Practice Essentials
Amenorrhea is the absence of menstrual bleeding. [1] Amenorrhea is a normal feature in prepubertal, pregnant, and postmenopausal females. In females of reproductive age, diagnosing amenorrhea is a matter of first determining whether pregnancy is the etiology. In the absence of pregnancy, the challenge is to determine the exact cause of absent menses. [2]
Primary amenorrhea is the failure of menses to occur by age 16 years, in the presence of normal growth and secondary sexual characteristics. If by age 13 menses has not occurred and the onset of puberty, such as breast development, is absent, a workup for primary amenorrhea should start.
Secondary amenorrhea is defined as the cessation of menses sometime after menarche has occurred. Oligomenorrhea is defined as menses occurring at intervals longer than 35 days apart.
No consensus has been reached regarding the point at which oligomenorrhea becomes amenorrhea. Some authors suggest the absence of menses for 6 months constitutes amenorrhea, but the basis for this recommendation is unclear. For a post-menarchal girl or a reproductive-aged woman to experience a menstrual cycle interval of more than 90 days is statistically unusual. Practically speaking, this should be an indication for an evaluation to seek the cause.
Pathophysiology
The menstrual cycle is an orderly progression of coordinated hormonal events in the female body that stimulates growth of a follicle to release an egg and prepare a site for implantation if fertilization should occur. Menstruation occurs when an egg released by the ovary remains unfertilized; subsequently, the soggy decidua of the endometrium (which was primed to receive a fertilized egg) is sloughed in a flow of menses in preparation for another cycle.
The menstrual cycle can be divided into 3 physiologic phases: follicular, ovulatory, and luteal. Each phase has a distinct hormonal secretory milieu. Consideration of the target organs of these reproductive hormones (hypothalamus, pituitary, ovary, uterus) is helpful for identifying the disease process responsible for a patient’s amenorrhea.
Follicular phase
In physiologic terms, the first day of menses is considered the first day of the menstrual cycle. The following 13 days of the cycle are designated the follicular phase. As levels of progesterone, estradiol, and inhibin decline 2-3 days before menses, the pituitary begins to release higher levels of follicle-stimulating hormone (FSH), which recruits oocytes for the next menstrual cycle. The hypothalamus is the initiator of the follicular phase via gonadotropin-releasing hormone (GnRH).
The GnRH pump in the hypothalamus releases GnRH in a pulsatile fashion into the portal vessel system surrounding the anterior pituitary gland. GnRH interacts with the anterior pituitary gland to stimulate release of FSH in the follicular phase. FSH is secreted into the circulation and communicates with the granulosa cells surrounding the developing oocytes.
As FSH increases during the early portion of the follicular phase, it meshes with granulosa cells to stimulate the aromatization of androgens into estradiol. The increase in estradiol and FSH leads to an increase in FSH-receptor content in the many developing follicles.
Over the next several days, the steady increase of estradiol (E2) levels exerts a progressively greater suppressive influence on pituitary FSH release. Only one selected lead follicle, with the largest reservoir of estrogen, can withstand the declining FSH environment. The remaining oocytes that were initially recruited with the lead follicle undergo atresia.
Immediately prior to ovulation, the combination of E2 and FSH leads to the production of luteinizing-hormone (LH) receptors on the granulosa cells surrounding the lead follicle.
During the late follicular phase, estrogen has a positive influence on LH secretion, instead of suppressing pituitary LH secretion as it does early in the follicular phase. To have this positive effect, the E2 level must achieve a sustained elevation for several days. The LH surge promotes maturation of the dominant oocyte, the release of the oocyte and then the luteinization of the granulosa cells and the surrounding theca cells of the dominant follicle resulting in progesterone production.
The appropriate level of progesterone arising from the maturing dominant follicle contributes to the precise timing of the mid cycle surge of LH. E2 promotes uterine endometrial gland growth, which allows for future implantation.
Ovulatory phase
Ovulation occurs approximately 34-36 hours after the onset of the LH surge or 10-12 hours after the LH peak and 24-36 hours after peak E2 levels. The rise in progesterone increases the distensibility of the follicular wall and enhances proteolytic enzymatic activity, which eventually breaks down the collagenous follicular wall.
After the ovum is released, the granulosa cells increase in size and take on a yellowish pigmentation characteristic of lutein. The corpus luteum then produces estrogen, progesterone, and androgens and becomes increasingly vascularized.
Luteal phase
The lifespan and steroidogenic capacity of the corpus luteum depends on continued LH secretion from the pituitary gland. The corpus luteum secretes progesterone that interacts with the endometrium of the uterus to prepare it for implantation. This process is termed endometrial decidualization.
In the normal ovulatory menstrual cycle, the corpus luteum declines in function 9-11 days after ovulation. If the corpus luteum is not rescued by human chorionic gonadotropin (hCG) hormone from the developing placenta, menstruation reliably occurs 14 days after ovulation. If conception occurs, placental hCG interacts with the LH receptor to maintain luteal function until placental production of progesterone is well established.
Puberty
The orderly progression of puberty begins with breast budding (thelarche), accelerated growth, and menses (menarche). Adrenarche, sexual hair growth, is independent from GnRH function and typically occurs between breast budding and accelerated growth but may occur anywhere along the puberty timeline. Secretion of dehydroepiandrostenedione (DHEA) initiates adrenarche. In the United States, the average age of girls at menarche is 12.6 years, with a range of 9-15 years. (Age 15 years is 2 standard deviations above the mean, while age 16 years is 3 standard deviations above.) Progression of puberty requires exposure to estrogens.
Menses occurring on a predictable cyclic pattern are associated with follicle development and ovulation. At birth, female infants have a predetermined number of primordial follicles. During the first trimester of pregnancy, fetal oogonia increase in number by rapid mitosis. By the 16 to 20th week of pregnancy, up to 6 million oogonia are present and mitosis halts. After reaching a maximum number of oogonia cells at 20 weeks, supporting cells envelop the oocyte forming the primordial follicle. The oocyte within the primordial follicle will enter into meiosis I, arresting at the diplotene stage of prophase. Over the remaining 20 weeks of pregnancy, over 4 million oocytes will undergo spontaneous atresia resulting in 2 million oocytes available at birth. Atresia continues such that only 300,000 oocytes remain at the time of puberty. Up to 500 oocytes will ovulate during a woman’ reproductive life with the remainder undergoing apoptosis. The oocytes will remain arrested at Meiosis I unless the oocyte is chosen to progress to a primary follicle and eventual the dominant follicle chosen for ovulation. Upon the LH surge associated with ovulation, the oocyte is stimulated to complete meiosis 1 with subsequent arrest at metaphase of meiosis 2. Completion of meiosis 2 occurs after fertilization of a single sperm. At birth, female infants have a predetermined number of primordial follicles that are arrested during meiosis 1 at the diplotene stage of prophase until stimulation at puberty. Until puberty, the hypothalamus is in a quiescent state. At approximately age 8 years, the GnRH pump is reactivated under the primary control of Kisspeptin. [3]
Menarche and sustained menstrual cycles requires normal function of the endocrine axis comprising the hypothalamus, pituitary, and ovaries (see the image below). Any disruption in this axis may result in amenorrhea. Defining the level of primary dysfunction is critical in determining the pathophysiology of amenorrhea.
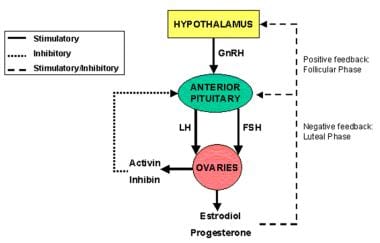 Hypothalamus, pituitary and ovaries form a functional endocrine axis, known as HPO axis with hormonal regulations and feedback loops.
Hypothalamus, pituitary and ovaries form a functional endocrine axis, known as HPO axis with hormonal regulations and feedback loops.
Types of amenorrhea based on HPO axis etiology
Hypothalamic amenorrhea
Hypothalamic dysfunction results in decreased or inhibited GnRH secretion, which affects the pulsatile release of pituitary gonadotropins, LH and FSH, causing anovulation. A common cause of amenorrhea is functional hypothalamic amenorrhea. [4] It is characterized by abnormal hypothalamic GnRH secretion, decreased gonadotropin pulsations, low or normal LH concentrations, absent LH surges, abnormal follicular development, and low serum estradiol. Serum FSH concentrations are usually in the normal range, with high FSH-to-LH ratio. [5]
Functional hypothalamic amenorrhea can be caused by eating disorders, exercise, or high levels of prolonged physical or mental stress. This can also include major psychiatric disorders such as depression. Hypothyroidism, hyperthyroidism, sarcoidosis, galactosemia or any severe chronic medical condition may result in amenorrhea. [1]
Idiopathic hypogonadotropic hypogonadism leads to low gonadotropin (FSH/LH) levels. When this occurs with anosmia, it is diagnosed as Kallmann syndrome, the signs of which include midline facial defects, renal agenesis, and neurologic deficiencies. Kallmann syndrome results from a failure of GnRH cells to migrate to the forebrain, a phenomenon associated with mutations in the genes KAL1, FGFR1, FGF8, PROKR2, and PROK2. Kallmann syndrome most commonly occurs as an X-linked recessive disorder caused by a KAL1 defect. Autosomal dominant and autosomal recessive inheritances are less common. [6, 7] For detailed information, see Gonadotropin-Releasing Hormone Deficiency in Adults.
Evidence suggests a negative correlation between body fat levels and menstrual abnormalities. A critical body fat level must be present for the reproductive system to function normally.
In some female athletes, the synergistic effects of excessive exercise and disordered eating cause severe suppression of GnRH, leading to low estradiol levels. The female athletic triad, as defined by the American College of Sports Medicine, is characterized by low energy availability with or without disordered eating, amenorrhea, and osteoporosis. [4] A 2009 study by DeSouza et al found that about half of exercising women could be amenorrheic. [8] Amenorrhea can be the initial presentation of female athlete triad. [9]
Anorexia nervosa is a serious psychiatric disease with severe medical complications including primary amenorrhea (15%), osteopenia (52%), and osteoporosis (35%). [10]
Functional causes of amenorrhea include severe chronic disease, rapid weight loss, malnutrition, depression or other psychiatric disorders, recreational drug abuse, and psychotropic drug use.
Pituitary amenorrhea
A deficiency in FSH and LH may result from GnRH receptor gene mutations, although such mutations are rare. Mutations in the FSH beta gene have also been associated with amenorrhea; women with these mutations have low FSH and estradiol levels and high LH levels.
Primary amenorrhea caused by hyperprolactinemia is a rare condition characterized by the onset of thelarche and pubarche at appropriate ages but arrest of pubertal development before menarche. [11] Hyperprolactinemia is associated with suppression of the GnRH from the hypothalamus and subsequent inhibition of LH and FSH, suppressed gonadal function and galactorrhea. Prolactinomas are the most common cause of persistent hyperprolactinemia, accounting for 40-50% of pituitary tumors. [12] Prolactinomas are more commonly noted in secondary amenorrhea.
Pituitary tumors may suppress gonadotropin secretion, such as in Cushing disease or hypothalamic tumors, craniopharyngioma, or germinoma. Brain injury or cranial irradiation may also result in amenorrhea. Other pituitary causes include empty sella syndrome, pituitary infarct, hemochromatoses, and sarcoidosis.
Ovarian causes of primary amenorrhea
Gonadal dysgenesis is characterized by the congenital loss or underdevelopment of germ cells within the gonad during organogenesis. The gonads usually contain only fibrous tissue and are called streak gonads. In females, the most common form of gonadal dysgenesis is Turner syndrome (45,X), in which gonadotropin levels, especially the FSH levels, are high during early childhood and after age 9-10 years.
Additional anomalies associated with Turner syndrome include short stature, webbed neck, coarctation of the aorta (10%), renal abnormalities (50%), hypertension, pigmented nevi, short forth metacarpal and metatarsals, Hashimoto thyroiditis, obesity, and osteoporosis. [4] Depletion of ovarian follicles causes amenorrhea.
Spontaneous 46,XX primary ovarian insufficiency (POI), (also known as premature ovarian failure [POF] and premature menopause) affects 1 in 10,000 women by age 20 years, 1 in 1000 women by age 30 years, 1 in 250 women by age 35 years, and 1 in 100 women by age 40 years. [13] POI is hypergonadotropic hypogonadism, characterized by oligomenorrhea/amenorrhea, estrogen deficiency, and its associated symptoms such as hot flashes, vaginal dryness, dyspareunia, and insomnia. For more detailed information, see Spontaneous Primary Ovarian Insufficiency and Premature Ovarian Failure.
The fragile X permutation accounts for approximately 6% of cases of overt POI. It is caused by an increased number of CGG repeats in the FMR1 gene located on the long arm of the X chromosome. In the permutation, the number of CGG repeats ranges from 55-200. Approximately 21% of permutation carriers have POF/POI compared with 1% in the general population. [14] Autoimmune oophoritis occurs in 3-4% of POI cases. [15]
Amenorrhea is also seen in pure 46, XX gonadal dysgenesis and in 46,XY gonadal dysgenesis. These women have significantly elevated FSH levels due to the absence of ovarian follicles and reduction in negative feedback on FSH from estradiol and inhibin A and B.
The early stages of testicular formation require the action of several genes, of which one of the earliest and most important is the sex-determining region of the Y chromosome (SRY). In Swyer syndrome, a testicular regression syndrome that occurs very early in embryogenesis, the fetus has a 46,XY karyotype but with mutations of the SRY gene such that the testes never form and anti-müllerian hormone is not produced, thereby resulting in a female phenotype.
These individuals have a vagina, uterus, and fallopian tubes. Germ cells in the gonads are lost before birth. The streak gonads must be surgically removed because of the increased risk for developing germ cell tumor. Pure gonadal dysgenesis occurs when the syndrome affects the gonads only and no other dysmorphic features are noted.
Polycystic ovarian syndrome (PCOS) usually presents as secondary amenorrhea, but in some cases may present as primary amenorrhea. See Polycystic Ovarian Syndrome for more information.
Congenital and anatomical abnormalities
A uterus and patent vaginal tract are needed for normal menstrual flow to occur. Female reproductive tract abnormalities account for about one fifth of primary amenorrhea cases. Cyclic pelvic pain is common in girls with disorders of the reproductive tract involving outflow obstruction. Imperforate hymen causes an outflow obstruction. These patients can have blood in the vagina that collects and can result in a perirectal mass. Transverse vaginal septum can be anywhere along the tract between the hymenal ring and cervix.
Vaginal agenesis, or müllerian dysgenesis (also known as Mayer-Rokitansky-Kuster-Hauser [MRKH] syndrome) is caused by agenesis or partial agenesis of the müllerian duct system. It is characterized by congenital aplasia of the uterus and upper two thirds of the vagina in women showing normal development of the secondary sexual characteristics and a normal 46,XX karyotype. [16] The first sign is primary amenorrhea. It affects 1 of 4500 women. It can be associated with renal, vertebral, and, to a lesser extent, auditory and cardiac defects. [16]
Receptor and enzyme defects
Congenital adrenal hyperplasia as a result of 17 alpha-hydroxylase deficiency (CYP17) causes an excess of deoxycortisone to be produced and deficiency of cortisol and adrenal and gonadal sex steroids. Patients with this disorder who experience primary amenorrhea can be either genotypic males (XY) or females (XX). [4]
Complete androgen insensitivity syndrome is caused by a defective androgen receptor. Although patients with this syndrome have a 46,XY karyotype and produce testicularly derived testosterone, the testosterone cannot activate cellular transcription; therefore, the patient has female external genitalia. In most cases the disorder is X-linked. The testes, located internally and sometimes in the labia or inguinal area, also produce müllerian-inhibiting hormone, so all müllerian-derived structures (ie, the fallopian tubes, uterus, upper third of the vagina) are absent. [16, 17]
Gonadotropin resistance is rare, but inactivating mutations of the receptors for LH and FSH can cause anovulatory amenorrhea. [18]
Aromatase deficiency is also a rare disorder. Aromatase catalyzes the conversion of androgen to estrogen. When estrogen synthesis cannot occur, increased levels of testosterone result and virilization of the female occurs. Often, girls have cystic ovaries and resultant amenorrhea. [19]
Etiology
Amenorrhea after puberty can be divided into 2 groups: (1) amenorrhea without evidence of associated androgen excess and (2) amenorrhea with evidence of androgen excess (eg, hirsutism, virilization, sexual ambiguity). For a review of the causes of amenorrhea associated with androgen excess, see Polycystic Ovarian Syndrome.
Causes of primary amenorrhea
First and foremost, it is imperative to rule out pregnancy. Additional diagnoses of primary amenorrhea usually result from a genetic or anatomic abnormality. The relative prevalence of primary amenorrhea (percentages rounded to the nearest tenth) includes hypergonadotropic hypogonadism (48.5% of cases), hypogonadotropic hypogonadism (27.8%), and eugonadism (pubertal delay with normal gonadotropins; 23.7%). [20]
The hypergonadotropic hypogonadism category includes patients with abnormal sex chromosomes (ie, Turner syndrome), who make up 29.7% of all primary amenorrhea cases, and those with normal sex chromosomes. The latter group includes both patients who are 46,XX (15.4%) and those who are 46,XY (3.4%).
Hypogonadotropic hypogonadism includes the following:
-
Congenital abnormalities
-
Endocrine disorders
-
Tumor
-
Systemic illness (2.6%)
-
Eating disorder (2.3%)
Congenital abnormalities that can cause hypogonadotropic hypogonadism include the following:
-
Isolated GnRH deficiency (8.3%)
-
Forms of hypopituitarism (2.3%)
-
Congenital central nervous system (CNS) defects (0.8%)
-
Constitutional delay (6%)
Endocrine disorders that can cause hypogonadotropic hypogonadism include the following:
-
Congenital adrenal hyperplasia (CAH) (0.8%)
-
Cushing syndrome (0.4%)
-
Pseudohypoparathyroidism (0.4%)
-
Hyperprolactinemia (1.9%)
Tumors that can cause hypogonadotropic hypogonadism include the following:
-
Unclassified pituitary adenoma (0.8%)
-
Craniopharyngioma (1.1%)
-
Unclassified malignant tumor (0.4%)
Eugonadism may result from anatomic abnormalities or intersex disorders. Anatomic abnormalities include congenital absence of the uterus and vagina (CAUV; 16.2%) and cervical atresia (0.4%). Intersex disorders include androgen insensitivity (1.5%), 17-ketoreductase deficiency (0.4%), and inappropriate feedback (5.3%).
Causes of secondary amenorrhea
Disorders associated with a low or normal FSH, which account for 66% of cases of secondary amenorrhea, include the following: [21]
-
Weight loss/anorexia
-
Nonspecific hypothalamic
-
Chronic anovulation including PCOS
-
Hypothyroidism
-
Cushing syndrome
-
Pituitary tumor, empty sella, Sheehan syndrome
Disorders in which the FSH is high (12%) include the following:
-
46,XX spontaneous POI
-
Premature ovarian failure due to abnormal karyotype (45,X mosaic/ring chromosome)
-
Pure gonadal dysgenesis
Disorders associated with a high prolactin level make up 13% of cases. Anatomic disorders (ie, Asherman syndrome) account for 7%.
Hyperandrogenic states as a cause of secondary amenorrhea (2%) include the following:
-
Polycystic ovarian syndrome (PCOS)
-
Ovarian tumor
-
Non-classic CAH
-
Undiagnosed
Epidemiology
The incidence of primary amenorrhea in the United States is less than 1%. [22] Each year, approximately 5-7% of menstruating women in the United States experience 3 months of secondary amenorrhea. About one third of cases of secondary amenorrhea can be attributed to functional hypothalamic amenorrhea. [23]
No evidence indicates that the prevalence of amenorrhea varies according to national origin or ethnic group. However, local environmental factors related to nutrition and the prevalence of chronic disease undoubtedly have an effect. For instance, the age of the first menses varies by geographic location, as demonstrated by a World Health Organization study comparing 11 countries, which reported a median age of menarche of 13-16 years across centers.
Increases in the rates of childhood obesity around the world may also contribute to earlier onset of menarche and increased prevalence of obesity-related menstrual disorders, especially in areas where obesity is more prevalent. [24] Exposure to environmental toxins, namely hormonally active endocrine disruptors, may also result in increased rates of menstrual and reproductive disorders in endemic areas. [25]
Prognosis
Loss of menstrual regularity has been associated with an increased risk of wrist and hip fractures related to reduced bone density, even without the development of amenorrhea. A later menarche and menstrual cycle intervals longer than 32 days have both been associated with increased fracture rates in later years. Young women with ovarian insufficiency that is unresponsive to therapy require hormone replacement to maintain bone density.
Adolescence is a critical period for bone accretion as over half of peak bone mass is achieved during the teenage years. [26] Regular menses is a sign that the ovaries are producing normal amounts of estrogen, androgens, and progesterone, all of which play an important role in building and maintaining bone mass. Late menarche has been associated with a 3-fold increase in the risk of wrist fracture. [27]
In some cases, loss of menstrual regularity is an early sign of declining fertility and impending premature ovarian failure. Also in some cases, follicle depletion progresses to cause irreversible infertility. Approximately 10% of women evaluated for amenorrhea in a tertiary center are found to have premature ovarian failure.
Women with PCOS have many long-term health issues, including higher risk of diabetes and cardiovascular disease, that should be monitored and treated. [28, 29]
The results of a study by Shufelt et al revealed endothelial dysfunction, a preclinical marker of cardiovascular disease, in 1 out of 3 young women with functional hypothalamic amenorrhea. [30]
Patient Education
For patients with ovarian insufficiency that persists despite appropriate evaluation and treatment, careful counseling is warranted to stress the need for ongoing attention to the factors that help maintain bone density. Hormone replacement therapy is important for these patients. Other factors to consider are the need for adequate calcium and vitamin D intake (1200-1500 mg/d of elemental calcium and 1000 IU/d of vitamin D) and the need for 20-30 minutes of weight-bearing exercise each day.
-
Hypothalamus, pituitary and ovaries form a functional endocrine axis, known as HPO axis with hormonal regulations and feedback loops.
-
Primary amenorrhea flowchart.









