Practice Essentials
Protein C deficiency is a congenital or acquired condition that leads to increased risk for thrombosis. Congenital protein C deficiency is one of several inherited thrombophilias, which are a heterogeneous group of genetic disorders associated with an elevated risk of venous thromboembolism. [1] Other inherited thrombophilias include the following:
-
Factor V Leiden mutation
-
Prothrombin gene mutation
See also Hereditary and Acquired Hypercoagulability.
This article focuses on the pathophysiology, prevalence, clinical manifestations, diagnosis, and treatment of hereditary protein C deficiency. Causes of acquired protein C deficiency are also addressed.
For patient education information, see the Deep Vein Thrombosis Health Center.
Pathophysiology
The protein C pathway
Protein C is a 62-kD, vitamin K–dependent glycoprotein synthesized in the liver. It circulates in the blood as an inactive zymogen at a concentration of 4 μg/mL. Its activation into the serine-protease-like enzyme, activated protein C (aPC), is catalyzed by thrombin when it is bound to the endothelial proteoglycan thrombomodulin. [1, 2, 3] The protein C pathway is illustrated in the image below.
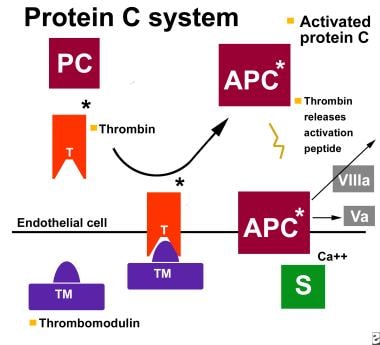 The protein C pathway. APC = activated protein C; PC = protein C; S= protein S; T = thrombin; TM = thrombomodulin; Va = factor Va; VIII = factor VIIIa.
The protein C pathway. APC = activated protein C; PC = protein C; S= protein S; T = thrombin; TM = thrombomodulin; Va = factor Va; VIII = factor VIIIa.
aPC exerts its anticoagulant activity primarily through inactivation of coagulation factors Va and VIIIa, which are required for factor X activation and thrombin generation. The catalytic activity of aPC is greatly enhanced by the vitamin K–dependent cofactor protein S. [4]
Aside from its role in coagulation, aPC subserves anti-inflammatory and cytoprotective functions, which are mediated through the endothelial protein C receptor and the protease-activated receptor–1 (PAR-1). [5, 6]
A deficiency of aPC disturbs the delicate balance between procoagulant and anticoagulant proteins and engenders a prothrombotic environment. The role of aPC and other anticoagulant proteins in this balance appears to be especially important in the slow-flowing venous circulation, in which procoagulant proteins and platelet phospholipids have prolonged exposure to the vessel wall. This may explain, in part, why protein C deficiency appears to be associated primarily with venous thrombosis.
Genetics of protein C deficiency
Heterozygous protein C deficiency is inherited in an autosomal dominant fashion, however, in families with individuals with complete deficiency, the mode of inheritance is autosomal recessive. [7, 8] The gene for protein C is located on the long arm of chromosome 2 and nearly 200 pathogenic mutations of this gene have been described. [9] These mutations are divided into 2 types—type I and type II—on the basis of whether they cause a quantitative (type I) or functional (type II) deficiency of protein C.
Type I deficiency
Type I protein C deficiency refers to a quantitative deficiency in the plasma protein C concentration. Heterozygous individuals typically demonstrate protein C antigen and activity levels that are approximately one half that of normal patient plasma. A range of causative genetic alterations within the protein C promoter region and splice sites as well as in the coding sequence of the protein C gene itself have been reported. [9]
There is marked phenotypic variation among families with heterozygous type I protein C deficiency. Some families exhibit a severe thrombotic tendency, whereas others remain asymptomatic. [10, 11, 12] Interestingly, this variability is seen even among different pedigrees that harbor the same protein C mutation, suggesting that the mutation itself does not fully explain the phenotypic variability. [13] The presence of a second thrombophilic mutation such as factor V Leiden has been associated with a more severe phenotype in some protein C–deficient kindreds. [14]
Type II deficiency
Type II protein C deficiency is less common than type I disease and is associated with decreased functional activity and normal immunologic levels of protein C. A number of point mutations within the protein C gene giving rise to this disorder have been described. [9] .
Individuals who are homozygous or compound heterozygous for a mutation or other genetic defect affecting the protein C, typically due to the inheritance of abnormal alleles from both parents, can experience neonatal purpura fulminans, intracranial thromboembolism, and thrombosis. [15]
Etiology
Protein C deficiency may be congenital or acquired. The genetic basis of congenital protein C deficiency is reviewed in Pathophysiology.
Acquired Protein C Deficiency
Causes of acquired protein C deficiency include the following:
-
Acute thrombosis
-
Warfarin therapy
-
Liver disease (eg, liver fibrosis from chronic hepatitis C [16]
-
Vitamin K deficiency
-
Sepsis
-
Certain chemotherapeutic agents (eg, L-asparaginase)
-
Uremia - Uremic patients may have a normal levels of protein C with low levels of protein C anticoagulant activity due to a antibodies against protein C [17]
Cases of acquired protein C deficiency in association with the development of a protein C autoantibody [18] and hematopoietic stem cell transplantation [19] have also been reported.
A severe form of acquired protein C deficiency associated with purpura fulminans may be observed in patients with meningococcemia and other causes of severe sepsis. [20]
Causes of increased protein C include the following:
Epidemiology
Frequency
Studies in cohorts with no clinical history of venous thromboembolism (VTE) have found protein C deficiency in 1 in 200 to 1 in 500 persons. [23, 24] In patients presenting with VTE, approximately 3-5% may have protein C deficiency. [25, 26, 27] Severe homozygous or compound heterozygous protein C deficiency occurs in approximately 1 in 500,000 to 1 in 750,000 live births. [23, 24, 25, 26, 27] Severe congenital protein C deficiency (SCPCD) is a rare autosomal recessive disorder with an estimated incidence of 1 per 4 million births. [8]
Race
Congenital protein C deficiency is recognized as a cause of thrombophilia around the world. Studies in blacks and Asians suggest that its prevalence in these populations is on par with its frequency of occurrence in whites. [28, 29] In contrast, the factor V Leiden and prothrombin gene mutations (see Hereditary and Acquired Hypercoagulability) occur with substantially greater frequency in white than in nonwhite populations.
Sex
As would be expected for an autosomal genetic disorder, the prevalence of hereditary protein C deficiency is similar in men and women. However, pregnancy, the postpartum state, and estrogen-containing hormonal therapy are important risk factors for the development of VTE that are unique to women.
Age
Preterm infants have protein C levels approximately 10-15% of normal adult levels; neonates, approximately 35%; and adolescents, 80%. Protein C levels increase approximately 4% per decade in adulthood [30, 31] ; nonetheless, the risk of thrombosis in individuals with heterozygous protein C deficiency increases with age. The median age at onset of VTE in heterozygous individuals is 30-40 years, and thrombosis is rare before age 20 years. [32]
In contrast, homozygous or compound heterozygous protein C deficiency classically manifests as NPF in the first several hours to days of life. Rare patients with homozygous or compound heterozygous deficiency may present with VTE during childhood or adolescence. [33, 16]
Prognosis
Clinical manifestations of heterozygous protein C deficiency include VTE and warfarin-induced skin necrosis (WISN). Whether the risk of pregnancy loss is increased in this disorder is controversial. Heterozygous protein C deficiency does not appear to be associated with an elevated risk of arterial thrombosis.
Homozygous and compound heterozygous protein C deficiency are classically associated with neonatal purpura fulminans (NPF); intracranial thromboembolism may also occur in neonates. [34] Occasionally, patients present with VTE in childhood or adolescence.
Venous thromboembolism
The cardinal clinical manifestation of heterozygous protein C deficiency is VTE. The risk of VTE in this population is roughly seven-fold higher than that of the general population. [35, 36] Approximately 40% of patients with VTE have one of the usual thrombotic risk factors, such as pregnancy, the postpartum state, hormonal therapy, surgery, or immobilization. [37] The remaining 60% present with unprovoked VTE.
The most common sites of thrombosis are the deep veins of the lower extremities, although an elevated risk of mesenteric vein and cerebral sinus thrombosis is also well-documented. [38, 39, 40] Approximately 40% of patients with protein C deficiency present with evidence of pulmonary embolism, and roughly 60% suffer recurrent thrombosis if anticoagulation is discontinued. [37]
The risk of VTE increases with age and, among heterozygotes, thrombosis is unusual before age 20 years. Rare homozygotes and compound heterozygotes who do not manifest NPF in infancy may present with VTE later in childhood or adolescence. [33]
The occurence of arterial thromboembolism in patients with protein C deficiency is low but has been reported in patients with venous thromboembolism. [41]
Warfarin-induced skin necrosis
WISN is a potentially catastrophic complication of warfarin therapy that arises as a consequence of the different half-lives of the vitamin K–dependent proteins. One day after initiation of usual doses of warfarin, protein C activity is reduced by approximately 50%. Owing to their longer half-lives, the levels of the vitamin K-dependent clotting factors II, IX, and X decline more slowly (factor VII activity declines at approximately the same rate as protein C).
The reduced level of protein C activity relative to these other procoagulant molecules creates a transient hypercoagulable state. This effect is more pronounced when large loading doses of warfarin are administered. Indeed, WISN typically occurs during the first few days of warfarin therapy, often when daily doses in excess of 10 mg are administered. [42, 43]
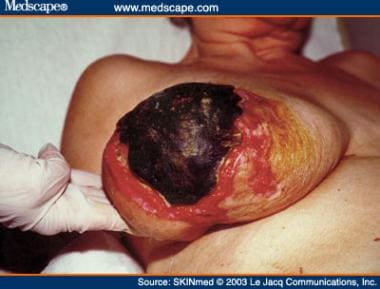
The skin lesions of WISN arise on the extremities, torso, breasts, and penis. They begin as erythematous macules and, if appropriate therapy is not initiated promptly, evolve to become purpuric and necrotic (see image below). Dermal biopsy demonstrates ischemic necrosis of the cutaneous tissue with cutaneous vessel thrombosis and surrounding interstitial hemorrhage. [44]
Although protein C deficiency is a strong risk factor for the development of WISN, approximately two thirds of patients with WISN do not have underlying hereditary protein C deficiency. [45] Other conditions reported in association with WISN include acquired protein C deficiency (see Causes) [46] and heterozygous protein S deficiency. [47]
See Prevention for the discussion of prevention and treatment of WISN.
Pregnancy loss
Protein C deficiency may be weakly associated with late and recurrent pregnancy loss. In the European Prospective Cohort on Thrombophilia, the odds ratio (OR) for stillbirth (defined as pregnancy loss at > 28 weeks' gestation) among women with an inherited thrombophilia was 3.6 (95% confidence interval [CI] 1.4-9.4), whereas the risk of miscarriage before 28 weeks' gestation in this cohort was not significantly different than that of nonthrombophilic women. [48] Among the subgroup of thrombophilia subjects with congenital protein C deficiency, the OR of stillbirth was 2.3 (95% CI 0.6-8.3). [49, 48] In a meta-analysis of 633 subjects with hereditary protein C deficiency, the association with recurrent fetal loss was likewise nonsignificant (OR 1.57; 95% CI 0.23-10.54). [50]
Arterial thrombosis
There are several case reports of arterial stroke [51, 52] and myocardial infarction [53] occurring in young adults with congenital protein C deficiency. However, the results of larger studies are conflicting [54, 55, 56, 57, 58] and the existence of an association between protein C deficiency and arterial thrombosis remains controversial.
Peripheral arterial disease
In a study of 106 patients with peripheral arterial disease (PAD) and 44 with abdominal aortic aneurysm (AAA), Komai and colleagues found that the incidences of protein C deficiency was 4.7% in patients with PAD and 4.% in patients with AAA — higher rates than those observed in the general population. Protein C activity levels were significantly lower in PAD patients with critical limb ischemia than in those with intermittent claudication. In multivariate logistic regression analysis, lower protein C activity and female gender were determinant factors of critical limb ischemia. [59]
Neonatal purpura fulminans
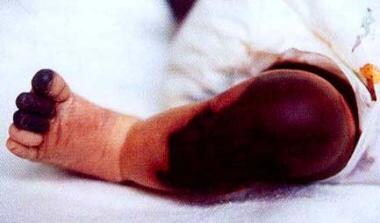
NPF is a life-threatening condition that occurs in newborns with homozygous or compound heterozygous protein C deficiency, usually during the first several days of life. Affected neonates present with diffuse ecchymoses (see image below). Skin biopsy demonstrates extensive thrombosis of cutaneous venous and arterial channels, much as is observed in WISN. [60, 61] Laboratory testing reveals severe deficiency (< 1% of normal) of immunologic protein C levels. [62] Expeditious treatment with an exogenous source of protein C, (discussed in Treatment, Medical Care, is paramount.
Many infants with severe congenital protein C deficiency have retinal and cerebral vessel thrombosis, and blindness is common. Cerebral vessel thrombosis results in cerebral infarction, often with secondary hemorrhage and hydrocephalus, causing long-term neurologic sequelae. [8]
Patient Education
Patients with protein C deficiency should be advised of the presenting signs and symptoms of VTE. Patients who are not maintained on anticoagulation should speak with their physician about thromboprophylaxis during events associated with an elevated risk of thrombosis such as surgery, trauma, immobilization, pregnancy, and the postpartum period.
Patients on warfarin should be advised of the importance of maintaining a regular diet and notifying their physician when changes to their medications have been made.
For patient education information, see the Deep Vein Thrombosis Health Center, as well as Pulmonary Embolism.
-
The protein C pathway. APC = activated protein C; PC = protein C; S= protein S; T = thrombin; TM = thrombomodulin; Va = factor Va; VIII = factor VIIIa.
-
A patient with warfarin-induced skin necrosis.
-
A patient with neonatal purpura fulminans.