Practice Essentials
Pancreatic cancer is the tenth most common cancer in men and the seventh most common in women, but it is the fourth leading cause of cancer deaths in men and the third leading cause in women; it accounts for about 3% of all cancers in the United States but is responsible for about 8% of all cancer-related deaths. Both the incidence and the death rates for pancreatic cancer are increasing. [1] The disease remains elusive to effective screening approaches and in 80% of cases presents at an unresectable or incurable stage. See the image below.
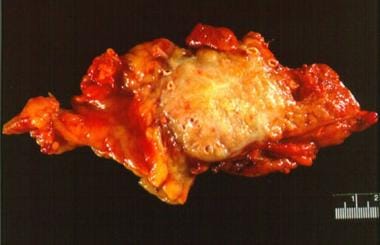 Pancreatic cancer. Gross section of an adenocarcinoma of the pancreas measuring 5 × 6 cm resected from the pancreatic body and tail. Although the tumor was considered to have been fully resected and had not spread to any nodes, the patient died of recurrent cancer within 1 year.
Pancreatic cancer. Gross section of an adenocarcinoma of the pancreas measuring 5 × 6 cm resected from the pancreatic body and tail. Although the tumor was considered to have been fully resected and had not spread to any nodes, the patient died of recurrent cancer within 1 year.
Signs and symptoms
The initial manifestations of pancreatic cancer are often nonspecific, and consequently are often misinterpreted. Patients with pancreatic cancer may present with the following signs and symptoms:
-
Significant weight loss
-
Mid-epigastric pain: Common symptom of pancreatic cancer, sometimes with radiation of the pain to the midback or lower-back region
-
Nighttime pain: Often a predominant complaint
-
Onset of diabetes mellitus within the previous 2 years
-
Painless obstructive jaundice
-
Pruritus
-
Migratory thrombophlebitis (ie, Trousseau sign) and deep venous thrombosis
-
Palpable gallbladder (ie, Courvoisier sign)
-
Presence of ascites, a palpable abdominal mass, hepatomegaly from liver metastases, or splenomegaly from portal vein obstruction
-
Paraumbilical subcutaneous metastases (or Sister Mary Joseph nodule or nodules)
See Presentation for more detail.
Diagnosis
Pancreatic cancer is notoriously difficult to diagnose in its early stages. [2]
Testing
Laboratory findings in patients with pancreatic cancer are usually nonspecific. Occasionally, the presence of pancreatic cancer is suggested by elevated liver transaminase, bilirubin, and alkaline phosphatase levels on testing performed for other purposes. Patients with advanced pancreatic cancer and weight loss have laboratory evidence of malnutrition (eg, low serum albumin or cholesterol level).
Potentially useful tests in patients with suspected pancreatic cancer include the following:
-
CBC count
-
Hepatobiliary tests - Patients with obstructive jaundice show significant elevations in bilirubin (conjugated and total), ALP, GGT, and, to a lesser extent, AST and ALT
-
Serum amylase and/or lipase levels - Elevated in less than 50% of patients with resectable pancreatic cancers and in only 25% of patients with unresectable tumors
-
Tumor markers - 75-85% of patients have elevated CA 19-9 levels; 40-45% have elevated CEA levels
-
Genetic testing - This includes tests for germline mutations such as BRCA1/2; testing for high microsatellite instability (MSI-H), which may indicate Lynch syndrome; and next-generation sequencing for somatic genetic alterations in tumor tissue, in patients with advanced disease
Imaging studies
Imaging studies that aid in the diagnosis of pancreatic cancer include the following:
-
CT scanning
-
Transcutaneous ultrasonography
-
Endoscopic ultrasonography
-
Magnetic resonance imaging
-
Endoscopic retrograde cholangiopancreatography
-
Positron emission tomography scanning
See Workup for more detail.
Management
Surgery is the only potentially curative treatment modality for early-stage pancreatic cancer. However, surgery is increasingly incorporated in a multimodality approach that includes neoadjuvant and adjuvant chemotherapy and/or radiation therapy.
Surgical options
Potentially curative resection options include the following:
-
Pancreaticoduodenectomy (Whipple procedure), with/without sparing of the pylorus
-
Total pancreatectomy
-
Distal pancreatectomy
Chemotherapy:
-
FOLFIRINOX (folinic acid [leucovorin], 5-fluorouracil [5-FU], irinotecan, oxaliplatin) - Neoadjuvant and adjuvant therapy, and first-line therapy in metastatic disease
-
Irinotecan liposome with oxaliplatin, 5-FU, and leucovorin, for first-line therapy in metastatic disease [5]
-
Gemcitabine monotherapy - Often in a palliative setting in frail patients
-
Gemcitabine plus capecitabine (GemCap) - In the adjuvant setting, for patients who cannot tolerate FOLFIRINOX
-
Erlotinib plus gemcitabine - Third-line therapy [6]
-
OFF (oxaliplatin, folinic acid, 5-FU) - Second- or third-line therapy
-
CapeOx (capecitabine, oxaliplatin) - Second- or third-line therapy
-
Larotrectinib or entrectinib - Second-line therapy in patients with tumors harboring NTRK fusions [7]
-
Pembrolizumab (with or without ipilimumab) - For MSI-H or deficient mismatch repair (dMMR) tumors or in patients with Lynch syndrome [8]
-
Gemcitabine + cisplatin - Only for known BRCA1/2 or PALB2 mutations)
Maintenance therapy:
-
Olaparib - Oral maintenance treatment for adults with deleterious or suspected deleterious germline BRCA-mutated metastatic pancreatic adenocarcinoma whose disease has not progressed on at least 16 weeks of a first-line platinum-based chemotherapy regimen. [9]
Supportive care:
-
Used in conjunction with active anticancer therapy or as a primary modality in patients approaching end of life
-
Pain relief - Non-opioid and opioid analgesics, celiac plexus lysis performed endoscopically or under CT guidance.
-
Endoscopic interventions for biliary or duodenal obstruction secondary to pancreatic carcinoma
-
Dietary and nutritional support, managment of pancreatic insufficiency
-
Behavioral support focusing on coping mechanisms, anxiety, and depression for both patients and their families
See Treatment and Medication for more detail.
For patient education information, see Pancreatic Cancer.
Pathophysiology
Pancreatic cancers can arise from the exocrine or endocrine portions of the pancreas, but the vast majority develop in the exocrine portion, including the ductal epithelium, acinar cells, connective tissue, and lymphatic tissue. Of all pancreatic malignancies, 90% are adenocarcinomas of the ductal epithelium (pancreatic ductal adenocarcinoma [PDAC]). [2] Less common histologic variants of PDAC, which usually present in a similar fashion, include acinar cell adenocarcinoma (AAC), adenosquamous carcinoma (ASCP) , and cystadenocarcinoma (serous and mucinous types). Rare pancreatic malignancies include the following [2]
-
Giant cell carcinoma
-
Invasive adenocarcinoma associated with cystic mucinous neoplasm or intraductal papillary mucinous neoplasm
-
Mixed type (ductal or acinar endocrine)
-
Mucinous carcinoma
-
Pancreatoblastoma
-
Papillary-cystic neoplasm (Frantz tumor); this tumor has lower malignant potential and may be curable with surgery alone
-
Papillary mucinous carcinoma
-
Signet ring carcinoma
-
Small cell carcinoma
-
Undifferentiated carcinoma
Pancreatic neoplasms with borderline malignancy include the following [2] :
-
Intraductal papillary mucinous tumor with dysplasia
-
Mucinous cystic tumor with dysplasia
-
Pseudopapillary solid tumor
AAC is a rare variant of pancreatic adenocarcinoma. [10] It is more common in young adults and adolescents. While it usually presents at an advanced and unresectable stage, it does portend a somewhat better prognosis than PDAC; 5-year survival is around 45%, much higher than the 7-10% for PDAC. [11]
Unlike PDAC, ACCs often harbor potentially targetable genetic abnormalities such as in BRCA1/2 (somatic and germline up to 22%), PALB2, BAP1, ATM, JAK1, BRAF, and ID3. [12, 13] The molecular and genomic signature of ACC is characterized by relative rarity of mutations in KRAS (2%), TP53 (15%), CDKN2 (14%), and SMAD (16%). [14] At the clinicopathologic level, these malignancies are characterized by a relative lack of desmoplastic reaction and stroma. [10]
ASCP represents another rare and distinct variant. [15] All ASCPs exhibit mutations in KRAS. Other characteristic features include a high frequency of mutations in TP53 (50%) and SMAD4 (25%), high TOPO2A expression, and low ERCC1 and TS expression (suggestive of sensitivity to platinum, TOPO2 inhibitors, and fluoropyrimidines). [16] Histologically, ASCP is distinguished by a characteristic mixed pathology with glandular and squamous epithelium.
Surveillance, Epidemiology, and End Results (SEER) data suggest that patients with ASCP have poorer outcomes than similarly staged patients with PDAC.Patients usually present with advanced-stage disease, elevated levels of CEA (in addition to CA 19.9), a high degree of lymph node involvement, and hypercalcemia. [17]
Metastasis
Typically, pancreatic cancer first metastasizes to regional lymph nodes, then to the liver and, less commonly, to the lungs. It can also directly invade surrounding visceral organs such as the duodenum, stomach, and colon, or it can metastasize to any surface in the abdominal cavity via peritoneal spread. Ascites may result, and this has an ominous prognosis. Pancreatic cancer may spread to the skin as painful nodular metastases. Metastasis to bone is uncommon.
Pancreatic cancer rarely spreads to the brain, but it can produce meningeal carcinomatosis.
Etiology
Tobacco smoking is the most common recognized risk factor for pancreatic cancer. [18] Other factors include the following [19] :
-
Obesity
-
Diabetes mellitus
-
Chronic pancreatitis
-
Family history of pancreatic cancer
-
Hereditary syndromes [19]
-
Lifestyle factors [20]
Less than 5% of all pancreatic cancers are related to underlying chronic pancreatitis. [21] Alcohol consumption does not appear to be an independent risk factor for pancreatic cancer unless it is associated with chronic pancreatitis.
Whether hepatitis B infection is a risk factor for pancreatic cancer has been controversial. However, more recent data support a link between the two diseases. [22, 23]
Smoking
Smoking is the most common environmental risk factor for pancreatic carcinoma. Estimates indicate that smoking accounts for up to 30% of cases of pancreatic cancer. [18]
People who smoke have at least a 2-fold greater risk for pancreatic cancer than do nonsmokers. Current smokers with over a 40 pack-year history of smoking may have up to a 5-fold risk greater risk for the disease. Smokeless tobacco also increases the risk of pancreatic cancer.
Obesity and dietary factors
In a number of studies, obesity, especially central, has been associated with a higher incidence of pancreatic cancer. For example, Li et al found that being overweight or obese during early adulthood was associated with a greater risk of pancreatic cancer and a younger age of disease onset, while obesity at an older age was associated with lower overall survival. [24] Several other studies have supported a link between early obesity and the risk of pancreatic cancer. [25, 26]
The incidence of pancreatic cancer is lower in persons with a diet rich in fresh fruits and vegetables. Fruits and vegetables rich in folate and lycopenes (such as tomatoes) may be especially good at reducing the risk of pancreatic cancer. [27, 28]
Consumption of red meat, especially of the processed kinds, is associated with a higher risk of pancreatic cancer. Poultry and dairy product consumption does not increase the risk of this disease. [29]
Despite early reports to the contrary, coffee consumption is not associated with an increased risk of pancreatic cancer. [30]
Diabetes mellitus
In patients recently diagnosed with diabetes mellitus, risk for pancreatic cancer is 5.4 fold above average. It has been suggested that diabetes may be at least in part a consequence or an early manifestation of pancreatic cancer. [31] However, the International Pancreatic Cancer Case-Control Consortium reported that a 30% excess risk for pancreatic cancer persists for more than 2 decades after diabetes diagnosis, which supports the hypothesis that the relation between diabetes and pancreatic cancer is bidirectional. [32]
A systematic review of 30 studies concluded that patients with diabetes mellitus of at least 5-years' duration have a 2-fold increased risk of developing pancreatic carcinoma. Pancreatic cancer may follow 18-36 months after a diagnosis of diabetes mellitus in elderly patients with no family history of diabetes mellitus.
The National Comprehensive Cancer Network (NCCN) acknowledges long-standing diabetes mellitus as a risk factor for pancreatic cancer. The NCCN also notes an association between sudden onset of type 2 diabetes mellitus in an adult older than 50 years and a new diagnosis of pancreatic cancer, although in those cases the diabetes is thought to be caused by the cancer. [33]
Chronic pancreatitis
Long-standing, chronic pancreatitis is a substantial risk factor for the development of pancreatic cancer. A multicenter study of more than 2000 patients with chronic pancreatitis showed a 26-fold increase in the risk of developing pancreatic cancer. This risk increased linearly with time, with 4% of patients who had chronic pancreatitis for 20 years' duration developing pancreatic cancer. [34]
The risk of pancreatic cancer is even higher in patients with hereditary pancreatitis. The mean age of development of pancreatic cancer in these patients is approximately 57 years, compared with age 70 years for pancreatic cancer in general. The relative risk of pancreatic cancer in hereditary pancreatitis is increased more than 50-fold, and the cumulative risk rate of pancreatic cancer by age 70 years is 40%.
This cumulative risk increases to 75% in persons whose family has a paternal inheritance pattern. [35]
Chronic pancreatitis from alcohol consumption is also associated a much higher incidence and an earlier age of onset of pancreatic carcinoma. [36]
Genetic factors
Approximately 5-10% of patients with pancreatic carcinoma have some genetic predisposition to developing the disease. [37]
Certain precursor lesions have been associated with pancreatic tumors arising from the ductal epithelium of the pancreas. The main morphologic form associated with ductal adenocarcinoma of the pancreas is pancreatic intraepithelial neoplasia (PIN). These lesions arise from specific genetic mutations and cellular alterations that contribute to the development of invasive ductal adenocarcinoma. [38]
The initial alterations appear to be related to KRAS2 gene mutations and telomere shortening. Thereafter, p16/CDKN2A is inactivated. Finally, the inactivation of TP53 and MAD4/DPC4 occur. These mutations have been correlated with increasing development of dysplasia and thus with the development of ductal carcinoma of the exocrine pancreas.
Based on more recent data from sequencing of human tumors, pancreatic cancer is a genetically complex and heterogeneous disease. [39] This is confounded by considerable variability in terms of the genetic malformations and pathways involved between individual tumors. In addition, the long time from early to clinically manifested disease (21.2 y on average) allows for an accumulation of complex genetic changes, which probably explains the fact that it is often resistant to chemotherapy and radiation therapy. [40, 41]
The inherited disorders that increase the risk of pancreatic cancer include the following:
-
Familial atypical multiple mole melanoma ( FAMMM) syndrome
-
Germline mutations in the BRCA1 and BRCA2 genes
Hereditary pancreatitis has been associated with a 40% cumulative risk of developing pancreatic cancer at 40%. [35] MEN-1 and VHL are other genetic syndromes associated with pancreatic endocrine tumor development.
About 50% of individuals with MEN-1 develop symptomatic pancreatic endocrine tumors, and those pancreatic tumors are noted to be the leading cause of disease-specific mortality. [42] In patients with VHL, 17% of masses found in the pancreas have proved to be malignant. [43]
Syndromes associated with an increased risk of the development of colon cancer, such as HNPCC and FAP (and Gardner syndrome), have also shown an increased correlation with pancreatic cancer, but the statistics have not been impressive.
In a cohort study of 1391 patients with FAP, only 4 developed pancreatic adenocarcinoma. No statistics are available to show the incidence of pancreatic cancer in patients with HNPCC. [44]
FAMMM has been shown to increase relative risk of developing pancreatic cancer by 13- to 22-fold and the incidence in sporadic cases to be 98%. [45]
The above disorders have specific genetic abnormalities associated with the noted increased risk of pancreatic cancer. Pancreatic cancer in hereditary pancreatitis is associated with a mutation in the PRSS1 gene. Pancreatic cancer appearing in FAP and HNPCC has been associated with a mutation in the APC gene and MSH2 and MLH1 genes respectively. FAMMM and pancreatic cancer has been associated with a mutation in CDKN2A. Endocrine tumors of the pancreas associated with VHL are thought to develop by way of the inactivation of the VHL tumor suppressor gene. [37]
Germline mutations in BRCA1 and BRCA2 have been shown to moderately increase the risk of developing pancreatic cancer by 2.3- to 3.6-fold, but BRCA2 has been associated more commonly with pancreatic cancer, at an incidence of 7%. [37]
The NCCN recommends genetic evaluation and germline testing in every patient diagnosed with exocrine pancreatic cancer, as well as their first-degree relatives, and in patients diagnosed with neuroendocrine pancreatic tumors. [46] This is important because pancreatic cancer is thought to have a hereditary component in about 10% of cases, and family history is often poorly predictive.
Race-related factors
Black men in the United States have the highest incidence rate of pancreatic cancer. [47] (See Epidemiology.) The reasons for this are unclear. Certainly, differences in risk factors for pancreatic cancer, such as dietary habits, obesity, and the frequency of cigarette smoking, are recognized among different population groups and may contribute to the higher incidence of this disease among Blacks.
However, Arnold et al found that excess pancreatic cancer in Blacks cannot be attributed to currently known risk factors, suggesting that as-yet undetermined factors play a role in the disease process. [48] One possibility is a difference in the underlying frequency of predisposing genetic mutations for pancreatic cancer.
Epidemiology
Incidence in the United States
The American Cancer Society estimates that in the United States in 2024, about 66,440 new cases of pancreatic cancer (34,530 in men and 31,910 in women) will be diagnosed. [1] Over 2010-2019, age-adjusted rates for new pancreatic cancer cases rose on average 0.9% each year. [47]
International incidence
Worldwide, pancreatic cancer ranks 11th in incidence but 7th as a cause of cancer death. [49] The age-standardized rate (ASR) incidence ranges widely, from 7.7 per 100,000 population in Europe to 2.2 per 100,000 population in Africa. Among individual countries, ASRs range from 0.81 per 100,000 in males in India to 15.3 per 100,000 in males in Latvia and the Republic of Moldova. [49]
Race predilection
From 2016 to 2020, the highest incidence rate of pancreatic cancer in the United States was 17.6 cases per 100,000 persons per year, in Black men. The incidences in men in other racial/ethnic groups were as follows [47] :
-
White: 15.1
-
Hispanic: 12.7
-
American Indian/Alaska Native: 16.5
-
Asian/Pacific Islander: 10.9
The incidences in US women during that period were as follows [47] :
-
Black: 14.9
-
White: 11.8
-
Hispanic: 11.3
-
American Indian/Alaska Native: 11.0
-
Asian/Pacific Islander: 9.2
Age predilection
In the absence of predisposing conditions, such as familial pancreatic cancer and chronic pancreatitis, pancreatic cancer is unusual in persons younger than 45 years. After age 50 years, the frequency of pancreatic cancer increases linearly. About 90% of patients are age 55 years or older at diagnosis; the median age at diagnosis is 70 years. [47]
However, emerging data are showing a disproportionate increase in early-onset pancreatic cancer (ie, before age 50-55 years), especially in women. [50, 51] Risk factors such as obesity and smoking may be driving this trend. [52, 50]
Mortality
Although pancreatic cancer constitutes only about 3% of all cancers in the United States, it is the fourth leading cause of cancer deaths in both men and women, being responsible for 8% of all cancer-related deaths.The American Cancer Society estimates that in the United States in 2024, about 51,750 people (27,270 men and 24,480 women) will die of pancreatic cancer. [53] During 2008 to 2017, the death rate for pancreatic cancer increased slightly (by 0.4% per year) in Whites and decreased slightly (by 0.5% per year) in Blacks. [53]
Prognosis
Pancreatic carcinoma is unfortunately usually a fatal disease. For all stages combined, the relative 5-year survival rate is only 13%, although this ranges from 8% for exocrine tumors to 72% for neuroendocrine tumors. [1] Survival is also much better in patients with cystic neoplasms of the pancreas, such as mucinous cystadenocarcinomas or intraductal papillary mucinous neoplasms [IPMN], than in patients with pancreatic adenocarcinoma
By stage, 5-year relative survival is 44.3% for localized disease, 16.2% for regional disease, and 3.2% for distant disease. [47] At the time of diagnosis, 51% of patients have distant disease. [53]
The occasional patient with metastatic disease or locally advanced disease who survives beyond 2-3 years may die of complications of local spread, such as bleeding esophageal varices.
In patients able to undergo a successful curative resection (about 20% of patients), median survival time ranges from 12-19 months, and the 5-year survival rate is 15-20%.
Lee et al developed and validated a model for preoperative prediction of early recurrence risk for resected pancreatic cancer. The model uses clinical, radiographic, and CT radiomics features. Among the features predictive of early pancreatic cancer recurrence were Ca19-9 level > 500 U/mL (odds ratio [OR] 2.91), abutment of the tumor to the portal vein and/or superior mesenteric vein (OR 2.14), and adjacent organ invasion (OR, 2.39). [54]
Tingle et al reported that in patients with unresectable pancreatic ductal adenocarcinoma, the combination of the neutrophil-albumin ratio (NAR) and the Ca19-9 level allows stratification into three groups with significantly different overall survival, as follows [55] :
-
NAR ≤ 0.13 and Ca19-9 ≤ 770 U/mL - Median survival 20.5 months
-
NAR > 0.13 or Ca19-9 > 770 - Median survival 9.7 months
-
NAR > 0.13 and Ca19-9 > 770 - Median survival 4.1 months
Patient Education
Smoking is the most significant reversible risk factor for pancreatic cancer. To help lower the risk of pancreatic cancer, the American Cancer Society recommends not smoking, as well as following a healthy eating pattern, getting regular physical activity, limiting alcohol consumption, and limiting exposure to certain chemicals in the workplace. [56]
Alcohol consumption does not increase the risk of pancreatic cancer unless it leads to chronic pancreatitis. A multicenter study of more than 2000 patients with chronic pancreatitis showed a 26-fold increase in the risk of developing pancreatic cancer. [34]
For patient education information, see Pancreatic Cancer.
-
Pancreatic cancer. Gross section of an adenocarcinoma of the pancreas measuring 5 × 6 cm resected from the pancreatic body and tail. Although the tumor was considered to have been fully resected and had not spread to any nodes, the patient died of recurrent cancer within 1 year.
-
Pancreatic cancer. Hematoxylin and eosin stain of a pancreatic carcinoma. Note the intense desmoplastic response around the neoplastic cells. The large amount of fibrotic reaction in these tumors can make obtaining adequate tissue by fine-needle aspiration difficult.
-
Pancreatic cancer. T staging for pancreatic carcinoma. T1 and T2 stages are confined to the pancreatic parenchyma. T3 lesions invade local structures such as the duodenum, bile duct, and/or major peripancreatic veins, and T4 lesions invade surrounding organs (eg, stomach, colon, liver) or invade major arteries such as the superior mesenteric or celiac arteries.
-
Pancreatic cancer. Computerized tomographic scan showing a pancreatic adenocarcinoma of the pancreatic head. The gallbladder (gb) is distended because of biliary obstruction. The superior mesenteric artery (sma) is surrounded by tumor, making this an unresectable T4 lesion.
-
Pancreatic cancer. Abdominal CT scan of a small, vaguely seen, 2-cm pancreatic adenocarcinoma (mass) causing obstruction of both the common bile duct (cbd) and pancreatic duct (pd).
-
Pancreatic cancer. Endoscopic ultrasound of a 2.2-cm pancreatic adenocarcinoma of the head of the pancreas obstructing the common bile duct (CBD) but not invading the portal vein (PV) or superior mesenteric vein (SMV). Findings from endoscopic ultrasound–guided fine-needle aspiration revealed a moderately to poorly differentiated adenocarcinoma. Abdominal CT findings did not show this mass, and an attempt at endoscopic retrograde cholangiopancreatography at another institution was unsuccessful.
-
Algorithm for evaluation of a patient with suspected pancreatic cancer. CT scanning for definitive diagnosis and staging must be with thin-cut, multidetector, spiral CT scanning using dual-phase contrast imaging to allow for maximal information. This schema varies among institutions depending on local expertise, research interest, and therapeutic protocols for pancreatic carcinoma.
-
Pancreatic cancer. Tip of linear array echoendoscope (Pentax FG 36UX) with 22-gauge aspiration needle exiting from biopsy channel. Insert shows magnification of aspiration needle tip. Note that the needle exits from the biopsy channel such that it appears continuously in the view of the ultrasonic transducer on the tip of the echoendoscope.
-
Pancreatic cancer. Cytologic samples from fine-needle aspirations (rapid Papanicolaou stain) of pancreatic adenocarcinomas. (A) Well differentiated, (B) moderately differentiated, (C) moderate to poorly differentiated, (D) poorly differentiated tumor.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Approach Considerations
- Laboratory Findings
- Tumor Markers
- Computed Tomography
- Transcutaneous Ultrasonography
- Endoscopic Ultrasonography
- Endoscopic Retrograde Cholangiopancreatography
- Magnetic Resonance Imaging
- PET Scanning
- Biopsy
- Histologic Findings
- Germline Testing and Molecular Analysis
- Staging
- Evaluation Algorithm
- Show All
- Treatment
- Guidelines
- Medication
- Questions & Answers
- Media Gallery
- References








