Background
The term central sleep apnea encompasses a heterogeneous group of sleep-related breathing disorders in which respiratory effort is diminished or absent in an intermittent or cyclical fashion during sleep. [1] In most cases, central sleep apnea is associated with obstructive sleep apnea syndromes or is caused by an underlying medical condition, recent ascent to high altitude, or narcotic use. Primary central sleep apnea is a rare condition, the etiology of which is not entirely understood.
During polysomnography (PSG), a central apneic event is conventionally defined as cessation of airflow for 10 seconds or longer without an identifiable respiratory effort. In contrast, an obstructive apneic event has a discernible ventilatory effort during the period of airflow cessation.
In general, treatment of central sleep apnea is often more difficult than treatment of obstructive sleep apnea and treatment varies according to the specific syndrome. The International Classification of Sleep Disorders, Third Edition (ICSD-3) [2] describes several different entities grouped under central sleep apnea with varying signs, symptoms, and clinical and polysomnographic features. Those that affect adults include primary central sleep apnea, Cheyne-Stokes breathing-central sleep apnea (CSB-CSA) pattern, high-altitude periodic breathing, central sleep apnea due to medical conditions other than Cheyne-Stokes, and central sleep apnea due to drugs or substances. The primary sleep apnea of infancy primarily affects premature newborns and is excluded from this discussion.
Pathophysiology
Knowledge of normal ventilatory control mechanisms is important for understanding the pathophysiology of central sleep apnea. Normal ventilation is tightly regulated to maintain levels of arterial oxygen (PaO2) and carbon dioxide (PaCO2) within narrow ranges. This is achieved by feedback loops that involve peripheral and central chemoreceptors, intrapulmonary vagal receptors, the respiratory control centers in the brain stem, and the respiratory muscles.
During wakefulness, signals from cortical areas of the brain influence respiration, a mechanism termed behavioral control. Many nonchemical stimuli, which include pulmonary mechanoreceptors and behavioral or awake stimulation, are known to modulate this phenomenon. During sleep, behavioral control is lost and chemical control is the major mechanism regulating ventilation, PaCO2 being the major stimulus for ventilation. Central sleep apnea is most often seen during non–rapid eye movement (NREM) sleep, when behavioral influence is least, followed by rapid eye movement (REM) sleep, while a fully awake person is least likely to manifest it. Despite these changes, ventilatory control during sleep remains similar to that during wakefulness.
Sleep is characterized by elevation of arterial carbon dioxide tension (PaCO2) and a higher PaCO2 apneic threshold, the PaCO2 below which apnea occurs. Reduction of PaCO2 just a few mm Hg below the PaCO2 set point can result in apneas. Central apneic events commonly occur during the transition between wake and sleep, a period during which the PaCO2 set point adjusts.
Two types of pathophysiologic phenomena can cause central sleep apnea syndromes: 1) ventilatory instability or 2) depression of the brainstem respiratory centers or chemoreceptors.
Ventilatory instability is the mechanism behind CSB-CSA, high-altitude periodic breathing, and probably primary central sleep apnea. [3] As with any system that is regulated by feedback loops, the respiratory system is vulnerable to instability. The occurrence and perpetuation of ventilatory instability in the pathogenesis of central sleep apnea can be visualized in the context of loop gain, an engineering term that describes the overall gain of a system controlled by feedback loops.
A system with high loop gain responds rapidly and intensely to a trigger, whereas a low loop gain system responds more gradually and weakly. Loop gain is affected by controller gain and plant gain. Controller gain represents the degree of response to a given disturbance, whereas plant gain reflects the efficiency of the response. In the respiratory system, controller gain is manifested as chemoresponsiveness, whereas plant gain is the effectiveness of a given minute ventilation to eliminate carbon dioxide.
The concept of loop gain can be illustrated by the way a thermostat-controlled air conditioner maintains room temperature within a narrow range. Minor temperature changes trigger a sensitive thermostat to turn the air conditioner on or off. The degree to which a thermostat responds to a change in room temperature represents a controller gain. Plant gain represents the effect of the response on the system, the temperature change in the room as a result of the cooling effect of the air conditioner. A system with high plant gain may have a stronger air conditioner or a smaller room to cool, resulting in a faster response and a greater likelihood of overshooting the limits.
Loop gain is defined as the response to a disturbance/disturbance itself. In a thermostat-controlled air conditioner system with high loop gain, a small increase in room temperature quickly results in cooling that may overshoot the range for which the thermostat is set, soon causing the air conditioner to be turned off. Such a system would be relatively unstable, with the air conditioner frequently being turned on and off, and the room experiencing swings in temperature.
In the system of ventilatory regulation, controller gain is the degree of ventilatory response to a given change in hypercapnia or hypoxia and is mediated by chemoreceptors. Plant gain is represented by the effect of a ventilatory response on arterial oxygen and carbon dioxide tensions. If a patient has low dead space, a low metabolic rate, a low functional residual capacity, or a high PaCO2, the effect of ventilatory changes is more marked, resulting in a higher plant gain.
For the ventilatory system, loop gain can be defined as demonstrated in the image below.
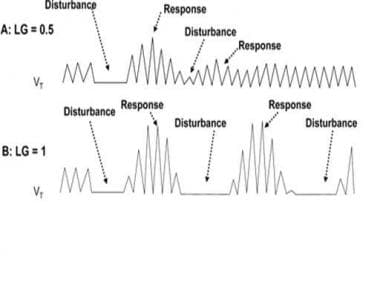 The role of loop gain in determining respiratory instability. A) When loop gain is less than 1, the tendency for an overshoot of the corrective response to an apnea or hypopnea is lessened, and ventilation returns to a steady pattern. B) When loop gain is greater than or equal to 1, the vigorous responses to respiratory disturbances result in continuous oscillation between the events and the corrections, resulting in an unstable periodic breathing pattern. Adapted from White DP Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. Dec 1 2005;172(11):1363-70.
The role of loop gain in determining respiratory instability. A) When loop gain is less than 1, the tendency for an overshoot of the corrective response to an apnea or hypopnea is lessened, and ventilation returns to a steady pattern. B) When loop gain is greater than or equal to 1, the vigorous responses to respiratory disturbances result in continuous oscillation between the events and the corrections, resulting in an unstable periodic breathing pattern. Adapted from White DP Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. Dec 1 2005;172(11):1363-70.
Loop gain = hyperpnea (response to disturbance)/apnea or hypopnea (disturbance)
If loop gain is less than 1, responses to apneas or hypopneas are more gradual and smaller, allowing ventilation to return to a steady pattern. If loop gain is greater than 1, the large responses to apneas and hypopneas result in swings of hyperventilation and apnea/hypoventilation, causing a state of instability termed periodic breathing. During waking, behavioral control may override periodic breathing patterns, so that the effect of high loop gain on the ventilatory system is most evident during sleep.
In addition to high loop gain, a delay must occur between the detection of a disturbance and the actuation of the response for a system to become unstable. This condition exists for the respiratory system because of the delay between change in PaCO2 in the pulmonary venous system and detection of the change in the carotid bodies and brainstem. Prolonged circulation time in some patients with congestive heart failure may accentuate the delay, predisposing them to an unstable ventilatory condition, CSB-CSA.
The ventilatory system is at particular risk of instability when the resting PaCO2 approaches the PaCO2 apneic threshold. In the situation of either high controller gain or high plant gain in association with a low baseline PaCO2 close to the apneic threshold, a minor disruption in the system can give rise to a cyclic appearance of central apneas and hyperpneas. Patients with hypocapnia and heart failure and those ascending to high altitudes often develop these conditions, predisposing them to a periodic breathing pattern. The credibility to this concept is supported by the observations that increasing the dead space, increasing the inhaled concentration of PaCO2, or providing increased baseline ventilation by acetazolamide are, under some circumstances, protective against periodic breathing.
Patients with heart failure and central sleep apnea have been shown to have an augmented ventilatory response to change in PaCO2 compared with patients with heart failure and obstructive sleep apnea. Hypoxia augments the ventilatory response to changes in PaCO2 (increases the slope of response) and predisposes to instability in ventilation. A change in PaCO2 may be more important than the low PaCO2 because patients with chronic liver disease also have low PaCO2 but do not develop central sleep apnea. In patients with heart failure and central sleep apnea, increased ventilatory response to exercise has been reported that was proportional to the severity of CSB-CSA failure, suggesting augmented peripheral and central chemoreceptor responsiveness. [4]
Central sleep apnea-hypoventilation syndromes such as those associated with narcotic use or brainstem lesions are due to disturbances of the central respiratory pattern center or peripheral chemoreceptors or both that may become more evident during sleep because of the suppression of wakefulness or behavior drive.
The respiratory "control center" involves several areas of the medulla. During NREM sleep, breathing is controlled by an automatic system that is primarily influenced by chemical stimuli. In REM sleep, both inhibitory and excitatory influences are exerted on the medullary respiratory neurons that are manifested by irregular breathing and occasional "physiologic" central apneas.
Primary disorders of the central nervous system such as meningitis or hemorrhage and tumors or strokes that involve the brainstem can result in an ataxic breathing pattern, referred to as Biot respiration. The Biot pattern may be irregular without any type of periodicity or it can consist of runs of similar-sized breaths alternating with central apneas, as demonstrated in the image below.
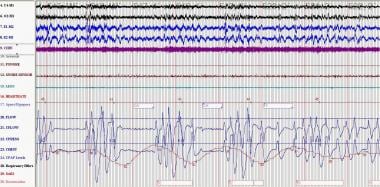 This polysomnogram demonstrates central sleep apnea and Biot respiration in a patient receiving long-term morphine for chronic pain. The Biot pattern may be irregular without any type of periodicity, or it can consist of runs of similar-sized breaths alternating with central apneas.
This polysomnogram demonstrates central sleep apnea and Biot respiration in a patient receiving long-term morphine for chronic pain. The Biot pattern may be irregular without any type of periodicity, or it can consist of runs of similar-sized breaths alternating with central apneas.
Narcotics such as heroin, morphine, and methadone cause respiratory depression via stimulation of the opioid Mu receptors on neurons located in the medullary respiratory complex. Although tolerance develops for many central nervous system effects of opioids, studies [5] have demonstrated abnormal hypercapnic and hypoxic ventilatory responses in chronic narcotic users, and several reports have demonstrated that central sleep apnea is common in individuals on long-term opioids. [6, 7] The possibility that Mu-receptor inhibition of the carotid bodies and other peripheral chemoreceptors plays a role in causing a more subtle form of respiratory depression in long-term narcotic use has been suggested. [7]
The mechanisms responsible for central sleep apnea and obstructive sleep apnea overlap, and patients with central apneas often have obstructive events. Studies have shown that the hypopharynx may be considerably narrowed during a central apneic event. During normal inspiration, a neuronal discharge occurs to the diaphragm and to the upper airway muscles that stiffens and dilates the pharynx to keep it open. If a decrease in activity occurs in both the diaphragm and upper airway dilators, the result could be a central or obstructive apnea. If, despite a lack of activation of the pharyngeal muscles, the upper airway remains open, the event will be a central apnea. If the upper airway is closed during central apnea and diaphragmatic activity resumes before pharyngeal dilator muscle tone is restored, a mixed apnea results. [8]
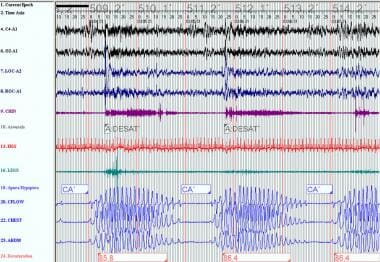
Thus, the susceptibility to upper airway collapse may determine whether central or obstructive apneas occur with cycling due to ventilatory instability. The conversion of obstructive apneas to a Cheyne-Stokes breathing pattern with the introduction of continuous positive airway pressure (CPAP) is an example of this phenomenon. See the images below.
 Obstructive sleep apnea (OSA): This polysomnogram demonstrates typical hypopneas occurring in OSA prior to continuous positive airway pressure titration. In OSA, airflow is absent or reduced, but ventilatory effort persists.
Obstructive sleep apnea (OSA): This polysomnogram demonstrates typical hypopneas occurring in OSA prior to continuous positive airway pressure titration. In OSA, airflow is absent or reduced, but ventilatory effort persists.
Etiology
This discussion includes the differentiation of various central sleep apnea syndromes from one another. Central sleep apnea in various forms can be seen in the following conditions or events:
Cheyne Stokes breathing-central sleep apnea
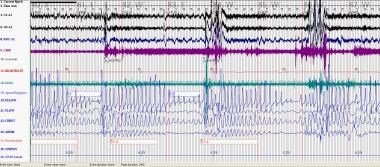
CSB-CSA is characterized by classic a crescendo-decrescendo pattern that typically occurs with a periodicity of 45 second or greater cycles (see image below). The ICSD-3 [2] specifies that at least 10 central apneas and hypopneas per hour of sleep should occur, accompanied by arousals and derangement of sleep structure. The arousals occur at the peak of the hyperpnea phase. Patients usually have predisposing factors such as heart failure, stroke, or renal failure, as well as a lower resting PaCO2 than normal. See the following:
Central sleep apnea due to a medical condition
The patient has a history of an underlying disorder other than heart failure or renal failure. Patients with stroke can have either classic CSB-CSA or central apneas without a crescendo-decrescendo pattern. See the following:
-
Stroke
-
Diabetes mellitus
-
Multiple system atrophy or Shy-Dragger syndrome
-
Familial dysautonomia
-
Damage to medullary respiratory centers by tumor, infarction, or infection
-
Cervical cordotomy
-
Myasthenia gravis
-
Idiopathic cardiomyopathy
High-altitude periodic breathing
The single most important feature is that high-altitude periodic breathing occurs only with recent ascent to high altitudes. Many patients develop this at an altitude of 5000 meters or greater, while almost everyone develops it at an elevation of 7600 meters. The cycle length is shorter than in CSB-CSA, 12-34 seconds.
Use of opiates and other CNS depressants
This is most easily recognized by a history of opiate use. Thirty percent of participants in a stable methadone maintenance program had a central apnea index (CAI) of greater than 5 and 20% had a CAI of greater than 10. [6] Methadone blood concentration was significantly associated with the severity of central sleep apnea.
Primary central sleep apnea
This is an uncommon condition in which 5 or more central apneas, lasting 10 seconds or more, occur per hour of sleep. Patients have a low-normal PaCO2. The central apneas terminate abruptly with a large breath and without associated hypoxemia. They do not have a crescendo-decrescendo pattern of breathing.
Complex sleep apnea
Central sleep apnea may emerge during titration of CPAP in patients previously diagnosed with obstructive sleep apnea. This syndrome, termed complex sleep apnea, has become a controversial topic in the sleep literature [9] and has been raised as a possible type of difficult-to-treat obstructive sleep apnea. As many as 6.5% of patients with obstructive sleep apnea may develop emergent or persistent central sleep apnea with CPAP treatment. CPAP emergent central sleep apnea is generally transitory and is eliminated after eight weeks of CPAP therapy. Persistent CPAP-related central sleep apnea has been observed in approximately 1.5% of treated patients. [10] Similarly, complex sleep apnea can occur following a tracheostomy for obstructive sleep apnea. Central apneas have been found initially after a tracheostomy, but after an extended period, central sleep apnea decreased on repeat PSG. [11]
Physiologically normal apneic events
Central sleep apnea during sleep-wake transition
Up to 40% of healthy individuals may exhibit central apneas during sleep-wake transition. The central apneas occur during the period that chemoreceptors are resetting and instability of ventilation control occurs. They are usually brief and not associated with significant oxygen desaturation. The clinical significance of this entity is unknown. Once stable sleep is reached, normal individuals should not have more than 5 central apneas per hour of sleep.
Postarousal central apnea or postsigh central apnea
During a PSG review, central apneas are commonly seen following an arousal or after a sigh and are usually inconsequential. They are thought to be a result of Herring-Breuer reflex or hypocapnia induced by hyperventilation caused by a sigh or arousal.
Epidemiology
US frequency
Predominant central apnea is uncommon and is seen in less than 10% of patients presenting for PSG. In the general population, the prevalence of central sleep apnea is less than 1%. [12] CSB-CSA has been reported in 25-40% of patients with heart failure and in 10% of patients who have had a stroke. One study [6] has reported the prevalence rate of central sleep apnea at 30% in a population of patients in a stable methadone maintenance program.
Race
No data are available on the racial distribution of central sleep apnea syndromes.
Sex
CSB-CSA shows a striking male preponderance. Sex distribution in other types of central sleep apnea syndromes has not been studied. Central sleep apnea is uncommon in premenopausal women. One explanation for this discrepancy is the presence of a lower apneic threshold of PaCO2 in women compared with men. Thus, women require a greater reduction in their PaCO2 to initiate apnea than do men.
Age
Primary central sleep apnea mostly affects middle-aged or elderly individuals. CSB-CSA increases in prevalence among individuals older than 60 years. [13] Age distribution in other central sleep apnea syndromes is unknown
Prognosis
The mortality and morbidity associated with primary central apnea remains unknown; however, these individuals are unlikely to develop significant hypercarbia or hypoxia to the detriment of pulmonary circulation or cor pulmonale. Patients with heart failure and CSB-CSA have a higher mortality rate than those without it. In one study, the 2-year survival rate for patients in heart failure with concomitant CSB-CSA higher than in those without CSB-CSA. [14] A more recent study demonstrated a higher mortality rate in congestive heart failure patients with central sleep apnea than those with no sleep apnea. However, the observed difference was no longer significant after adjusting for age and New York Heart Association functional class. [15]
A study of sleep-disordered breathing and nocturnal cardiac arrhythmias in older men documented that the likelihood of atrial fibrillation or complex ventricular ectopy increased along with the severity of sleep-disordered breathing, which included obstructive sleep apnea and CSB-CSA. [16] Different forms of sleep-disordered breathing were associated with different types of arrhythmias, and central sleep apnea was strongly associated with atrial fibrillation/flutter. The odds of atrial fibrillation (P = .01) and of complex ventricular ectopy (P< .001) increased with increasing quartiles of the respiratory disturbance index (a major index including all apneas and hypopneas).
-
The role of loop gain in determining respiratory instability. A) When loop gain is less than 1, the tendency for an overshoot of the corrective response to an apnea or hypopnea is lessened, and ventilation returns to a steady pattern. B) When loop gain is greater than or equal to 1, the vigorous responses to respiratory disturbances result in continuous oscillation between the events and the corrections, resulting in an unstable periodic breathing pattern. Adapted from White DP Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. Dec 1 2005;172(11):1363-70.
-
This polysomnogram demonstrates central sleep apnea and Biot respiration in a patient receiving long-term morphine for chronic pain. The Biot pattern may be irregular without any type of periodicity, or it can consist of runs of similar-sized breaths alternating with central apneas.
-
Obstructive sleep apnea (OSA): This polysomnogram demonstrates typical hypopneas occurring in OSA prior to continuous positive airway pressure titration. In OSA, airflow is absent or reduced, but ventilatory effort persists.
-
Cheyne Stokes: This polysomnogram represents Cheyne Stokes breathing and occurred subsequent to continuous positive airway pressure titration for OSA in the same patient in the previous media file. Cheyne Stokes breathing has a classic crescendo-decrescendo breathing pattern.