Practice Essentials
Twin-twin transfusion syndrome (TTTS) is a severe complication of monochorionic twinning. If TTTS is left untreated, mortality is 80-100% in severe cases. [1] Fetoscopic laser photocoagulation (FLP) of placental anastomoses is the standard treatment of TTTS. (See the image below.)
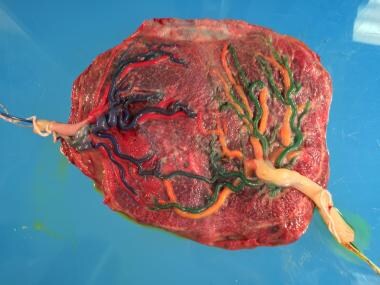 A monochorionic placenta with the vasculature of twin A in blue/red and that of twin B in green/yellow. This placenta is shown after laser ablation of shared vessels. Close examination reveals the line of photocoagulation using the Solomon technique. Courtesy of Anthony Johnson, DO.
A monochorionic placenta with the vasculature of twin A in blue/red and that of twin B in green/yellow. This placenta is shown after laser ablation of shared vessels. Close examination reveals the line of photocoagulation using the Solomon technique. Courtesy of Anthony Johnson, DO.
Signs and symptoms
Fundal height larger than dates is a common finding because of the production of excess amniotic fluid by the recipient twin.
See Presentation for more detail.
Diagnosis
Chorionicity is best determined prior to 14 weeks' gestation. From 16 weeks until delivery, monochorionic twin pregnancies should be screened for TTTS every 2 weeks.
TTTS is defined as polyhydramnios (single deepest pocket [SDP] >8 cm) in one twin and oligohydramnios (SDP < 2 cm ) in the co-twin.
The Quintero system is used to stage twin-twin transfusion [2, 3] :
-
Stage I: Polyhydramnios (deepest pocket >8 cm)/oligohydramnios (deepest pocket < 2 cm )
-
Stage II: Donor bladder not visualized
-
Stage III: Doppler abnormalities
-
Stage IV: Hydrops in either twin
-
Stage V: Death of one or both twin
See Workup for more detail.
Management
Patients with TTTS before 26 weeks' gestation should be referred to a center for fetal intervention for FLP.
Serial amnioreduction may have a role in TTTS identified too late for fetoscopic laser treatment. Septostomy has no role in the treatment of TTTS.
The demise of one twin places the surviving twin at risk for death (15%) and significant neurologic morbidity (26%); however, once the death of one twin is identifed, immediate delivery of the surviving twin has not been shown to improve outcomes.
See Treatment for more detail.
Background
Twin-twin transfusion syndrome (TTTS) is a serious complication of monozygotic monochorionic twinning in which shared placental blood vessels allow shunting of blood away from one twin (the donor) to the co-twin (the recipient).
Monozygotic twinning occurs when a single fertilized egg divides to form 2 embryos. The timing of the division determines the degree of differentiation between the 2 embryos. The earlier the split occurs, the greater the differentiation. Twins that split between days 0 and 3 have 2 amnions and 2 placentas (diamniotic dichorionic). Between days 4 and 7 the twins will share a placenta but will have separate amniotic sacs (diamniotic monochorionic). (See the images below.) Between days 7 and 13 the twins will share both a placenta and an amniotic sac (monoamniotic monochorionic), and after day 13 the twins will be conjoined.
When twins share a placenta, there will always be vascular anastomoses between the circulations of the twins. When the anastomoses are unbalanced, with preferential shunting from one twin to the other, TTTS is the result. The donor twin is usually smaller and has oligohydramnios due to decreased urine production, whereas the recipient is larger with polyhydramnios due to polyuria.
Left untreated, TTTS has high morbidity and mortality.
Pathophysiology
Monochorionic placentas have anastomotic vascular connections between the twins. These anastomoses can be artery to artery (AA), vein to vein (VV), or artery to vein (AV).
AA and VV anastomoses are on the surface of the placenta and usually have bidirectional flow. AV anastomoses are typically within the placenta and are at least partially responsible for unbalanced transfer of fluid volume to the recipient twin because they only allow flow in one direction. AA anastomoses are believed to compensate for AV anastomoses. [1, 4] Placental dye injection studies show that monochorionic placentas in pregnancies that develop TTTS have fewer AA anastomoses. [5]
Over time, the unbalanced transfer of fluid volume has significant but different effects on both twins. As the donor twin becomes hypovolemic, decreased renal perfusion causes decreased urine output and oligohydramnios. The renin-angiotensin system is activated to increase reabsorption in the renal tubules, and angiotensin-2 causes vasoconstriction in an attempt to maintain circulating volume in the donor twin.
Vasoconstriction leads to uteroplacental insufficiency and intrauterine growth restriction (IUGR) of the donor twin. Blood flow is redistributed to protect the brain, and vasoconstriction results in abnormal umbilical artery Doppler studies. The donor may develop hydrops because of severe anemia.
The recipient twin experiences an increase in circulating volume, which causes synthesis of atrial natriuretic peptide. Glomerular filtration is increased, and reabsorption from the renal tubules decreases. Urine production is increased, which results in polyhydramnios. Hydrops may occur because of volume overload and high output failure.
The cardiovascular system of the recipient twin experiences changes in overall function and in preload and afterload. In Quintero stages I and II TTTS, left ventricular filling pressures are increased with decreased systolic function. In stages III and IV TTTS, right ventricular filling pressures become elevated. This finding demonstrates that the heart of the recipient twin is affected even in the early stages of the disease. [6, 7]
Twin anemia polycythemia sequence (TAPS) is a variant of TTTS. TAPS differs from TTTS in that polyhydramnios and oligohydramnios do not occur. TAPS may occur spontaneously (3-6%) or as a side effect of incomplete ablation of an anastomosis during laser photocoagulation (16%). Use of the Solomon technique reduces the postablation rate to 3%. [8]
TAPS is the result of chronic, slow, unidirectional transfusion of blood from one twin to the other via a few small (< 1 mm in diameter) anastomoses. One fetus becomes anemic and the other polycythemic, but no difference in the amount of amniotic fluid occurs. In contrast to TAPS, the flow in TTTS is bidirectional though unbalanced and the anastomoses are larger.
TAPS is generally unrecognized because no recommendation exists to screen for TAPS in monochorionic diamniotic pregnancies. Screening is done by evaluating the peak systolic velocity (PSV) in the middle cerebral artery (MCA). Criteria for diagnosis are based on the difference between twins (the delta) in the MCA-PSV. [9] The table below lists the stages of TAPS.
Table 1. Stages of Twin Anemia Polycythemia Sequence (TAPS) (Open Table in a new window)
Stage 1 |
Delta MCA-PSV >0.5 MOM; no signs of fetal compromise |
Stage 2 |
Delta MCA-PSV >0.7 MOM; no signs of fetal compromise |
Stage 3 |
Stage 1 or 2 with hydrops of the donor |
Stage 4 |
Hydrops of the donor |
Stage 5 |
Death of one or both twins preceded by TAPS |
MCA-PSV = middle cerebral artery peak systolic velocity; MOM = multiples of the median.
Intervention is recommended for stage 2 and higher TAPS, with intrauterine transfusion or laser photocoagulation.
Epidemiology
United States data
Monozygotic twins occur in 3-5 per 1000 pregnancies. Approximately 75% of monozygotic twins are monochorionic. TTTS occurs in 10-15% of monochorionic twins. [10, 11]
Prognosis
Morbidity/mortality
Untreated severe TTTS (stage 3 or higher) has a 60-100% fetal or neonatal mortality rate. Mild to moderate TTTS (stage 2) is frequently associated with premature delivery. Fetal demise of one twin is associated with neurologic sequelae in 25% of surviving twins. Fetal blood pressure instability can lead to brain ischemia in either the donor or the recipient twin. Periventricular leukomalacia, porencephaly, microcephaly, and cerebral palsy have all been identified in survivors of TTTS. [1, 12]
Survival of at least one twin approaches 75% with treatment by fetoscopic laser photocoagulation (FLP). [13]
In a review of 135 monochorionic twin pregnancies with single intrauterine death (sIUD), whether spontaneous or procedure related, O'Donoghue et al found that death of the co-twin followed in 22.9% of cases. [14] In the pregnancies that continued after sIUD, the frequency of antenatally acquired brain injury in the co-twin was significantly lower after procedure-related than spontaneous sIUD: 2.6% versus 22.2% (P = .003). The investigators concluded that the risk of brain injury is reduced but not negated by procedures that restrict inter-twin transfusion.
The risk of prematurity continues after FLP treatment and is as high as 50% for birth before 34 weeks. The risk of preterm premature rupture of the membranes (PPROM) before 34 weeks ranges between 20% and 30%. [15]
Complications
Heart disease
Heart disease is a major cause of death in TTTS, representing 50% of the fetal deaths that occur in the postnatal period. If TTTS is treated with amnioreduction rather than fetoscopic laser photocoagulation (FLP), the risk of heart failure in the recipient twin is 2.7-fold higher. [16] This increased risk is due to the fact that FLP corrects the underlying placental pathology whereas amnioreduction does not. Among patients who survive after undergoing FLP, up to 85-87% will have a normal cardiac evaluation. [17]
When patients are treated with FLP, cardiac function usually normalizes by about 15 months of age. However, these patients are at higher risk for long-term cardiac pathology than the general population. The recipient twin has a 4.46% increased risk of pulmonary stenosis compared with the general population. The risk of atrial septal defects is also higher than in the general population, most likely because of pressure differences that result from volume overload. [18]
Neurologic sequelae
Neurologic abnormalities related to TTTS include periventricular leukomalacia and intraventricular hemorrhage. During childhood, neurologic sequelae include intellectual disability, language delay, strabismus, and cerebral palsy. [17, 19] It is theorized that placental abnormalities such as arteriovenous malformations lead to hemodynamic disturbances. These disturbances disrupt normal blood flow and may lead to hemorrhagic brain lesions and ischemia. [12, 20] Other complications associated with TTTS result from low birth weight and prematurity.
After FLP, the rate of significant neurologic impairment is 10% at 2 years of age. A higher Quintero stage and a lower gestational age at birth are associated with worse cognitive performance. [10, 17]
-
Monozygotic twins with monochorionic, diamniotic placentation.
-
Monozygotic twins with monochorionic, monoamniotic placentation.
-
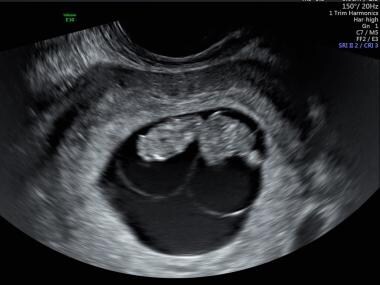
This image of diamniotic monochorionic twins shows the dividing membrane and a single placenta.
-
A monochorionic placenta with the vasculature of twin A in blue/red and that of twin B in green/yellow. This placenta is shown after laser ablation of shared vessels. Close examination reveals the line of photocoagulation using the Solomon technique. Courtesy of Anthony Johnson, DO.
-
The 2 separate amniotic sacs of diamniotic twins are prominent in this image.
-
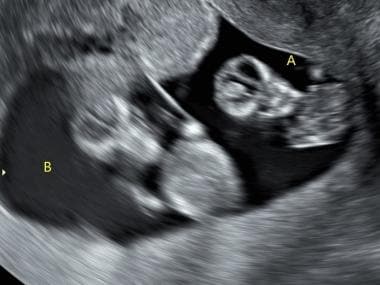
Twins showing the dividing thin membrane
-
A recipient twin with hydrops at 22 weeks' gestation. Fluid surrounds the heart and lungs, and the skin is thickened.