Practice Essentials
Gonorrhea is a purulent infection of the mucous membrane surfaces caused by Neisseria gonorrhoeae. N gonorrhoeae is spread by sexual contact or through transmission during childbirth. The Centers for Disease Control (CDC) recommends that all patients with gonorrheal infection also be treated for presumed co-infection with Chlamydia trachomatis. [1]
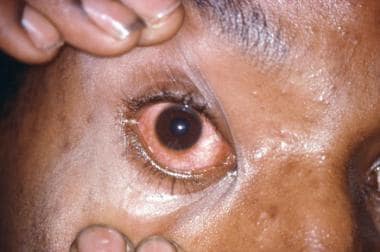 This patient presented with gonococcal urethritis, which became systemically disseminated, leading to gonococcal conjunctivitis of the right eye. Courtesy of the CDC/Joe Miller, VD.
This patient presented with gonococcal urethritis, which became systemically disseminated, leading to gonococcal conjunctivitis of the right eye. Courtesy of the CDC/Joe Miller, VD.
See Visual Findings of 9 Sexually Transmitted Infections, a Critical Images slideshow, to help make an accurate diagnosis.
Signs and symptoms
History
In women, the major genitourinary symptoms of gonorrhea include the following:
-
Vaginal discharge: The most common presenting symptom of gonorrhea, vaginal discharge from endocervicitis is usually described as thin, purulent, and mildly odorous; however, many patients have minimal or no symptoms from gonococcal cervicitis
-
Dysuria
-
Intermenstrual bleeding
-
Dyspareunia (painful intercourse)
-
Mild lower abdominal pain
If the infection progresses to pelvic inflammatory disease (PID), symptoms may include the following:
-
Lower abdominal pain: Most consistent symptom of PID
-
Increased vaginal discharge or mucopurulent urethral discharge
-
Dysuria: Usually without urgency or frequency
-
Cervical motion tenderness
-
Adnexal tenderness (usually bilateral) or adnexal mass
-
Intermenstrual bleeding
-
Fever, chills, nausea, and vomiting (less common)
In males, the major genitourinary symptoms of gonorrhea include the following:
-
Urethritis: The major manifestation of gonococcal infection in men; initial characteristics include burning upon urination and a serous discharge; a few days later, the discharge usually becomes more profuse, purulent, and, at times, tinged with blood
-
Acute epididymitis: Usually unilateral and often occurs in conjunction with a urethral exudate
-
Urethral strictures: Have become uncommon in the antibiotic era, but they can present with a decreased and abnormal urine stream, as well as with the secondary complications of prostatitis and cystitis
-
Rectal infection: May present with pain, pruritus, discharge, or tenesmus
In males and females, the classic presentation of disseminated gonococcal infection (DGI) is an arthritis-dermatitis syndrome. Joint or tendon pain is the most common presenting complaint in the early stage of infection. The second stage of DGI is characterized by septic arthritis. The knee is the most common site of purulent gonococcal arthritis.
In neonates, in whom bilateral conjunctivitis (ophthalmia neonatorum) often follows vaginal delivery from an untreated mother with a gonococcal infection, symptoms of gonococcal conjunctivitis include the following:
-
Eye pain
-
Redness
-
Purulent discharge
Physical examination
Look for the following genitourinary symptoms during physical examination in females:
-
Mucopurulent or purulent vaginal, urethral, or cervical discharge
-
Vaginal bleeding; vulvovaginitis in children
-
Cervical friability - Tendency to bleed upon manipulation
-
Cervical motion tenderness during bimanual pelvic examination
-
Fullness and/or tenderness of the adnexa, unilateral or bilateral (eg, ovaries, fallopian tubes)
-
Lower abdominal pain/tenderness, with or without rebound tenderness
-
Possible low back pain - More common in progression to PID
-
Upper right abdominal tenderness (with perihepatitis)
Look for the following genitourinary symptoms during physical examination in males:
-
Mucopurulent or purulent urethral discharge: Obtained by milking the urethra along the shaft of the penis
-
Possible epididymitis: Unilateral epididymal tenderness and edema, with or without penile discharge or dysuria
-
Penile edema without other overt inflammatory signs
-
Urethral stricture: Uncommon; more often seen in the preantibiotic era with urethral irrigation using caustic liquids
See Clinical Presentation for more detail.
Diagnosis
Culture is the most common diagnostic test for gonorrhea, followed by the deoxyribonucleic acid (DNA) probe and then the polymerase chain reaction (PCR) assay and ligand chain reaction (LCR). The DNA probe is an antigen detection test that uses a probe to detect gonorrhea DNA in specimens.
Specific culture of a swab from the site of infection is the gold standard for diagnosis at all potential sites of gonococcal infection. Cultures are particularly useful when the clinical diagnosis is unclear, when a failure of treatment has occurred, when contact tracing is problematic, and when legal questions arise.
In patients who may have DGI, all possible mucosal sites should be cultured (eg, pharynx, cervix, urethra, rectum), as should blood and synovial fluid (in cases of septic arthritis). Three sets of blood cultures should also be obtained.
See Workup for more detail.
Management
For uncomplicated urogenital, anorectal, and pharyngeal gonococcal infection, a drug regimen using ceftriaxone plus either azithromycin or doxycycline may be used. Antimicrobial drugs used alone or in various combinations in other gonococcal infections include the following:
-
Gonococcal arthritis: Ceftriaxone
-
Gonococcal conjunctivitis: Ceftriaxone
-
Gonorrhea contributing to PID: Cefoxitin, ceftriaxone, doxycycline, metronidazole, cefotetan, clindamycin, gentamicin
-
Gonococcal epididymitis: Ceftriaxone, doxycycline
-
DGI: Ceftriaxone, cefotaxime, ceftizoxime
-
Gonococcal meningitis and endocarditis: Ceftriaxone
See Treatment and Medication for more detail.
Background
Gonorrhea, an important public health problem and the second most common notifiable disease in the United States, is a purulent infection of mucous membrane surfaces caused by the gram-negative diplococcus Neisseria gonorrhoeae. Although gonorrhea (known colloquially as the clap and the drip) is most frequently spread during sexual contact, it can also be transmitted from the mother's genital tract to the newborn during birth, causing ophthalmia neonatorum and systemic neonatal infection.
In women, the cervix is the most common site of gonorrhea, resulting in endocervicitis and urethritis, which can be complicated by pelvic inflammatory disease (PID). In men, gonorrhea causes anterior urethritis. Gonorrhea can also spread throughout the body to cause localized and disseminated disease. Complications include ectopic pregnancy and increased susceptibility to human immunodeficiency virus (HIV) infection. Most commonly, the term gonorrhea refers to urethritis and/or cervicitis in a sexually active person.
Gonococcal infections after sexual and perinatal transmission are a major source of morbidity worldwide. In the developed world, where prophylaxis for neonatal eye infection is standard, the vast majority of infections follow genitourinary mucosal exposure.
In the pediatric population, the importance of gonorrhea is 3-fold, as follows:
-
As a common and preventable sexually transmitted disease (STD) in the sexually active teenage population
-
As a perinatal infection at childbirth
-
As a forensic aid in investigating sexual abuse
Gonococcemia
Gonococcemia is defined as the presence of N gonorrhoeae in the bloodstream, which can lead to the development of disseminated gonococcal infection (DGI). Gonococcemia occurs in about 0.5-3% of patients with gonorrhea.
 This patient presented with gonococcal urethritis, which became systemically disseminated, leading to gonococcal conjunctivitis of the right eye. Courtesy of the CDC/Joe Miller, VD.
This patient presented with gonococcal urethritis, which became systemically disseminated, leading to gonococcal conjunctivitis of the right eye. Courtesy of the CDC/Joe Miller, VD.
The clinical manifestations of this process are biphasic, with an early bacteremic phase consisting of tenosynovitis, arthralgias, [2] and dermatitis, followed by a localized phase consisting of localized septic arthritis. Other potentially severe clinical complications include osteomyelitis, meningitis, endocarditis, adult respiratory distress syndrome (ARDS), [3, 4] and fatal septic shock. [5] Polymyositis is also a rare complication of gonococcemia. (See Pathophysiology, Prognosis, and Presentation.)
Patients who are pregnant or menstruating may be particularly prone to gonococcemia. Other populations at risk for infection include women and individuals with complement deficiencies, HIV disease, or systemic lupus erythematosus (SLE). DGI is an important, potentially life-threatening, and easily treatable clinical entity that remains the most common cause of acute septic arthritis in young, sexually active adults.
Pathophysiology
The pathophysiology of N gonorrhoeae and the relative virulence of different subtypes depend on the antigenic characteristics of the respective surface proteins. Certain subtypes are able to evade serum immune responses and are more likely to lead to disseminated (systemic) infection.
Well-characterized plasmids commonly carry antibiotic-resistance genes, most notably penicillinase. Plasmid and nonplasmid genes are transmitted freely between different subtypes. The ensuing exchange of surface protein genes results in high host susceptibility to reinfection. The exchange of antibiotic resistance genes has led to extremely high levels of resistance to beta-lactam antibiotics. Fluoroquinolone resistance has also been documented on multiple continents and in widespread populations within the United States. [1]
Infection of the lower genital tract, the most common clinical presentation, primarily manifests as male urethritis and female endocervicitis. Infection of the pharynx, rectum, and female urethra occur frequently but are more likely to be asymptomatic or minimally symptomatic. Retrograde spread of the organisms occurs in as many as 20% of women with cervicitis, often resulting in pelvic inflammatory disease (PID), with salpingitis, endometritis, and/or tubo-ovarian abscess. Retrograde spread can lead to frank abdominal peritonitis and to a perihepatitis known as Fitz-Hugh-Curtis syndrome.
Long-term sequelae of PID, such as tubal factor infertility, ectopic pregnancy, and chronic pain, may occur in up to 25% of affected patients. Epididymitis or epididymo-orchitis may occur in men after gonococcal urethritis. Lower genital infection is a risk factor for the presence of other sexually transmitted diseases (STDs), including human immunodeficiency virus (HIV).
Conjunctivitis can occur in adults, as well as children, following direct inoculation of organisms (usually as a result of hand-eye inoculation in adults) and can lead to blindness.
Disseminated gonococcal infection
Disseminated gonococcal infection (DGI) occurs following approximately 1% of genital infections. Patients with DGI may present with symptoms of rash, fever, arthralgias, migratory polyarthritis, septic arthritis, tendonitis, tenosynovitis, endocarditis, or meningitis.
N gonorrhoeae organisms spread from a primary site, such as the endocervix, the urethra, the pharynx, or the rectum, and disseminate to the blood to infect other end organs. Usually, multiple sites, such as the skin and the joints, are infected. Neisserial organisms disseminate to the blood due to a variety of predisposing factors, such as host physiologic changes, virulence factors of the organism itself, and failures of the host's immune defenses. [7]
For example, changes in the vaginal pH that occur during menses and pregnancy and the puerperium period make the vaginal environment more suitable for the growth of the organism and provide increased access to the bloodstream. (Three fourths of the cases of DGI occur in women; susceptibility is increased if the primary mucosal infection occurs during menstruation or pregnancy.) [8, 9]
Defects in the host's immune defenses are also involved in the pathophysiology, with certain patients more likely to develop bacteremia. Specifically, patients with deficiency in terminal complement components are less able to combat infection, as complement plays an important role in the killing of neisserial organisms. As many as 13% of patients with DGI have a complement deficiency.
A study of 22 patients with DGI revealed that total serum complement activity was greater than 25% below the normal mean. Other causes of immunocompromise (eg, HIV, SLE) also predispose to dissemination of infection.
In addition, certain strains of gonorrhea causing asymptomatic genital infections are seen in association with DGI. [10]
Etiology
N gonorrhoeae is a gram-negative, intracellular, aerobic diplococcus; more specifically, it is a form of diplococcus known as the gonococcus. N gonorrhoeae is spread by sexual contact or through vertical transmission during childbirth. It mainly affects the host’s columnar or cuboidal epithelium. Virtually any mucous membrane can be infected by this microorganism. The physiologic ectopy of the squamocolumnar junction onto the ectocervix in the adolescent female is one factor that causes particular susceptibility to this infection.
Many factors influence the manner in which gonococci mediate their virulence and pathogenicity. Pili help in attachment of gonococci to mucosal surfaces and contribute to resistance by preventing ingestion and destruction by neutrophils. Opacity-associated (Opa) proteins increase adherence between gonococci and phagocytes, promote invasion into host cells, and possibly down-regulate the immune response.
Porin channels (porA, porB) in the outer membrane play key roles in virulence. Gonococcal strains with porA may have inherent resistance to normal human serum and an increased ability to invade epithelial cells, explaining their association with bacteremia.
Certain acquired plasmids and genetic mutations enhance virulence. TEM-1–type beta-lactamase (penicillinase) affects penicillin binding and efflux pumps and confers resistance to penicillin. [11, 12] TetM protects the ribosome and confers resistance to tetracycline. Alterations in gyrA and parC genes result in fluoroquinolone resistance by efflux activation and decreased antibiotic cell permeation. [11]
Gonococci attach to the host mucosal cell (pili and Opa proteins play major roles) and, within 24-48 hours, penetrate through and between cells into the subepithelial space. A typical host response is characterized by invasion with neutrophils, followed by epithelial sloughing, formation of submucosal microabscesses, and purulent discharge. If left untreated, macrophage and lymphocyte infiltration replaces the neutrophils. Some gonococcal strains cause an asymptomatic infection, leading to an asymptomatic carrier state in persons of either sex.
The ability to grow anaerobically allows gonococci, when mixed with refluxed menstrual blood or attached to sperm, to secondarily invade lower genital structures (vagina and cervix) and progress to upper genital organs (endometrium, salpinx, ovaries).
Sexually transmitted infection
Gonococcal infection usually follows mucosal inoculation during vaginal, anal, or oral sexual contact. It also may be caused by inoculation of mucosa by contaminated fingers or other objects. Transmission through penile-rectal contact is fairly efficient.
The risk of transmission of N gonorrhoeae from an infected woman to the urethra of her male partner is approximately 20% per episode of vaginal intercourse and rises to 60-80% after 4 or more exposures. In contrast, the risk of male-to-female transmission approximates 50-70% per contact, with little evidence of increased risk with more sexual exposures.
Persons who have unprotected intercourse with new partners frequently enough to sustain the infection in a community are defined as core transmitters.
Neonatal and pediatric gonococcal infection
Neonatal gonococcal infection may follow conjunctival infection, which is obtained during passage through the birth canal. In addition, direct infection may occur through the scalp at the sites of fetal monitoring electrodes.
In children, infection may occur from sexual abuse by an infected individual or possibly nonsexual contact in the child's household or in institutional settings.
Autoinoculation
Autoinoculation can occur when a person touches an infected site (genital organ) and contacts skin or mucosa.
Risk factors
Risk factors for gonorrhea include the following:
-
Sexual exposure to an infected partner without barrier protection (eg, failure to use a condom or condom failure) [13]
-
Multiple sex partners
-
Male homosexuality
-
Low socioeconomic status
-
Minority status - Blacks, Hispanics, and Native Americans have the highest rates in the United States
-
History of concurrent or past STDs
-
Exchange of sex for drugs or money
-
Use of crack cocaine
-
Early age of onset of sexual activity
-
Pelvic inflammatory disease (PID) - Use of an intrauterine device (IUD)
Epidemiology
Occurrence in the United States
CDC estimates that approximately 1.6 million new gonococcal infections occurred in the United States in 2018, with a significant number of cases likely unreported. [14] Per the CDC, gonorrhea is the second most commonly reported communicable disease. [15] The national average in 2009 was 99.1 cases per 100,000 population, a 10.5% decrease from 2008, with considerable state-to-state variation. [16, 17] Rates of reported gonorrhea have increased 92.0% since the historic low in 2009. [18] Men were apparently less likely than women to be tested for gonorrhea, 20.7% vs 50.9%, respectively. [19] However, the infection rates between men and women were similar (105.8 vs 108.7 cases per 100,000). [20] Infection rates in men appear to be on the rise.
CDC report estimated the annual cost of gonorrhea and its complications to be $271 million.
In the United States, the number of gonococcal infections peaked in the 1970s, the era of the sexual revolution. With the onset of the HIV epidemic and the practicing of safe sex techniques, the incidence dramatically decreased from 468 cases per 100,000 population in 1975 to 100-150 cases per 100,000 population at the turn of the century. The rate of reported gonorrhea cases was at its lowest in 2009 but has been increasing overall since then. [21] The increased numbers have been attributed to increased cases in males and persistently high rates in adolescents, young adults, and certain racial/ethnic groups in defined geographic areas. [21]
Within the United States, carriage rates highly depend on the geographic area, the racial and ethnic group, and sexual preferences. The rate of gonorrhea is much higher in African Americans than in other racial groups [22] and is much higher in the rural southeastern United States and in inner cities, presumably because of an association with socioeconomic and behavioral factors, as well as with social networks.
In 2016, rates of infection ranged from about 479.9 cases per 100,000 population in the District of Columbia to 20.1 cases per 100,000 population in Vermont. [23] The CDC supports a campaign (Healthy People 2020) that targets a decreased incidence rate of 251.9 cases per 100,000 population among females aged 15-44 years and 194.8 per 100,000 population among males of the same age by the year 2020.
In children who have been sexually abused, rates of recovery of gonorrhea range from 1% to 30%. In female adolescents who are sexually active, asymptomatic carriage of gonorrhea occurs in 1-5%.
Resistant gonorrhea
The incidence of antibiotic-resistant strains of N gonorrhoeae has been rising since the late 1940s. Of greatest concern is the rise in the percentage of cases due to N gonorrhoeae with higher minimum inhibitory concentrations (MICs) to ceftriaxone, the current treatment of choice. As per the Gonococcal Isolate Surveillance Project (GISP) only 0.1% of isolates displayed elevated ceftriaxone minimum inhibitory concentrations (MICs) in 2019. [1, 24]
In May 2016, the first cluster of highly resistant gonorrhea infections in the United States was identified in Hawaii. Most of the isolates showed decreased susceptibility to ceftriaxone, and all cases had very high-level resistance to azithromycin. [25]
International occurrence
An estimated 98 million new cases of gonorrhea occur annually, according to World Health Organization estimates. In comparison, an estimated 62 million new cases occurred in 1999 and 88 million in 2005. In 1999, the number of new cases of gonococcal infection diagnosed in North America was 1.56 million; in Western Europe, 1.11 million; in South and Southeast Asia, 27.2 million; and in Latin America and the Caribbean, 7.27 million.
Gonorrhea was the most common STD worldwide for at least most of the 20th century, although since the mid-1970s, public health initiatives in the industrialized world have resulted in declining incidence of the disease. As noted earlier, however, gonococcal infection is still the second most common notifiable disease in the United States, and Western European rates approximate those in the United States. [26, 27, 28]
Although the frequency data are unknown in most developing nations, these countries are considered to have the highest rates of gonorrhea and its complications. Gonococcal infection rates in pregnant women in the Central African Republic and South Africa were 3.1% and 7.8%, respectively.
Resistant gonorrhea
The incidence of antibiotic-resistant strains has been rising since the late 1940s. Of greatest concern historically has been the high percentage of cases due to penicillinase-producing N gonorrhoeae. However, fluoroquinolone resistance has increased rapidly over the past decade on most continents and within the United States. The CDC reported fluoroquinolone resistance in 6.8% of 2004 isolates, 9.4% of 2005 isolates, and 13.3% of 2006 isolates. [1]
Race-related demographics
All sexually active populations are at risk for gonococcal infection, and the level of risk rises with the number of sexual partners and the presence of other STDs.
Although race has no intrinsic effect on susceptibility to gonorrhea, the frequency of gonorrhea in the United States is increased among urban dwellers, individuals of lower socioeconomic status, and minorities of any population. This may be due to decreased access to diagnosis and treatment; lack of adequate care (ie, education, diagnosis, and treatment), leading to increased transmission rates; and/or reflection bias due to data collection site preference (eg, urban emergency departments and STD clinics), as well as true differences in prevalence.
Overall, the African American–to–White ratio of gonococcal infections declined from 23:1 in 2002 to 18:1 in 2006. Infection rates have trended downward since 1998; however, between 2005 and 2006, the CDC noted a 6.3% increase in the rate of gonococcal infections in African Americans. Subsequently, rates have begun to downtrend once again.
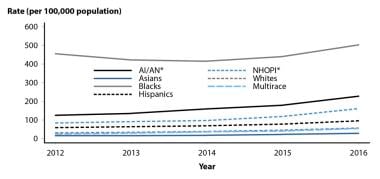 Gonorrhea rates by race/ethnicity, United States, 2012-2016. Courtesy of the Centers for Disease Control and Prevention (CDC).
Gonorrhea rates by race/ethnicity, United States, 2012-2016. Courtesy of the Centers for Disease Control and Prevention (CDC).
Compared with reported incidence in Whites, the rate of reported cases was 8.6 times higher in Blacks, 3 times higher in native Hawaiians/Pacific Islanders, 1.7 times higher in Hispanics, and 0.5 times higher in Asians. [29] From 2012-2016, the gonorrhea rate increased among all races and ethnic groups.
Sex-related demographics
The male-to-female ratio for gonorrhea is approximately 1:1.4; however, females may be asymptomatic, whereas males are rarely asymptomatic. From 2015 to 2016, rates among males increased approximately 22%, whereas rates in females increased 13.8%. The large increase in diagnosis in males is attributed to either increased transmission or increased case documentation (increased extra-genital screening) among men who have sex with men (MSM). [29]
Serious sequelae are much more common in women, in whom pelvic inflammatory disease (PID) may lead to ectopic pregnancy or infertility and in whom DGI is more likely, owing to menstruation, pregnancy, and a higher incidence of occult infection.
Age-related demographics
The highest incidence of gonococcal infection in the United States in 2016 was among adults aged 20-24 years, in both men and women. [16, 17] This is likely due to the following:
-
Increased numbers of sexual partners
-
Decreased access to or use of health care
-
Physiologic ectopy of the squamocolumnar junction in females
-
Decreased use of barrier contraceptives
Infection in children is a marker for child sexual abuse and should be reported as such, although a 2007 review provided some support for nonsexual transmission between children and for transmission from adults to children related to poor hand hygiene. [30, 31]
Gonococcemia remains an important disease in the adolescent and young adult population, with a peak incidence in males aged 20-24 years and in females aged 15-19 years.
Prognosis
With adequate early therapy, complete cure and return to normal function are the rule. Most gonococcal infections respond quickly to cephalosporin therapy. Late, delayed, or inappropriate therapy may lead to significant morbidity or, on rare occasions, death.
Complications in males
Urethral strictures secondary to gonococcal infection in men are less common than previously thought. Some strictures in the preantibiotic era likely resulted from treatment by urethral irrigation using caustic compounds rather than from the gonorrhea itself.
Other complications, such as penile lymphangitis, periurethral abscess, acute prostatitis, seminal vesiculitis, and infection of the Tyson and Cowper glands, are now rare.
Complications in females
Tubal scarring and infertility are the major complications of gonococcal infection in females. The incidence of involuntary infertility is estimated at 15% after one attack of pelvic inflammatory disease (PID) and approximately 50%-80% after 3 attacks. (However, infertility may be more common after chlamydial PID than after gonococcal PID, presumably because the more acute inflammatory signs associated with gonorrhea prompt women to seek diagnosis and treatment sooner.) Despite clinical and microbiological cure of infection, one study showed 13% infertility rates in females with PID due to N gonorrhoeae infection. [32]
Failure to diagnose PID can result in acute morbidity, including tuboovarian abscess, endometritis, Fitz-Hugh-Curtis syndrome (perihepatitis), and other chronic sequelae. Perihepatitis secondary to gonorrhea presents as right upper quadrant pain and nausea.
The incidence of ectopic pregnancy is increased from 7-fold to 10-fold in women with previous salpingitis, with resultant increased fetal and maternal mortality rates.
Gonococcal infections in women may also manifest as gonococcal urethritis or infection of periurethral (Skene) or Bartholin glands.
Pelvic inflammatory disease
PID is generally the most feared complication of gonococcal infection, because it is one of the leading causes of female infertility and often leads to hospitalization. This can be devastating to any woman, especially an adolescent who potentially has many years of childbearing ahead of her. In a 2011 study, female adolescents with PID were more likely than older women to have a rapid recurrence of PID or to become pregnant despite reporting more consistent condom use. [33] Ten to twenty percent of patients diagnosed with cervical gonorrhea may develop PID.
Tubo-ovarian abscess and, rarely, tubal perforation with peritonitis and death, can occur, especially if the tubo-ovarian abscess was recurrent. Females with recurrent PID have high rates of ectopic pregnancy and infertility.
Epididymitis and orchitis
Epididymitis and orchitis occur infrequently in males who go untreated. These conditions usually respond well to the same antibiotics used for uncomplicated urethritis, but the drugs are administered for a longer course.
Arthritis
Gonorrhea is the most common cause of arthritis in the adolescent; however, arthritis (septic or reactive) is a rare complication of this disease.
Because it mimics septic arthritis, excluding the possibility of gonococcal infection in any adolescent with acute onset of pyogenic arthritis is important. Adequate diagnosis may require culturing extraarticular sites for N gonorrhoeae.
Endocarditis
Endocarditis is a rare but serious complication of disseminated gonococcal infection, affecting 1%-2% of cases. Before the era in which antibiotics were the primary treatment, median survival was 6-8 weeks.
Additional complications
Complications of gonococcal infections also include the following [34] :
-
Corneal scarring after ocular gonococcal infections
-
Destruction of cardiac valves in gonococcal endocarditis
-
Death from congestive heart failure related to endocarditis
-
Central nervous system (CNS) complications of gonococcal meningitis
It has been suggested that a person with a gonococcal infection may be at a 3- to 5-fold increased risk of acquiring HIV infection, if exposed to the virus.
DGI is an acute illness that causes fever, asymmetrical polyarthralgias, and skin pustules overlying small joints in patients with gonorrhea. Disseminated infection may also lead to meningitis or endocarditis.
In newborns, vertical transmission can cause conjunctivitis, known as ophthalmia neonatorum, and permanent damage and blindness, if untreated.
Oral sex with an infected partner can result in pharyngitis, and, similarly, anal infection can arise from anal sex or local spread from a vaginal source.
Patient Education
Discuss safe sexual practices with all individuals in whom gonorrhea is suspected. Proper education to prevent gonorrhea may be more effective than simplistic instructions to avoid sex, especially in the teenaged population. Teenagers involved with abstinence-only campaigns have unchanged STD rates and disproportionately acquire anal and oral infections, rather than vaginal infections (the perception being that if an activity is not vaginal sex, it is not sex). Stress that oral or anal sex can also transmit disease.
Patients should know the method of disease transmission and the adverse impact of recurrent infections on future fertility, they should be counseled about the risks of complications following gonococcal infection and the risk of other STDs, and they should always be instructed to refer any sex partners for prompt evaluation and treatment.
In addition, these individuals should be aware that they should avoid sexual contact until medication is finished and until their partners are fully evaluated and treated. Thereafter, they should avoid unprotected contact.
The discussion of responsible sexual behavior should not be limited or withheld because of personal religious or moral views, because these may not be shared by the patient, and teenagers are notorious for sexual experimentation; evidence suggests that offering only limited discussion does the teenage population a huge disservice. This advice is especially pertinent in states where sexual education is almost nonexistent in the school system because of abstinence-only teaching, which is misleading and factually inaccurate.
In one study in Peru, a bundle of interventions that included extensive public health efforts, including training of local medical personnel, specific and presumptive treatment, outreach to female sex workers, and supply of barrier contraception, may have been effective at reducing the prevalence of several STDs, although the effect did not reach statistical significance overall.
The effects were more greatly pronounced (and significant) among female sex workers and young adult women. The study was hampered by several methodologic limitations, such as comparing different cities as controls, which made drawing conclusions from the data difficult. [35]
Abstinence education
Although the most effective STD prevention is abstinence from sex, this is oftentimes an unrealistic expectation, especially in the teenaged population. In fact, 88% of teenagers who pledged abstinence in middle and high school still engaged in premarital sex. Moreover, they tend to have riskier, unprotected sex because of their lack of education. Those who pledge before having sex have been found to have a 33% higher prevalence rate of STDs than have those who had sex and then retrospectively pledged, with nonpledgers falling in between. This is despite a lower number of partners and an older age at first intercourse in pledgers.
Moreover, pledgers are less likely to be aware of their STD status and are less likely to seek testing, even if their STD rates are similar overall (again, highlighting a lack of appropriate sexual education).
Of course, abstinence should be explained to be the best option, but a more practical expectation is abstinence from sex with someone known or suspected of having an STD until treatment is obtained and completed. In light of the difficulty of knowing a potential partner's sexual history (or honesty), strongly recommend the use of condoms as a reasonable alternative to abstinence. [13]
Risks of unprotected sex
Patients should also be counseled about the additional risks of unprotected sex, including the acquisition of more serious or lifelong infections such as herpes, hepatitis B, and HIV, and, of course, about the risks of pregnancy. The emotional aspect of sexual relationships may also need to be addressed, especially in teenage girls. Teenagers are vulnerable in that they are sexually mature but not yet emotionally mature.
For patient education information, see the Sexual Health Center, as well as Sexually Transmitted Diseases, Gonorrhea, and Chlamydia.
Patient education materials are also available at The Centers for Disease Control and Prevention (CDC) Website (Sexually Transmitted Diseases – Gonorrhea) and from many local public health departments.
-
This patient presented with gonococcal urethritis, which became systemically disseminated, leading to gonococcal conjunctivitis of the right eye. Courtesy of the CDC/Joe Miller, VD.
-
Gonorrhea rates, United States, 1941-2016. Courtesy of the Centers for Disease Control and Prevention (CDC).
-
Gonorrhea rates by race/ethnicity, United States, 2012-2016. Courtesy of the Centers for Disease Control and Prevention (CDC).
-
Rates of gonococcal infection per 100,000 by state and outlying regions (2016). Courtesy of the Centers for Disease Control and Prevention (CDC).
-
Disseminated gonococcemia, acral pustules.
-
Cytologic smear of cutaneous acral pustule showing gram-negative, intracellular diplococci.
-
Rates of reported gonorrhea cases by age group and sex, United States, 2016. Courtesy of the Centers for Disease Control and Prevention (CDC).
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Approach Considerations
- Smears With Gram Stain
- Isolation Via Culture
- Imaging Studies
- Nucleic Acid Amplification Tests
- Nucleic Acid Probe Signal Amplification
- Antibody-Antigen Testing
- Procedures
- Histologic Findings
- Testing for Gonorrhea in Males
- Testing for Gonorrhea in Females
- Testing for Gonorrhea at Extragenital Sites
- Show All
- Treatment
- Guidelines
- Medication
- Questions & Answers
- Media Gallery
- References








