Practice Essentials
Benign salivary gland tumors represent a diverse group of neoplasms with varied clinical behaviors. In 2022, the World Health Organization (WHO) introduced the fifth edition of their classification for salivary gland tumors, [1] which included multiple newly described benign tumors. Successful management of these neoplasms relies on accurate clinical and diagnostic assessment, as well as appropriate therapeutic intervention. A strong understanding of the biologic behavior of the various types of lesions allows the development of an appropriate treatment plan tailored to each individual patient affected.
Tumors of the major and minor salivary glands account for only 2-4% of head and neck neoplasms. Most (~70%) salivary gland tumors originate in the parotid gland, with the bulk of the remainder arising in the submandibular gland (~8%) and in the minor salivary glands (~22%). [2, 3] Although 75% of parotid gland tumors are benign, slightly more than 50% of tumors of the submandibular gland and 60-80% of minor salivary gland tumors are found to be malignant. [4]
Benign minor salivary gland tumors thus are relatively uncommon neoplastic entities. Of these, pleomorphic adenoma, cystadenoma, and canalicular adenoma are the most frequently encountered. [5]
The ubiquitous deposition of the minor salivary glands complicates the diagnosis and management of salivary gland tumors. The approach to a suspected tumor of the minor salivary glands begins with a thorough history and a physical examination. Radiographic imaging (computed tomography [CT] with or without magnetic resonance imaging [MRI]) and a histopathologic diagnosis (obtained via fine-needle aspiration [FNA] cytology) often provide useful diagnostic information prior to definitive surgical therapy.
The general consensus is that definitive surgical therapy is indicated for most benign tumors of the minor salivary glands (with some notable exceptions). (See Treatment.) The primary contraindication for surgical treatment of benign salivary gland tumors is the presence of associated medical comorbidities that preclude the use of general anesthesia.
Anatomy
The minor salivary glands consist of 600-1000 smaller, unnamed glands distributed throughout the upper aerodigestive tract (ie, the palate, lip, pharynx, nasopharynx, larynx, and parapharyngeal space). The majority (70-90%) are located in the oral cavity and oropharynx, including the lateral tongue, lips, buccal mucosa, palate, and retromolar pad. The greatest glandular densities are located within the hard (250) and soft (150) palates. The remaining glands are located in the nose, paranasal sinuses, pharynx, and larynx.
As compared with the major salivary glands, the minor salivary glands are more numerous, are reduced in volume with regard to tissue size, have an abbreviated duct system, and have a paucity of capsular tissue. Overall, they contribute about 8-10% of the volume of unstimulated and stimulated whole saliva.
The minor salivary glands are commonly classified according to their anatomic location—for example, labial glands (upper and lower lips), buccal glands, and so forth. Palatal minor salivary glands are the most common anatomic subsite affected by minor salivary gland tumors; most series identify a nearly equal distribution between benign and malignant tumors. [6]
Pathophysiology
There are four major theories of salivary gland neoplastic pathogenesis, as follows:
-
Basal reserve theory - Basal cells of excretory and intercalated ducts are stem cells involved in the differentiation of mature salivary functional units and therefore are capable of tumor formation [7]
-
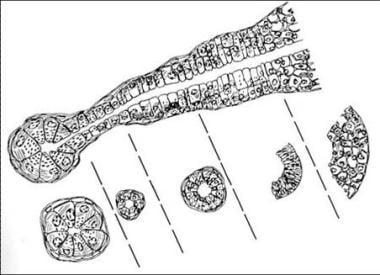
Pluripotent unicellular reserve cell theory - Basal cells of the excretory duct responsible for the formation of the salivary gland functional unit (see the image below) are implicated in tumor induction [7]
-
Semipluripotent bicellular reserve cell theory - Excretory and intercalated duct reserve cells are responsible for salivary gland tumors; tumors arise from one of these two stem cell populations, with squamous cell carcinomas and mucoepidermoid carcinomas arising from stem cells of the excretory duct and other tumor types arising from intercalated duct reserve cells [8]
-
Multicellular theory - Lesions originate from many different cell types at different stages of differentiation [9] ; mixed tumors originate from the intercalated duct cells and myoepithelial cells, oncocytic tumors from the striated duct cells, acinic cell tumors from the acinar cells, and mucoepidermoid and squamous cell tumors from the excretory duct cells
Etiology
Etiologic factors for the development of benign salivary gland neoplasms are not well understood; however, both environmental and genetic factors have been proposed as potential causes.
Low-dose radiation has been implicated in the development of benign salivary gland tumors. The strongest evidence for this association is derived from analysis of the incidence of salivary gland neoplasms in atomic bomb survivors. [10] Supporting evidence is also provided by analysis of the relative risk conferred by exposure to early therapeutic external beam irradiation.
Exposure to diagnostic dental radiographs has also been found to confer an increased risk for salivary gland neoplasms, but this higher risk may be attributable to the greater radiation exposure inherent in older technology as compared with the reduced exposure characteristic of current low-dose diagnostic radiographs. [11]
The latency period for the development of a radiation-induced salivary gland tumor appears to be in the range of 15-20 years.
Epstein-Barr virus (EBV) may be a factor in the development of lymphoepithelial tumors of the salivary glands.
The molecular genetic basis for the development of benign salivary gland neoplasms has become more widely understood and reported. As a result, molecular analysis of tumors is increasingly commonplace. Cytogenetic studies demonstrate a high incidence of translocations and intra-chromosomal rearrangements affecting 8q12 (50% of cases) and 12q13-15 (10-15% of cases) specific for benign pleomorphic adenomas, resulting in gene fusions involving the transcription factor oncogenes PLAG1 (a zinc finger protein) and HMGA2. [12]
One study detected increased immunoglobulin G4 (IgG4) plasma cells in patients with lymphadenoma; this finding suggests that an immune reaction is implicated in the pathogenesis. [13]
Many more genetic alterations in salivary tumors have been described. As additional molecular changes are identified, more classes of salivary neoplasms will surely be defined in the future.
Epidemiology
Between 60% and 80% of all minor salivary gland tumors are malignant. Overall, adenoid cystic carcinoma is the most common malignant tumor of all minor salivary glands. [14] In a study of 485 cases of minor salivary gland tumors from northeastern China, pleomorphic adenoma was found to be the most common type of benign tumor, and adenoid cystic carcinoma was the most common type of malignant tumor. [15]
Most benign salivary gland tumors (95%) occur in adults, with the clear majority pathologically identified as pleomorphic adenomas. In children, the most common benign tumors of mesenchymal origin are hemangiomas, and the most common benign epithelial tumors are pleomorphic adenomas. [16]
Prognosis
With appropriate treatment of benign salivary gland tumors, the outcome is excellent, and the recurrence rate is very low.
In general, pleomorphic adenoma has the highest risk for malignant transformation and recurrence of all benign salivary gland tumors. Basal cell adenomas also carry a risk of malignant transformation, albeit a much smaller one, whereas the other types virtually never develop into malignancy. Recurrence is unlikely for oncocytoma, canalicular adenoma, myoepithelioma, and basal cell adenoma (membranous subtype). Recurrence is extremely rare for lymphadenoma, cystadenoma, and basal cell adenoma (solid, trabecular, and tubular subtypes). [17]
-
Histologic architecture of the salivary gland unit.
-
Coronal magnetic resonance imaging (MRI) scan demonstrating a large, benign lesion of the right parapharyngeal space.