ambulation.
In the setting of major trauma, fractures are likely nonpathologic. The bony fragments can compress the neural elements.
Vascular
Epidural spinal hematoma may be posttraumatic; in anticoagulated patients, minor trauma presumably may be the cause. Alternatively, epidural spinal hematoma may result from a dural vascular malformation. Arteriovenous malformations may cause a hemorrhage that affects the conus medullaris but is less likely to affect individual roots.
Infarction may result from disruption of the radicular vessels as a result of atherosclerotic disease of the aorta or another disease. The cord is especially at risk if infarction affects the L2 vessel (ie, the artery of Adamkiewicz, usually on the left).
Infectious
Presentation in patients with epidural abscess may include fever, back pain, and localized tenderness. Risk factors include intravenous drug abuse, diabetes, and renal failure. Staphylococcus aureus is the major causative organism. Typically, patients have an elevated erythrocyte sedimentation rate and white blood cell (WBC) count.
Vertebral osteomyelitis is an infection of the vertebral bodies. The lumbar area is affected most commonly. Tuberculous osteomyelitis or spondylitis is referred to as Pott disease.
Diskitis is an infection of the nucleus pulposus. Causative organisms include Escherichia coli and Staphylococcus, Streptococcus, and Pseudomonas species. Diskitis may be spontaneous or may occur following a procedure, such as discectomy.
Autoimmune and degenerative disorders
These disorders include transverse myelitis, multiple sclerosis, viral infection or its sequela, amyotrophic lateral sclerosis, and Guillain-Barré syndrome.
Workup Overview
The diagnostic workup overlaps, in part, with the workup presented in Cauda Equina and Conus Medullaris Syndromes and Neoplasms, Spinal Cord.
In general, findings from the history and physical examination are most helpful in categorizing symptoms, developing a differential diagnosis, and guiding the diagnostic workup. Timely diagnosis and treatment are imperative for optimal outcomes and for avoiding medicolegal ramifications. [17] [18]
Tumors of the conus and cauda equina quite often have an insidious and potentially progressive nature. Because these symptoms may affect sexual capacity and bowel and bladder function, they are sometimes discounted by patients or dismissed as psychological. In women, symptoms may be attributed to a cystocele or to another urologic disease; in older men, they can be attributed to prostate disease. The correct diagnosis often is not made until profound neurologic deficits have occurred.
A heightened index of suspicion should be maintained when patients have undiagnosed chronic conditions, including lower extremity weakness, or when they have sensory problems and physical findings. Patients with evolving or rapidly progressing neurologic deficits should be examined carefully.
Most patients with back pain do not have spinal tumors. Frequently, this diagnosis is not considered until an imaging study is performed.
Emergency department (ED) workup for patients who present with persistent back pain or with subacute or chronic neurologic deficits should include an interview, careful examination, and timely referral if findings are indicative. [18]
The consensus is that laboratory studies are not helpful in obtaining a diagnosis; however, laboratory findings may guide screening for and possibly exclusion of other disease processes. For example, an abnormal erythrocyte sedimentation rate (ESR) or C-reactive protein level, and possibly an elevated WBC count, may lead to consideration of an epidural abscess.
With regard to studies of the urinary tract, back pain with positive urinalysis results may suggest a GU cause. Renal ultrasonography or a GU CT scan can be diagnostic.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) with gadolinium is the imaging study of choice because it provides a detailed assessment of neural tissue, other soft tissue, and bone. Tumors of the conus and cauda equina, such as meningioma, neurofibroma, and ependymoma, typically are enhanced with contrast. Magnetic resonance imaging provides information on the spinal canal and the anatomic location of the lesion, whether intradural in location and intramedullary or extramedullary. [6, 16, 19, 18]
The relationship of the lesion to normal structures (spinal cord and roots) can often be demonstrated. Intervertebral disks can be seen and can be assessed for concomitant disk disease.
In an MRI study of patients with spinal schwannoma or myxopapillary ependymoma, in the schwannoma group, all 24 tumors that were homogeneously hyperintense on T2-weighted (T2W) images showed rim enhancement on postcontrast T1-weighted (T1W) images. Moreover, all 14 of the schwannomas with homogeneous enhancement on postcontrast T1W images were isointense on T2W images. In the myxopapillary ependymoma group, however, all 8 tumors that were homogeneously hyperintense on T2W images showed homogeneous enhancement on their postcontrast T1W images. [6]
Plain Radiographs
Plain radiographs are of limited value but may be helpful in detecting bony lesions and other osseous abnormalities that are extra-axial or that have been present for a long time and have caused osseous changes.
Tumors of the cauda equina are frequently present for years before they are diagnosed. They can cause bone erosion, which may be visible on radiographs.
Computed Tomography
Computed tomography (CT) with myelography was used extensively until MRI became readily available.
Myelography is an invasive procedure that requires injection of contrast material directly into the subarachnoid space. As a result of dural puncture, the patient may experience headaches due to persistent spinal fluid leakage.
This modality does not allow visualization of the intramedullary spinal cord and therefore should be used only for patients who cannot tolerate an MRI (eg, those with a cardiac pacemaker).
Other Studies
Bone scans may be helpful in identifying inflammatory, infectious, or neoplastic lesions. However, they do not provide high-resolution anatomic detail, do not facilitate visualization of neural tissue, and are not typically used in the workup for spinal cord tumors.
Duplex ultrasound findings, in the setting of back pain or claudication, can help exclude an aortic aneurysm or other vascular causes of back or leg pain. However, ultrasonography is not usually employed as an initial diagnostic tool.
Electromyelography (EMG) and nerve conduction studies (NCSs) may be useful in the differential diagnosis of lower extremity pain, weakness, or sensory loss. Tumors of the low spine may cause a radiculopathy that is detectable on EMG. Images do not provide any anatomic detail regarding these lesions but may help the clinician to localize involvement of a particular motor root.
Treatment Approach
Surgical excision is the primary modality of treatment for spinal tumors. Urgent or emergent surgery should be performed in patients with rapidly progressing neurologic deficits.
Radiation therapy and intrathecal chemotherapy are reasonable adjuvants for the treatment of these tumors in patients with contraindications for surgical treatment.
Medical treatment of the patient is focused on optimizing the treatment of any chronic medical condition that may limit functional status. Very limited types of medications, including chemotherapies, are used to treat these diseases.
Tumor Resection
Surgical resection entails opening the dura mater and exposing the spinal cord. Once localized, typically with ultrasound, the spinal cord in incised at the midline. The lesion is entered and a biopsy specimen is obtained. If the lesion is an invasive neoplasm (astrocytoma), the procedure is halted if resection planes cannot be established. With benign lesions, resection along tissue planes may provide a gross total resection, which is the optimal goal.
Adjuvant radiation therapy or intrathecal chemotherapy is used in selected cases or for unresectable neoplasms.
Contraindications for surgery are related to the overall health and condition of the patient, as well as to the patient's overall life expectancy. Patients with significant comorbidity are poor candidates for any surgery, including spinal surgery. A relative contraindication for surgery is a tumor that is in a difficult anatomic location, especially on the ventral aspect of the cord. However, experienced surgeons can use microsurgical techniques to obtain appropriate exposure. Fortunately, the roots of the cauda equina may be retracted gently to enable exposure of most tumors in this area.
One study investigated radiographic changes in global spinal sagittal alignment (GSSA) and clinical outcomes following tumor resection with the spinous process-splitting laminectomy (SPSL) approach without fixation in patients with conus medullaris (CM) or cauda equina (CE) tumor. Tumor resection via SPSL did not affect the various GSSA parameters examined and resulted in satisfactory clinical outcomes, indicating that SPSL is a suitable surgical technique for patients with CM or CE tumor. [20]
Preoperative details
Informed consent should be obtained from patients or their guardians. A realistic portrayal of test results should be presented, and expectations of the patient should be addressed. Discussion should include the message that neurologic deficits may not improve and risk of worsening exists, including pain, paralysis, paresthesia, bowel or bladder problems, and sexual dysfunction.
When preparing for surgery, one should pay special attention to cardiopulmonary status and to correction of any coagulation, electrolyte, or metabolic disorders. A careful neurologic examination is required. The objective is to have a baseline assessment against which any postoperative neurologic deterioration can be detected.
Some surgeons routinely pretreat patients with one dose or several doses of steroids (eg, dexamethasone). Steroids may help with perioperative edema or may improve the tolerance of neurologic tissue, and patients may have improved outcomes.
Patients on anticoagulation therapy or aspirin prophylaxis should stop their medications prior to surgery. Laboratory confirmation should be obtained to demonstrate that the coagulation profile and bleeding time and the in vitro platelet function analysis are acceptable.
Intraoperative details
Prophylactic antibiotic is administered intraoperatively preferably 1 hour before skin incision. A posterior laminectomy is performed over the appropriate lumbar or thoracic region. An intraoperative ultrasound study is typically performed if a question remains about the extent or precise localization of the tumor.
The dura mater is opened and the spinal cord is divided at the midline. For delicate debulking of the tumor, an operating microscope and microsurgical instruments are used. An ultrasonic aspirator or a surgical laser may be helpful with intramedullary lesions, so as not to disrupt tissue planes.
The area around the tumor may have increased vascularity with enlarged veins. Special care should be taken when a myelotomy (opening of the spinal cord) is performed. Stay sutures in the pia may be used to help with exposure. Although a cavity is left after the procedure, the defect in the neural tissue does not need to be closed. The dura is closed in the usual watertight manner.
Attention to hemostasis is essential. Some intradural-intramedullary tumors separate out readily; others need to be removed in a piecemeal manner. Tumors of the cauda equina are usually easier to remove completely compared with those of the conus because they are extramedullary. Special care should be taken with removal of dermoid and epidermoid tumors. Spilling their irritating contents into the subarachnoid space may potentially cause adhesions, arachnoiditis, and inflammation.
Special intraoperative neuromonitoring techniques may be used for cauda equina and conus medullaris tumors (CECMT). Pudendal somatosensory evoked potential (SEP) and bulbocavernosus reflex (BCR) are useful tests for monitoring CECMT surgeries. BCR is an easily obtainable modality for preserving sacral functions and is recommended as a primary monitoring modality in conjunction with traditional neurophysiologic techniques during CECMT surgery. [21, 22]
Postoperative details
Attention should be given to any potential cardiovascular or pulmonary problems. Postoperatively, patients should be given prophylaxis for deep vein thrombosis. Some surgeons use a brief course of postoperative steroids and antibiotics. Serial neurologic examinations should be performed. In addition, resumption of normal bowel and bladder function should be monitored carefully. A postoperative imaging study (MRI) should be obtained to confirm resection of the lesion or extent of postoperative residual tumor.
Follow-up
Patients should receive appropriate follow-up care and instructions. The hospital should provide discharge instructions, with explicit instructions about wound infection and any neurologic deterioration. Follow-up visits for wound care should be conducted in a timely fashion.
Appointments for possible adjuvant chemotherapy or radiation therapy should be set up. [23, 24, 25]
The patient should be observed periodically for a period of several years to monitor for recurrence.
Complications of Surgery and Postsurgical Pain
Complications of spinal surgery are similar to those of any other major surgery, including risks of anesthesia, acute myocardial infarction, deep venous thrombosis, pulmonary embolism, pneumonia, and other pulmonary complications.
Careful preoperative screening and preparation of patients, attention to detail, and aggressive postsurgical rehabilitation can help minimize the risk of complications.
Pain
Postsurgical pain resulting from manipulation of neural tissue has been categorized as somatic or central.
Somatic or acute pain results from manipulation of nerves and nerve roots. One example is traumatic retraction of a nerve root during surgery upon manipulation and dissection. Steroids (eg, dexamethasone) may be helpful for easing the pain, presumably by minimizing inflammation. Prolonged use of narcotics should be avoided, but they can be used for short-term relief.
Central pain results from resection of intramedullary tumors and is a chronic, persistent pain that is difficult to control. The pain is described as gnawing, sometimes burning; it can be triggered by light touch and may extend well beyond the area of stimulation. The mechanisms are not well understood, and the pain does not respond well to drugs or stimulators.
Worsening of neurologic deficits
Exposure and manipulation of the cord and the dural sac, the spinal cord, or the nerve roots can cause worsening of neurologic deficits. Great care must be taken with dissection and manipulation of the spinal cord, especially when tumors are large and occupy most of the spinal canal.
Infection
Patients are at risk for wound infection or meningitis. Risk factors for infectious complications include diabetes, obesity, revision surgery, and prior radiation therapy or chemotherapy.
Postoperative hematoma
Hematoma in the surgical area is recognized by immediate progressive deterioration of spinal cord or nerve root function. Radiologic confirmation can be obtained by CT scan or MRI of the surgical area. If radiographic studies are not available, the surgeon should consider reexploring the surgical site. Aggressive management of a possible hematoma may protect against permanent neural deficits.
Postoperative spinal fluid leak
This complication occurs as the result of an incompletely closed dura, perhaps discovered after poor healing of the skin and soft tissue over the incision site is noticed. In general, a patient who has received prior radiation therapy is more likely to develop this problem.
Postoperative instability after extensive laminectomy
Small laminectomies of the lumbar region do not usually result in the development of instability; however, more extensive laminectomies (>3 levels) and those that are wide and may involve the articular facets may lead to instability. Younger patients are at higher risk for instability, as are patients with severe neurologic deficits.
Flexion/extension plain radiographs prior to surgery may be compared to postoperative films. If progressive subluxation or a swan-neck deformity is identified, a stabilization procedure is indicated. The earlier the intervention is performed, the better the results will be.
Residual neurologic complications
These may include paresis or paralysis, spasticity, contractures, sensory deficits, bowel and bladder dysfunction, and erectile dysfunction. Preoperative deficits may improve after surgical intervention.
Prognosis
In general, the earlier the detection of tumor and the more minor the neurologic deficit, the better is the prognosis for treatment and recovery. Advanced age and severe neurologic deficits are associated with a poor prognosis. Correlation of the American Spinal Cord Injury Association (ASIA) impairment scale score with prognosis is presented in Cauda Equina and Conus Medullaris Syndromes.
Series of patients have reported a low incidence of recurrence. In general, recurrence depends on the grade of the tumor, the stage at which it was recognized, the completeness of resection, and the use of adjuvant radiation therapy or chemotherapy.
Patients with cauda equina tumors fare extremely well because of the high incidence of complete resection, with a high survival rate and good recovery of function. Patients with conus medullaris tumors also have a good prognosis. Patients with a gross total resection have a better outcome. Patients with ependymoma of the conus medullaris have 5-year survival rates of 70 to 100%. Long-term recurrence rates are reported to be 0 to 30%.
For astrocytoma, the survival rate at 1-5 years is 20-70%. The tumor is infiltrative; therefore, identifying a plane of cleavage and dissection is difficult. Usually, astrocytomas have a high incidence of recurrence, and mortality is correlated directly with recurrence. Incidences of intra-axial dissemination are also reported with aggressive or high-grade astrocytoma (grades 3 and 4).
Several small series have reported complete cure of the lowest-grade astrocytomas (grade 1), referred to as pilocytic astrocytomas. Some series demonstrate that patients receiving adjuvant radiation therapy or chemotherapy tend to fare better. Other series demonstrate excellent results without adjuvant therapy.
In children with ependymoma, favorable results have been achieved without radiation therapy. Risk-to-benefit considerations may justify avoiding postoperative radiation therapy for pediatric patients. In one study, up to 18% of patients experienced postoperative neurologic deterioration; however, in most cases, such deterioration was transient. [26] The new postoperative neurologic deficits were permanent in only 6% of patients with neurologic deterioration. [26]
Reversal of neurologic deficits depends on extent of dysfunction at the time of presentation. Patients with severe neurologic impairment are less likely to improve than those who are less impaired. In some instances of severe deficits, complete or near-complete recovery occurs. Predicting which patients will have significant neurologic improvement is difficult. Patients at advanced ages do not fare as well as younger patients with similar lesions.
-
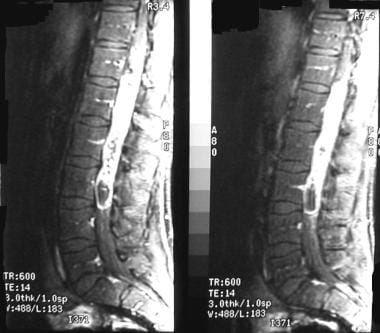
Tumor of the conus medullaris.
Tables
What would you like to print?
- Practice Essentials
- Anatomy
- Epidemiology
- Etiology
- Pathophysiology
- Presentation
- Differential Diagnosis
- Workup Overview
- Magnetic Resonance Imaging
- Plain Radiographs
- Computed Tomography
- Other Studies
- Treatment Approach
- Tumor Resection
- Complications of Surgery and Postsurgical Pain
- Prognosis
- Show All
- References