Practice Essentials
Ovarian cancer is the most common cause of cancer death from gynecologic tumors in the United States. Malignant ovarian lesions include primary lesions arising from normal structures within the ovary and secondary lesions from cancers arising elsewhere in the body. Primary lesions include epithelial ovarian carcinoma (70% of all ovarian malignancies). Current research suggests that the majority of these originate from the fallopian tubes.
Stromal tumors of the ovary include germ-cell tumors, sex-cord stromal tumors, and other more rare types. Metastases to the ovaries are relatively frequent; common sources are tumors in the endometrium, breast, colon, stomach, and cervix. See the image below.
Signs and symptoms
Early ovarian cancer causes minimal, nonspecific, or no symptoms. The patient may feel an abdominal mass. Most cases are diagnosed in an advanced stage.
Epithelial ovarian cancer presents with a wide variety of vague and nonspecific symptoms, including the following:
-
Bloating; abdominal distention or discomfort
-
Pressure effects on the bladder and rectum
-
Constipation
-
Vaginal bleeding
-
Indigestion and acid reflux
-
Shortness of breath
-
Tiredness
-
Weight loss
-
Early satiety
Symptoms independently associated with the presence of ovarian cancer include pelvic and abdominal pain, increased abdominal size and bloating, and difficulty eating or feeling full. [1] Symptoms associated with later-stage disease include gastrointestinal symptoms such as nausea and vomiting, constipation, and diarrhea. [2] Presentation with swelling of a leg due to venous thrombosis is not uncommon. Paraneoplastic syndromes due to tumor-mediated factors lead to various presentations.
See Presentation for more detail.
Diagnosis
Physical findings are uncommon in patients with early disease. Patients with more advanced disease may present with ovarian or pelvic mass, ascites, pleural effusion, or abdominal mass or bowel obstruction.
The presence of advanced ovarian cancer is often suspected on clinical grounds, but it can be confirmed only pathologically by removal of the ovaries or, when the disease is advanced, by sampling tissue or ascitic fluid.
Screening
The US Preventive Services Task Force (USPSTF) recommends against screening (with serum CA-125 level or transvaginal ultrasonography) for ovarian cancer in the general population. [3] The US Food & Drug Administration (FDA) recommends against the use of tests marketed for ovarian cancer screening. [4] The National Cancer Institute (NCI) cites evidence of lack of mortality benefit with screening, and potential harms relating to false-positive test results. [5]
Laboratory testing
No tumor marker (eg, CA-125, beta-human chorionic gonadotropin, alpha-fetoprotein, lactate dehydrogenase) is completely specific; therefore, use diagnostic immunohistochemistry testing in conjunction with morphologic and clinical findings. Also, obtain a urinalysis to exclude other possible causes of abdominal/pelvic pain, such as urinary tract infections or kidney stones.
Imaging studies
Imaging studies to consider, when clinically indicated, in patients with a presentation suspicious for ovarian cancer may include the following [6] :
-
Pelvic and abdominal magnetic resonance imaging: Increases specificity of imaging when sonography findings are indeterminate [9]
-
Chest radiography: Routine imaging to exclude lung metastases; chest CT preferred if there is preferred if there is particular concern for metastatic or disseminated disease [6]
-
Mammography: Part of preoperative workup for women older than 40 years who have not had one in the preceding 6-12 months; estrogen-producing tumors may increase the risk of breast malignancies, and breast cancers can metastasize to the ovaries and are often bilateral
In patients with diffuse carcinomatosis and GI symptoms, a GI tract workup may be indicated, including one of the following imaging studies:
-
Upper and/or lower endoscopy
-
Barium enema
-
Upper GI series
Procedures
Fine-needle aspiration (FNA) or percutaneous biopsy of an adnexal mass is not routinely recommended, as it may delay diagnosis and treatment of ovarian cancer. Instead, if a clinical suggestion of ovarian cancer is present, the patient should undergo laparoscopic evaluation or laparotomy, based on the presentation, for diagnosis and staging. An FNA or diagnostic paracentesis should be performed in patients with diffuse carcinomatosis or ascites without an obvious ovarian mass.
See Workup for more detail.
Management
Standard treatment for women with ovarian cancer involves aggressive debulking surgery and chemotherapy. The aim of cytoreductive surgery is to confirm the diagnosis, define the extent of disease, and resect all visible tumor. Neoadjuvant chemotherapy is increasingly used.
Surgery
The type of procedure depends on whether or not disease is visible outside the ovaries. When no disease is visible outside the ovaries, or no lesion greater than 2 cm is present outside of the pelvis, the patient requires formal surgical staging, including peritoneal cytology, multiple peritoneal biopsies, omentectomy, pelvic and para-aortic lymph node sampling, and biopsies of the diaphragmatic peritoneum.
If visible disease is noted, aggressive surgical debulking, with the intent to remove all visible disease should be undertaken. If the surgeon determines that optimal debulking is not possible, then neoadjuvant chemotherapy should be considered. For patients with stage IV disease, surgery should be individualized on the basis of presentation.
Surgical procedures that may be performed in women with ovarian cancer are as follows:
-
Surgical staging
-
Cytoreductive surgery
-
Interval debulking
-
Laparoscopic surgery
-
Secondary surgery
Chemotherapy
Postoperative chemotherapy is indicated in all patients with ovarian cancer, except those who have surgical-pathologic stage I disease with low-risk characteristics. Standard postoperative chemotherapy for ovarian cancer is combination therapy with a platinum compound and a taxane (eg, carboplatin and paclitaxel). Additional agents for recurrent disease include the following:
-
Liposomal doxorubicin
-
Etoposide
-
Topotecan
-
Gemcitabine
-
Vinorelbine
-
Ifosfamide
-
Fluorouracil
-
Melphalan
-
Altretamine
-
Bevacizumab
-
Olaparib
-
Niraparib
-
Rucaparib
-
Pazopanib
Adjunctive medications include the following:
-
Cytoprotective agents (eg, mesna)
-
Antiemetics (eg, ondansetron, granisetron, palonosetron, dexamethasone)
See Treatment and Medication for more detail.
For patient education information, see the Ovarian Cancer Health Center.
Background
Malignant lesions of the ovaries include primary lesions arising from normal structures within the ovary and secondary lesions from cancers arising elsewhere in the body. Primary lesions include epithelial ovarian carcinoma (70% of all ovarian malignancies), germ-cell tumors, sex-cord stromal tumors, and other more rare types. Metastases to the ovaries are relatively frequent, with the most common being from the endometrium, breast, colon, stomach, and cervix.
Although many histologic types of ovarian tumors have been described, more than 90% of ovarian malignancies are epithelial tumors. Many of these actually originate in the fallopian tubes. (See Pathophysiology.)
The precise cause of ovarian cancer is unknown. However, several risk and contributing factors (including both reproductive and genetic factors) have been identified. (See Etiology.)
Ovarian cancer is the most common cause of cancer death from gynecologic tumors in the United States. Around the world, more than 200,000 women are estimated to develop ovarian cancer every year and about 100,000 die from the disease. The lifetime risk of a woman developing epithelial ovarian cancer is 1 in 70. (See Epidemiology.)
Early disease causes minimal, nonspecific, or no symptoms. Therefore, most cases are diagnosed in an advanced stage. Prognosis in ovarian cancer is closely related to the stage at diagnosis; thus, overall, prognosis for these patients remains poor. (See Presentation and Prognosis.)
Standard treatment involves aggressive debulking surgery followed by chemotherapy. The incorporation of neoadjuvant chemotherapy has recently increased, with multiple studies indicating that in some situations it offers an improvement in morbidity and possibly survival.(See Treatment and Medication.)
Pathophysiology
Historically, most theories of the pathophysiology of ovarian cancer included the concept that it begins with the dedifferentiation of the cells overlying the ovary. During ovulation, these cells can be incorporated into the ovary, where they then proliferate. However, new evidence indicates that the majority of these tumors actually originate in the fimbria of the fallopian tube. Detailed pathologic studies have pushed much of the thinking about the origin of these tumors in this direction. [10]
Ovarian cancer typically spreads to the peritoneal surfaces and omentum. Spread can occur by local extension, lymphatic invasion, intraperitoneal implantation, hematogenous dissemination, or transdiaphragmatic passage. Intraperitoneal dissemination is the most common and recognized characteristic of ovarian cancer. Malignant cells can implant anywhere in the peritoneal cavity but are more likely to implant in sites of stasis along the peritoneal fluid circulation.
These mechanisms of dissemination represent the rationale to conduct surgical staging, debulking surgery, and intraperitoneal administration of chemotherapy. In contrast, hematogenous spread is clinically unusual early on in the disease process, although it is not infrequent in patients with advanced disease.
Epithelial tumors represent the most common histology (90%) of ovarian tumors. Other histologies include the following:
-
Sex-cord stromal tumors
-
Germ cell tumors
-
Primary peritoneal carcinoma
-
Metastatic tumors of the ovary
Epithelial ovarian cancer
Epithelial ovarian cancer is thought to arise from epithelium covering the fimbria of the fallopian tubes, or the ovaries, both of which are derived from the coelomic epithelium in fetal development. This coelomic epithelium is also involved in formation of the müllerian ducts, from which the fallopian tubes, uterus, cervix, and upper vagina develop.
Four main histologic subtypes, which are similar to carcinoma, arise in the epithelial lining of the cervix, uterus, and fallopian tube, as follows:
-
Serous (from fallopian tube)
-
Endometrioid (endometrium)
-
Mucinous (cervix)
-
Clear cell (mesonephros)
Some variation is observed in the patterns of spread and disease distribution within the various histologic subtypes.
Epithelial tumors are found as partially cystic lesions with solid components. The surface may be smooth or covered in papillary projections (see the image below), and the cysts contain fluid ranging from straw-colored to opaque brown or hemorrhagic.
Epithelial ovarian cancer most often spreads initially within the peritoneal cavity (see the image below). Metastatic disease often is found on the peritoneal surfaces, particularly on the undersurface of the diaphragms, the paracolic gutters, the bladder, and the cul-de-sac. Other common sites are as follows:
-
Surface of the liver
-
Mesentery and serosa of the large and small bowel
-
Omentum
-
Uterus
-
Para-aortic and pelvic lymph nodes
Outside the peritoneal cavity, epithelial ovarian cancer may spread to the pleural cavity, lungs, and groin lymph nodes. The presence of pleural effusion does not necessarily indicate disease in the chest, and malignancy can be diagnosed only cytologically. Mucinous tumors tend to form large dominant masses, while papillary serous tumors have a more diffuse distribution and are more commonly bilateral. Endometrioid and clear-cell variants more commonly exhibit local invasion, retroperitoneal disease, and hepatic metastases.
Increasing evidence suggests that a high proportion of high-grade serous carcinoma originates from distal fallopian tube epithelium or the tuboperitoneal junction rather than the ovarian surface epithelium. Serous intraepithelial or early invasive carcinoma has been found in up to 10% of fallopian tubes from BRCA mutation carriers who had undergone prophylactic bilateral salpingo-oophorectomies. Clinical, molecular, and genetic studies, as well as in vitro and animal models, have also supported a tubal origin for high-grade serous ovarian carcinoma. [10, 11]
Those findings have prompted the suggestion that prevention of ovarian cancer in selected women at high risk could be better accomplished with salpingectomy. A study comparing standard risk-reducing salpingo-oophorectomy with the combination of early risk-reducing salpingectomy and delayed oophorectomy in BRCA carriers is currently recruiting participants. [12]
Tumors of low malignant potential
Tumors of low malignant potential (LMP), or borderline tumors, are a distinct variety of epithelial ovarian cancer that behave in a much less aggressive fashion and have a very favorable prognosis. These tumors cause great anxiety to patients, and the concept of LMP sometimes is difficult to explain. They comprise approximately 20% of malignant ovarian tumors. The mean age of diagnosis is younger than for invasive epithelial ovarian cancer, at approximately 48 years, and no large peak of incidence is observed.
These tumors are staged identically to epithelial ovarian cancer, using the FIGO (Fédération Internationale de Gynécologie et d'Obstétrique; International Federation of Obstetrics and Gynecology) system. In contrast to epithelial ovarian cancer, however, most LMP tumors are stage I at presentation, with a distribution as follows:
-
Stage IA: 51%
-
Stage IB: 6%
-
Stage IC: 18%
-
Stages II-III: 15%
-
Stage IV: 2%
LMP tumors can cause a range of symptoms similar to epithelial ovarian cancer, including increasing abdominal girth, an abdominal mass, abdominal pain, abnormal uterine bleeding, urinary symptoms, and gastrointestinal symptoms. They may be asymptomatic and found on routine physical examination or ultrasound scan.
For more information, see Borderline Ovarian Cancer.
Malignant germ cell tumors
Malignant germ cell tumors (GCTs), which include dysgerminoma, endodermal sinus tumor, malignant teratoma, embryonal carcinoma, and choriocarcinoma, are thought to derive from primitive germ cells in the embryonic gonad. GCT of the ovary is much rarer than GCT of the testis in males, and much of the development of the management approach has been based on experience with male GCT.
Common characteristics of these tumors include rapid growth, a predilection for lymphatic spread, frequent mixtures of tumor types, and a predominantly unilateral pattern of ovarian involvement (except for dysgerminoma). GCT is much more common in young women but occasionally occurs in infants and older women.
Many GCTs produce tumor markers that can be measured in the blood and then used to monitor response to treatment and for follow-up care. Endodermal sinus tumors secrete alpha-fetoprotein and choriocarcinoma, and dysgerminomas occasionally secrete beta human chorionic gonadotropin (bHCG). Dysgerminoma may secrete lactate dehydrogenase and placental alkaline phosphatase.
No factors have been established related to etiology, apart from an increased incidence associated with dysgenetic gonads.
Although these tumors may be asymptomatic and present as a palpable mass, many patients present with abdominal pain. The mass may lead to acute pain due to torsion, rupture, or hemorrhage, or, patients may have abdominal distension, vaginal bleeding, or fever.
Most are stage I and confined to the ovary at the time of diagnosis.
Dysgerminoma
This is the most common malignant GCT and represents 3-5% of all ovarian malignancies. Ninety percent occur in people younger than 30 years, and 75% occur in the second and third decades, with a median age of 22 years.
Dysgerminomas are bilateral in 10-35% of cases. Five percent occur in phenotypic females with abnormal gonads. They may have a 46XY karyotype with pure gonadal dysgenesis or androgen insensitivity syndrome, or, they may have a 45X, 46XY karyotype with mixed gonadal dysgenesis. Dysgerminomas may be large and usually are solid, with a smooth external surface and a fleshy pink-tan color inside. The majority are confined to the ovary at diagnosis, but approximately 25% of otherwise stage I dysgerminomas have lymph node metastasis.
For more information, see Ovarian Dysgerminomas.
Cystic teratoma
Teratomas are germ cell tumors commonly composed of multiple cell types derived from one or more of the 3 germ layers. Inconsistent nomenclature often confuses discussions of various subtypes of teratomas. The word is derived from the Greek teras, meaning monster, which Virchow coined in the first edition of his book on tumors published in 1863. [13] Teratomas range from benign, well-differentiated (mature) cystic lesions to those that are solid and malignant (immature). Additionally, teratomas may be monodermal and highly specialized. Rarely, within some mature teratomas certain elements (most commonly squamous components) undergo malignant transformation.
In 1831, Leblanc coined the term dermoid cyst in the veterinary literature when he removed a lesion that resembled skin at the base of a horse's skull, which he called a “kyste dermoid.” [14] Both dermoid and teratoma, terms now more than a century old, remain in general use and often are used interchangeably with various preferences among subspecialties. The earliest implications were that dermoids comprised elements similar to skin and its appendages, whereas teratomas had no such limits. Dermoids now are recognized as often being trigeminal and containing practically any type of tissue.
For those who continue to make a distinction, dermoids are tumors that maintain rather orderly arrangements, with well-differentiated ectodermal and mesodermal tissues surrounding endodermal components. Teratomas, specifically solid teratomas, are essentially devoid of organization; thus, the presence of some degree of organization, a high degree of cellular differentiation, and cystic structure differentiates dermoids from teratomas (see the images below). [13]
For more information, see Teratoma, Cystic.
Immature teratoma
This is the second most common GCT. It occurs mostly in females aged 10-20 years but may occur after menopause. The tumor spreads most commonly to peritoneal surfaces.
Other germ cell tumors
Endodermal sinus tumor occurs at a mean age of 18 years, and one third occur before puberty. Embryonal carcinoma and choriocarcinoma are extremely rare.
Sex-cord stromal tumors
These include tumors arising from the sex cords; granulosa cells; Sertoli cells; and the specialized stroma of the genital ridge, theca, and Leydig cells. They comprise fewer than 5% of all ovarian tumors.
Although granulosa cell tumors are malignant and Sertoli-Leydig cell tumors less so, they behave in a much less malignant fashion than epithelial ovarian cancers. Benign tumors in the group include thecoma and fibroma. Granulosa cell tumors and pure Sertoli cell tumors commonly secrete estrogen, while Leydig cell tumors and combined Sertoli-Leydig tumors often secrete androgens.
Granulosa cell tumor
This is the most common malignant sex-cord stromal tumor. Ninety percent of granulosa cell tumors are stage I at the time of diagnosis. This tumor account for approximately 2% of all ovarian tumors and can be divided into adult (95%) and juvenile (5%) types based on histologic findings. Juvenile granulosa cell tumor is a variant of granulosa cell tumor that is rarely malignant. It most often presents in young girls with isosexual precocious puberty. The tumor is usually unilateral and confined to the ovary and can be managed with surgery alone.
Granulosa cell tumor can occur at any age, with a mean age of the early 50s. Because of the secretion of estrogen, the presenting features depend on the patient's age. Prepubertal girls typically present with precocious sexual development, women of reproductive age have heavy or irregular periods, and postmenopausal women may have postmenopausal bleeding. At all ages, the tumor may present with acute abdominal pain due to rupture or hemorrhage.
The tumors vary in size and may be solid or partially cystic (see the image below).
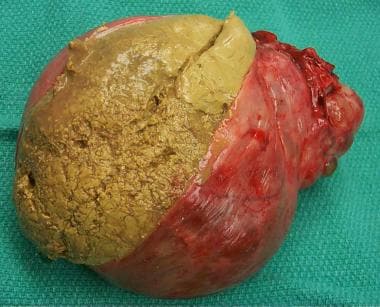
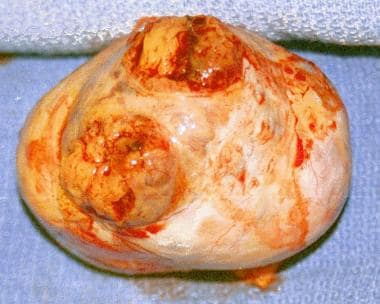 Granulosa cell tumor excised from a woman aged 44 years. Note the yellowish tumor that has eroded through, onto the surface of the ovary.
Granulosa cell tumor excised from a woman aged 44 years. Note the yellowish tumor that has eroded through, onto the surface of the ovary.
The cut surface may be gray-white or yellow, depending on lipid content. Necrosis and hemorrhage often are present, with cystic compartments filled with fluid or clotted blood (see the image below).
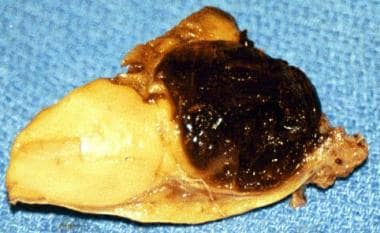 This photo shows a granulosa cell tumor, with the cut surface showing classic features of a hemorrhagic cyst and yellowish solid component.
This photo shows a granulosa cell tumor, with the cut surface showing classic features of a hemorrhagic cyst and yellowish solid component.
The microscopic features are granulosa cells in a wide variety of patterns, and characteristic Call-Exner bodies may be present.
For more information, see Granulosa-Theca Cell Tumors.
Sertoli-Leydig cell tumor
These tumors are rare. They are a form of low-grade malignancy that typically produces androgens and rarely estrogens.
Other rare tumors
Small-cell carcinoma is a rare type of carcinoma that occurs in females aged 2-46 years. It often is associated with hypercalcemia.
The most common form of sarcoma in the ovary is the mixed mesodermal sarcoma or carcinosarcoma.
Metastatic tumors of the ovary arise from direct extension and spread within the bloodstream or lymphatic system or within the peritoneal cavity. Sites of origin include the endometrium; cervix; and nongynecologic sites such as breast, colon, and stomach. The classic Krukenberg tumor refers to bilateral enlargement of the ovaries from metastases from a signet-ring carcinoma of the stomach.
Etiology
The precise cause of ovarian cancer is unknown, but several risk and contributing factors have been identified.
Hippisley-Cox and Coupland developed an algorithm to determine risk of ovarian cancer in women with and without symptoms. [15] In their cohort study, 10% of women with the highest predicted risk had 63% of all ovarian cancers diagnosed over the next 2 years.
Reproductive factors
Parity is an important risk factor. The risk of epithelial ovarian cancer is increased in women who have not had children and possibly those with early menarche or late menopause. Women who have been pregnant have a 50% decreased risk for developing ovarian cancer compared with nulliparous women. Multiple pregnancies offer an increasingly protective effect. Oral contraceptive use decreases the risk of ovarian cancer significantly.
These factors support the idea that risk for ovarian cancer is related to ovulation. Two theories regarding this relationship have been proposed. The incessant ovulation theory suggests that repeated ovarian epithelial trauma caused by follicular rupture and subsequent epithelial repair results in genetic alterations within the surface epithelium. The gonadotropin theory proposes that persistent stimulation of the ovaries by gonadotropins, coupled with local effects of endogenous hormones, increases surface epithelial proliferation and subsequent mitotic activity.
Thus, the probability of ovarian cancer may be related to the number of ovulatory cycles, and conditions that suppress the ovulatory cycle may play a protective role. Ovulation suppression has been shown to decrease cancer incidence. Although treatment with agents that induce ovulation in women with infertility has been suggested to increase the incidence of epithelial ovarian cancer, this is unproven.
Genetic factors
Family history plays an important role in the risk of developing ovarian cancer. The lifetime risk for developing ovarian cancer is 1.6% in the general population. This compares with a 4-5% risk when 1 first-degree family member is affected, rising to 7% when 2 relatives are affected. From 5-10% of cases of ovarian cancer occur in an individual with a family history of the disease. Only a small percentage of these patients have an inherited genetic abnormality, and the risk of this occurrence increases with the strength of the family history. Hereditary epithelial ovarian cancer occurs at a younger age (approximately 10 years younger) than nonhereditary epithelial ovarian cancer, but the prognosis may be somewhat better.
Integrated genomic analyses by the Cancer Genome Atlas Research Network have revealed high-grade serous ovarian cancer is characterized by TP53 mutations in almost all tumors. The findings also include the low prevalence but statistically recurrent somatic mutations in 9 further genes, including NF1, BRCA1, BRCA2, RB1, and CDK12, along with 113 significant focal DNA copy number aberrations and promoter methylation events involving 168 genes. Pathway analyses revealed defective homologous recombination in about half of all tumors, and that NOTCH and FOXM1 signaling are involved in serous ovarian cancer pathophysiology. [16]
Evidence from the Cancer Genome Atlas Network showed that serous ovarian tumors and breast basal-like tumors shared a number of molecular characteristics, such as the types and frequencies of genomic mutations, suggesting that ovarian and breast cancer may have a related etiology and potentially similar responsiveness to some of the same therapies. [17]
At least two syndromes of hereditary ovarian cancer are clearly identified, involving either (1) disorders of the genes associated with breast cancer, BRCA1 and BRCA2, or (2) more rarely, genes within the Lynch II syndrome complex. Breast/ovarian cancer syndrome is associated with early onset of breast or ovarian cancer. Inheritance follows an autosomal dominant transmission. It can be inherited from either parent.
Most cases are related to the BRCA1 gene mutation. BRCA1 is a tumor suppressor gene that inhibits cell growth when functioning properly; the inheritance of mutant alleles of BRCA1 leads to a considerable increase in risk for developing ovarian cancer.
Approximately 1 person in 4000 in the general population carries a mutation of BRCA1. Some populations have a much higher rate of BRCA1 and BRCA2 mutations, especially Ashkenazi Jews. In families with 2 first-degree relatives (mother, sister, or daughter) with premenopausal epithelial ovarian cancer, the likelihood of a female relative having an affected BRCA1 or BRCA2 gene is as high as 40%. The probability is much lower when the disease occurs in relatives postmenopausally.
Individuals with a BRCA1 gene mutation have a 50-85% lifetime risk of developing breast cancer and a 15-45% risk of developing epithelial ovarian cancer. Those with a BRCA2 gene mutation have a 50-85% lifetime risk of developing breast cancer and a 10-20% risk of developing epithelial ovarian cancer. Families with BRCA2 mutations are at risk for developing cancer of the prostate, larynx, pancreas, and male breast.
Germline mutations in the BRCA1 and BRCA2 genes are associated with increased risks of breast and ovarian cancers; however, in an investigation of a common genetic variation at the 9p22.2 locus, a decreased risk of ovarian cancer was noted in carriers of a BRCA1 or BRCA2 mutation. [18]
Families with Lynch II syndrome or hereditary nonpolyposis colorectal cancer are characterized by a high risk for developing colorectal, endometrial, stomach, small bowel, breast, pancreas, and ovarian cancers. This syndrome is caused by mutations in the mismatch repair genes. Mutations have been demonstrated in mismatch repair genes MSH2, MLH1, PMS1, and PMS2.
Women with a history of breast cancer have an increased risk of epithelial ovarian cancer.
In a study by Rafner et al, whole-genome sequencing identified a rare mutation in BRIP1, which behaves like a classical tumor suppressor gene in ovarian cancer. [19] This allele was also associated with breast cancer.
Hormone therapy
A nationwide prospective cohort study over 10 years that included all Danish women aged 50-79 years concluded that risk for ovarian cancer is increased with hormone therapy, regardless of duration of use, formulation, estrogen dose, regimen, progestin type, and administration route. [20] Nearly 1 million women without hormone-sensitive cancer or bilateral oophorectomy were followed. In an average of 8 years of follow-up, 3068 ovarian cancers were detected, of which 2681 were epithelial cancers.
Current users of hormones had incidence rate ratios for all ovarian cancers of 1.38 (95% confidence interval [CI], 11.26-1.51) compared with women who never took hormone therapy. Risk declined as years since last hormone use increased. Incidence rates in current and never users of hormones were 0.52 and 0.40 per 1000 years, respectively. This translates to approximately one extra ovarian cancer for approximately 8300 women taking hormone therapy each year.
Other factors
There is fair evidence that increased adult height and body mass index (BMI) are associated with a modestly increased risk of ovarian cancer. [21] A Danish study found that size in childhood may also affect risk, with increased risks of ovarian cancer overall in girls who were overweight or tall at 7 and 13 years, compared with average-sized girls at both ages. [22]
Endometriosis has been linked to an increased risk of ovarian cancer. [23] The association is stronger with nonserous histologic subtypes, specifically endometrioid and clear cell carcinomas. [21]
The use of talcum powder on the vulva and perineum may be associated with increased risk of epithelial ovarian cancer. [24] High lactose consumption has been associated with increased risk of ovarian cancer, but evidence linking lactose and specific dairy products with ovarian cancer remains contradictory. [25, 26]
According to the findings of a longitudinal study, women who experienced six or more symptoms of post-traumatic stress disorder (PTSD) at some point in life had a twofold greater risk of developing ovarian cancer compared with women who never had any PTSD symptoms (age-adjusted hazard ratio [HR]=2.10, 95% confidence interval [CI]=1.12, 3.95). Analyzing data from nearly 55,000 participants in the Nurses' Health Study II, Roberts et al reported that having higher levels of PTSD symptoms can be associated with increased risks of ovarian cancer even decades after a traumatic event. The study also showed that women who experienced 6-7 symptoms associated with PTSD were at a significantly higher risk of developing the high-grade serous histotype of ovarian cancer, the most aggressive form of ovarian cancer. [27]
Epidemiology
In the United States, the incidence of ovarian cancer is 10.6 per 100,000 women per year, based on 2015-2019 cases. [28] The incidence of ovarian cancer has decreased by about 1% per year since at least the mid-1970s among women younger than age 65, but only since the early 1990s in older women. [29] Ovarian cancer is more common in whites than in blacks (11.0 versus 9.1 cases per 100,000 women per year, respectively). [28] Epithelial ovarian cancer can occur in girls as young as 15 years, but the median age at diagnosis is 63 years, and most cases are diagnosed in women 55-64 years of age. [28] In the United States, the estimated lifetime risk is 1.22%. [5]
The American Cancer Society estimates that 19,88 new cases of ovarian cancer will be diagnosed in 2022 and 12,810 women will die from the disease. [29] Although ovarian cancer is the 18th most common cancer in women, it is the fifth most common cause of cancer death in women, accounting for 5% of cancer deaths—more than any other gynecologic cancer. [29, 28]
From 2010 to 2019, the death rate from ovarian cancer decreased by an average of 2.7% each year. Median age at death is 70 years. [28]
International statistics
Internationally, ovarian cancer is the eighth most common cancer in women and the 18th most common cancer overall, with more than 313,000 new cases and more than 207,000 deaths in 2020. [30, 31] The worldwide age-standardized rate is 6.6 cases per 100,000; the highest rate is 17.4, in Brunei. [30]
Prognosis
Although the 5-year survival rate for ovarian cancer has improved significantly in the past 30 years, the prognosis for ovarian cancer remains poor overall, with a modeled 53.9% 5-year relative survival rate in 2019. [28] The prognosis of ovarian cancer is closely related to the stage at diagnosis, [32, 33] as determined according to the staging system developed by the International Federation of Gynecology and Obstetrics (FIGO). (See Workup/Staging.) Approximately 20%, 5%, 58%, and 17% of women present with stage I, II, III, and IV, respectively.
The 5-year survival rates for epithelial ovarian carcinoma by FIGO stage are as follows [34] :
-
Stage I - 80-90%
-
Stage II - 40-60%
-
Stage III - 10-15%
-
Stage IV - < 5%
Bakhru et al found poorer survival among patients with ovarian cancer and diabetes. Although the underlying reason for this association is unknown, further studies are needed. [35]
Among women with high-grade serous ovarian cancer, BRCA2 mutation but not BRCA1 deficiency was associated with improved survival, improved chemotherapy response, and genome instability compared with BRCA wild-type. [36]
A study by Bolton et al found improved 5-year overall survival among carriers of BRCA1 or BRCA2, with BRCA1 having the best prognosis. [37]
A retrospective study found an association between statin use and reduced ovarian cancer–specific mortality. In this observational study, which included 10,062 women aged 18 and older diagnosed with incident ovarian cancer between 1995 and 2015, mortality was reduced by 40% in women who were taking any type of statin, and by 43% in those taking lipophilic statins, in comparison with mortality in women who had never used statins. The beneficial effect of statin use was observed across all stages, treatments, and subtypes of epithelial ovarian cancer. [38]
Prognosis of Tumors of Low Malignant Potential
Overall survival rate at 5 years according to FIGO is shown below. Others have reported better survival rates with 5-year, 10-year, 15-year, and 20-year survival for patients with serous LMP as 97%, 95%, 92%, and 89%, respectively.
Five-year-survival rate for LMP tumors by FIGO stage (survival percentages rounded to nearest whole number) are as follows:
-
Stage IA - 93%
-
Stage IB - 90%
-
Stage IC - 91%
-
Stage IIA - 88%
-
Stage IIB - 86%
-
Stage IIC - 100%
-
Stage IIIA - 29%
-
Stage IIIB - 75%
-
Stage IIIC - 62%
-
Stage IV - 30%
-
Overall survival rate - 86%
-
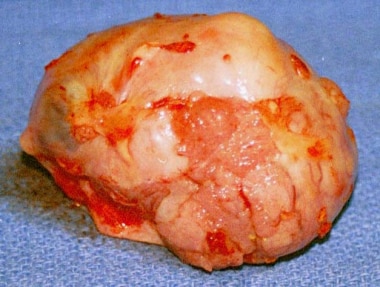
An enlarged ovary with a papillary serous carcinoma on the surface.
-
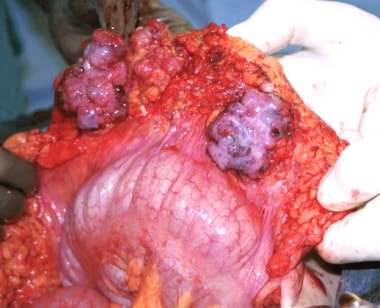
Metastases from epithelial ovarian carcinoma involving the omentum.
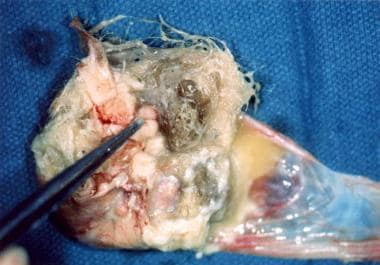
-
Inside of a large, smooth-surfaced tumor replacing the ovary. Final histologic studies indicated the tumor was a mucinous carcinoma of low malignant potential. Note the multiple cysts with thick septa between. This tumor was extensively sectioned and was a mucinous carcinoma of low malignant potential.
-
Granulosa cell tumor excised from a woman aged 44 years. Note the yellowish tumor that has eroded through, onto the surface of the ovary.
-
This photo shows a granulosa cell tumor, with the cut surface showing classic features of a hemorrhagic cyst and yellowish solid component.
-
Mature cystic teratoma of the ovary exhibiting multiple tissue types.
-
Mature cystic teratoma of the ovary with hair, sebaceous material, and thyroid tissue.
-
Dr. Oliver Zivanovic, MD, PhD, discusses the role of hyperthermic intraperitoneal chemotherapy in ovarian cancer. Courtesy of Memorial Sloan-Kettering Cancer Center.
-
Dr. Oliver Zivanovic, MD, PhD, demonstrates hyperthermic intraperitoneal chemotherapy for ovarian cancer. Courtesy of Memorial Sloan-Kettering Cancer Center.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Choosing Appropriate Surgery
- Surgical Staging
- Cytoreductive Surgery
- Interval Debulking
- Laparoscopic Surgery
- Secondary Surgery
- Chemotherapy Regimens
- Radiation Therapy
- Estrogen Replacement Therapy
- Second-Look Laparotomy
- Assessment of Response to Therapy and Relapse
- Management of Recurrent Disease
- Palliative Care
- Experimental Medications
- Management of Ovarian Cysts
- Management of Tumors of Low Malignant Potential (Borderline Tumors)
- Treatment of Malignant Germ Cell Tumors
- Treatment of Sex-Cord Stromal Tumors
- Treatment of Rare Tumors
- Long-term Monitoring
- Consultations
- Deterrence and Prevention
- Show All
- Guidelines
- Medication
- Questions & Answers
- Media Gallery
- References