Overview
The reconstruction of oromandibular defects (mandibular reconstruction) following surgical extirpation of oral cavity carcinoma presents a significant surgical challenge. Mandibular deformities and defects may result from trauma, infections, prior radiation exposure, neoplasms, and congenital defects; most mandibular deformities result from ablative surgery for neoplasms. Approximately 24,000 patients have new cancers of the oral cavity each year. [1] The resultant changes in normal oromandibular anatomy are often functionally disabling and socially isolating. Once complete resection of the primary and regional metastases has been undertaken, subsequent functional recovery and aesthetics are extremely important when reconstruction is considered.
Within the last few decades, many advances in head and neck surgical techniques, plate technology, and microvascular surgery have improved the functional restoration in this patient population. However, the expansion in knowledge and the development of techniques have left surgeons with a large number of reconstructive options, few of which are supported by findings from prospective randomized trials. [2] The purpose of this article is to assist surgeons with the selection of reconstructive techniques and patients to achieve the best possible outcome.
The rationale for reconstruction
Rehabilitation and reconstruction of the oral cavity following the resection of pathologic processes remains a complex challenge. The mandible assists in verbalization, oral competence, mastication, deglutination, and airway support, yet it is also a major aesthetic highlight of the face. With oromandibular pathology, a loss of not only bony support but also neighboring soft tissues, muscles, and nerves is frequently present. Thus, large complex defects can be created during resection. Functional and aesthetic outcomes become less favorable as the extent of resection increases. [3] Without reconstruction, patients may have functional and cosmetic impairments.
The reconstructive surgeon attempts to redefine the preoperative functions and facial aesthetic units to allow the patient to return to a normal family and social life. The approach includes the placement of dentures or osseointegrated implants to help shape the face and to assist with mastication and verbalization. [3] Other important factors for rehabilitation are intact motor and sensory functions of the tongue. Loss of these functions, independent of any mandibular continuity defect, can vastly hinder oral function and competence, and many patients receive little benefit in deglutination with aggressive mandibular reconstruction. [4] Therefore, attention must be given to total reconstruction and postoperative rehabilitation if the patient is to derive maximum benefit.
See the image below.
Preoperative Evaluations and Considerations
Preoperative evaluation may address the patient's use of tobacco and alcohol, nutritional status, medical history and comorbidities, preoperative dental status, age, history of radiation therapy, and, possibly, 3-dimensional stereolithographic (SLA) modeling for preoperative planning.
Tobacco and alcohol
Many patients with oral cancers or acquired mandibular defects use tobacco products, either alone or with alcohol. Patients who smoke tobacco are at an increased risk for poor wound healing, and evidence to support shortened flap survival in animals that are exposed to cigarette smoke exists. [5] This mechanism is likely multifactorial, involving such factors as the sympathomimetic effects of nicotine as well as the interference of the oxygen-hemoglobin interaction caused by carbon monoxide.
Cigarette smoking is a major risk factor for the initiation and progression of atherosclerosis, and it is associated with an increased incidence of mortality from coronary artery disease and an increased incidence of myocardial infarction.
Other effects on microcirculatory flow and coagulability have been demonstrated. These include increased postcapillary pressures and reduced capillary flow, decreased platelet survival, enhanced response to adenosine diphosphate (ADP)–induced platelet aggregation, and a decrease in platelet aggregate ratios. Major endothelial damage has also been noted with associated changes, such as endothelial cell elongation, uplifting of the basement membrane, endothelial loss, pitting and crater formation, and WBC invasion. [6]
In an attempt to address guidelines for clinical decision making in smokers who require free flaps, Reus et al conducted a retrospective study of 2 groups with similar demographic and comorbid factors. [7] The first group included smokers who had abstained in the perioperative period (104 flaps in 93 patients), and the second group included nonsmokers (58 flaps in 51 patients). They found that flap survival and anastomotic patency were not statistically different between the groups, but the smoking group did have more recipient site wound healing complications than the nonsmoking group.
Patients who consume large quantities of alcohol may have perioperative complications, such as withdrawal and delirium tremens. Associated poor nutritional status may compound these difficulties, contributing to decreased wound healing. Passeri and coworkers found that patients who used alcohol, intravenous drugs, or nonintravenous drugs had an increased complication rate when undergoing mandible fracture treatment. [8] If alcohol and tobacco use cannot both be halted, patients and physicians should focus on curtailing tobacco use several weeks prior to surgery to maximally improve pulmonary function before anesthesia. [1] Clinicians should remember that some patients require postoperative preventative therapy for alcohol, and possibly nicotine, withdrawal.
Nutritional status
As previously discussed, many patients with underlying substance abuse problems also have poor nutrition, including protein-energy malnutrition and vitamin and mineral deficiencies. These problems may be compounded by a recent history of anorexia and weight loss caused by the release of tumor factors and cytokines, such as interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor (TNF) alpha, and interferon (IFN) alpha. The fact that disease processes affecting the oral cavity and the jaw may easily lead to trismus and alterations in masticatory and deglutition abilities is intuitive. These mechanical abnormalities alone may contribute to significant weight loss and poor nutrition.
Malnutrition has been recognized as a poor prognostic indicator for cancer treatment–related mortality and morbidity, and it is reported to affect 30-50% of all patients with head and neck cancer. [9] Hammerlid and coworkers (1998) evaluated the nutritional status of 58 consecutive patients with head and neck cancer, 16 of whom had oral cancer; 10 of these 16 patients had malnutrition. [10] Research has shown that postoperative complications increase in relation to weight loss of more than 10% during the 6 months prior to hospitalization and surgery. A weight loss of this severity is also related to a decreased 3-year survival rate in men with head and neck cancer. [9]
If the patient has a questionable nutritional status, the clinician can evaluate the patient by using several readily available tests. Nitrogen balance can be evaluated with a 24-hour urine collection and a detailed consumption diary. Results of laboratory tests, such as those of prealbumin, albumin, transferrin, and total protein, are other indicators of overall nutritional status. Negative nitrogen balance and protein-energy malnutrition impair the overall immunological and proliferative aspects of wound healing. The result is a wound that has both reduced tensile strength and a higher rate of secondary infection. [11] If anemia is suspected, a complete blood count and iron studies can be used to determine the type of anemia. A clinical nutritionist can assist the patient and the surgeon before and after the operation with supplementation and feeding regimens that promote global nutrition and healing.
Medical history and comorbidities
Many patients with acquired mandibular and oral cavity defects have treated and untreated medical disease processes. Severe cardiovascular and pulmonary diseases may lead to poor tissue oxygenation, and, if they are severe enough, they can be a contraindication to general anesthesia. Pulmonary function tests, cardiac stress tests, and radionuclide cardiac scans may be necessary if cardiopulmonary disease is present or suspected. Peripheral vascular disease may prevent the use of osteocutaneous free flaps of the lower extremities because of associated morbidities at the donor site and the possibility of flap failure. Carotid artery disease and carotid plaques may also cause complications related to microvascular anastomoses.
An example of a common systemic disease that may cause difficulties before and after surgery is diabetes mellitus. About 16 million persons in the United States have diabetes mellitus. Diabetes contributes to poor wound healing in many ways, and the disease can cause local infection rates approaching 10%. [11] The abnormal carbohydrate metabolism associated with both type 1 diabetes and type 2 diabetes slows wound repair because glucose may be unavailable for cellular metabolism.
The growth factor properties of insulin are less available in insulin-dependent and insulin-resistant diabetes. This lack of availability further decreases collagen deposition within a surgical wound. The collagen deposition is abnormal because cross-linking of collagen fibrils is decreased and the ratio of type III collagen to type I collagen is increased. In patients without diabetes, this percentage decreases from 57% initially to 28% by the end of the second week of healing, but, in patients with diabetes, this decrease occurs much more slowly and wound tensile strength is less. Cellular infiltration can also be impaired in healing wounds in patients with diabetes because of small vessel disease, which affects the permeability of the vascular membranes and channels. [11]
Alterations of coagulability in patients with diabetes include increased platelet adhesiveness, increased thromboxane production by platelets, decreased endothelial prostacyclin levels, and reduced plasminogen activator production. [12] Unfortunately, the pathophysiologic impact of these abnormalities has not been related to free flap and tissue transfer survival because of the small number of patients with diabetes included in published clinical series. [6] Thus, while one may presume that diabetic and atherosclerotic disease is not an absolute contraindication to microsurgery and free tissue transfer, these disease processes may influence the choice of donor site and recipient vessel selection.
Some systemic medications may slow healing and decrease wound strength. Two major classes of agents that contribute to slowed healing are anticoagulants and corticosteroids. Many patients with chronic obstructive pulmonary disease and rheumatologic disorders use systemic corticosteroids; this practice has been proven detrimental to wound healing. Anticoagulants interfere with initial clot formation during wound healing, predisposing the patient to hematoma formation and deterring neomatrix production. [11] Other drugs, such as chemotherapeutic agents (eg, dactinomycin, doxorubicin, bleomycin, nitrosoureas), colchicines, high-dose salicylates, and nonsteroidals, also adversely affect wound healing. [11]
Preoperative dental status
Preoperative dental status is an important consideration in formulating a plan for the reconstruction of oromandibular defects. Edentulous mandibles tend to have much less density and vertical height compared with dentate mandibles. As the edentulous mandible atrophies, the mental foramen tends to migrate superior to the surface of the alveolus, and the inferior alveolar canal and the neurovascular bundle also demonstrate a markedly variable course through the bone. Therefore, careful dissection based on preoperative examination by Panorex is warranted. [13]
The quantity of blood supply to the mandible is well known to affect postsurgical outcome; while this is less of a consideration with vascularized bone flaps (VBFs), it is a major consideration when one considers the use of osseointegrated implants. Bradley studied the inferior alveolar artery in elderly cadaveric specimens (aged 60-80 y) using angiography. [14] He demonstrated that the inferior alveolar artery was abnormal in 56% of the specimens and absent in 37% of the specimens. He also demonstrated that, in these individuals, the loss of the inferior alveolar artery partially correlated with the loss of teeth. In these patients, the subperiosteal plexus represents the major supply to the mandible.
Thus, surgeons should remember that overzealous circumferential subperiosteal dissection in elderly edentulous patients may result in the disruption of the major blood supply to the mandible or remaining mandibular fragments that can contribute to nonunion and infection. This becomes a major consideration when one chooses the method of repair and reconstruction because vascularized bone grafts tend to heal better in this situation.
The routine practice of full-mouth extraction is no longer necessary for all patients prior to radiation therapy. Patient education; improved dental hygiene; use of fluoride trays and rinses; and use of intraradiation protective devices, such as splints, can preserve many teeth and allow the restoration of other teeth. Thus, many patients with cancer of the oral cavity may retain some of their dentition, which assists with verbalization, cosmesis, and mastication. Other patients may desire to undergo restoration with osseointegrated implants or restorative dentures. Both can assist with the rehabilitation of patients with cancer and their reintegration into family and community life.
Age
Many patients with oral defects are elderly, have poor health, and would benefit little from an extensive reconstructive procedure. In patients who are debilitated, simple reconstruction of the soft tissue defects may be adequate to restore bulk. However, when lateral and posterior mandibular defects are not treated with plates, mandibular drift and malocclusion are the functional sequelae. Surprisingly, this functional defect is well tolerated in many patients and interferes little with normal mouth opening. [2]
Shestak and Jones reported findings in one of the largest head and neck tissue transfer series in elderly patients. [15] Ninety-four free tissue transfers were performed in 92 patients aged 50-79 years; 75% of these flaps were used for head and neck reconstructions. They reported a 99% flap viability rate and a 45% medical or surgical complication rate. They identified a subset of patients at high risk for morbidity and mortality, that is, those with an American Society of Anesthesiologists (ASA) class 3 or 4 condition and who underwent operations lasting longer than 10 hours. However, the researchers emphasized that physiologic and biologic ages were more important than chronologic age.
Chick et al, who examined 31 patients older than 65 years who received free flaps, supported these findings. Medical morbidities and wound complications occurred twice as often in these patients as in 90 patients younger than 65 years. However, after these data were corrected for preexisting medical conditions, no significant differences were present. Chick and associates also found thick-walled calcific vessels with loose friable intimas. This finding led them to consider the use of vascular systems in the upper extremity and the trunk, when possible, to avoid the more atherosclerotic vessels of the lower extremities and the groin. [16]
Radiation therapy
Head and neck surgeons are often confronted with reconstructive procedures following surgical salvage of postradiation failures, after persistent malignancies of the oromandibular region, or with the realization that irradiation is required following free tissue transfer. Irradiation has been shown to affect large vessels by accelerating atherosclerosis and by causing obliterative endarteritis and thrombosis; however, experiments show confounding results in the comparison of microvascular anastomoses. While many experimental and clinical reports support the findings of similar anastomotic patency and free flap survival rates between irradiated and nonirradiated sites, others do not. [6]
Deutsch et al evaluated 140 patients who underwent mandibular reconstruction with a fibular free flap. [17] The patients were divided into 4 groups: preoperative x-ray therapy (XRT) with immediate reconstruction, preoperative XRT with delayed reconstruction, postoperative XRT, and no XRT. Complications included orocutaneous fistula, osteoradionecrosis, partial or complete flap loss, exposure of bone or hardware, and severe cervical contractures; 42% of patients experienced at least 1 of these complications. No difference occurred in the incidence of complications in patients receiving preoperative or postoperative XRT. However, those receiving no XRT had only a 28% complication rate. [17]
De Wilde et al (1983) used a scanning electron microscope to evaluate microanastomoses created in irradiated rat carotid arteries. [18] They found all of the anastomoses to be patent 10 weeks following irradiation but noted a higher incidence of platelet and fibrin deposits, nonocclusive thrombi, and delayed endothelial regeneration compared with those of control subjects. The same experiment was repeated after 1 year, and no significant changes from those observed in the short term were noted. [19]
Guelinckx and coworkers, who histologically evaluated biopsy specimens of 40 clinical cases with irradiated microvascular anastomoses, supported De Wilde et al's findings. [20] They additionally noted a significantly greater incidence of arterial wall thickness and intimal dehiscence compared with findings in nonirradiated anastomoses. They formulated specific surgical practices to minimize the risk of technical failures. According to Guelinckx, these included the following:
-
End-to-end anastomoses created by using a number 70 needle without vessel rotation
-
Passage of the needle from the inside to the outside on the irradiated side to minimize intramural dissection
-
Minimization of retrograde thrombosis near recipient vessels by avoiding electrocoagulation in their vicinity
-
Limitation of cross-clamping time to avoid stasis and microthrombi formation
The true risk of radiation therapy to free flap beds is unknown; however, some studies suggest that the use of careful technique may eliminate further risk to the free flap. Choi showed no differences between radiated and nonradiated patients who underwent fibular free flap reconstruction for mandibular defects. [21]
3-Dimensional stereolithography
The use of 3-dimensional stereolithographic (SLA) models has allowed for highly accurate presurgical planning. These medical models, derived from high-resolution CT images, are available through major device and plating manufacturers.
Since their advent over 20 years ago, popularity and ease of use has increased. [22] Frequently cited benefits include improved preoperative planning, decreased operative time, improved accuracy of skeletal osteotomies, increased efficiency, and better functional outcomes. [23] Xia et al found a reduction in over 40% of operative time with the use of medical models. In their analysis, they found that overall costs associated with medical modeling were below standard techniques when accounting for operative time. [24] Decreases in operative time are attributed to increased preparedness and efficiency, precise placement of osteotomies, [25] and prebending of plates and guides prior to entering the operating room. [26]
Some authors deny there is any time or cost benefit from use of SLA models. [27]
Preoperative details
For patients undergoing mandibular reconstruction, high-resolution CT imaging is required at a thickness of 1.2 mm or less. Options for medical modeling are available through most companies offering plates and screws for mandibular reconstruction. After receipt of the images, consultation with a clinical engineer allows the goals of the surgery to be communicated, as well as operative strategy, extent of defect, and desired reconstruction. An operative plan is finalized by the surgeon and engineer.
After approximately one week from consultation, the 3-dimensional models and cutting guides are delivered. Plates are conformed to the model using AO bending techniques prior to the operating room, or they may come preformed to the medical model using a technique called patient specific plating.
Intraoperative details
One of the benefits of SLA models is the ability of models to be sterilized and used as a visual reference during the case. The cutting guides are used intraoperatively for precise cutting and shaping of the mandible and osseocutaneous free flaps.
For cases of fibula free flap reconstructions, the following steps are used:
Exposure of the mandible and placement of the mandible cutting guides: Cutting guides are secured using monocortical screws and a sagittal saw is used to complete the skeletal osteotomies.
A skin paddle is outlined and harvested with the fibula free flap (if desired).
The harvested fibula free flap is cut from the native fibula using cutting guides. The harvested fibula is taken to the back table and the fibula-cutting guide is applied to the fibula on the lateral surface. This is secured with monocortical screws. A saw is then used with the cutting guides to perform osteotomies while taking care to protect the pedicle. The guides ensure proper angulation for the best fit of the neomandible to both the defect and the patient specific plate.
See the images below.
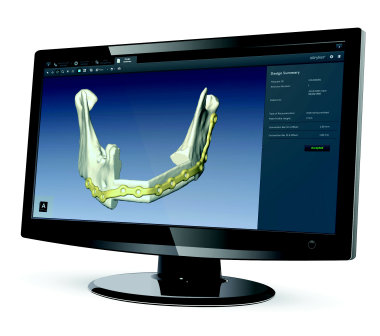
 Three-dimensional stereolithography design of a reconstruction plate, specific for a defect and to add height to the mandibular reconstruction. Courtesy of Stryker CMF (Craniomaxillofacial) (http://www.stryker.com/en-us/products/Craniomaxillofacial/index.htm).
Three-dimensional stereolithography design of a reconstruction plate, specific for a defect and to add height to the mandibular reconstruction. Courtesy of Stryker CMF (Craniomaxillofacial) (http://www.stryker.com/en-us/products/Craniomaxillofacial/index.htm).
Hardware and Techniques
Various techniques and hardware systems have been used to secure bone grafts to existing mandibular segments for reconstruction. These have included Kirschner wires, interosseous wires, miniplates, lag screws, dynamic compression plates, and 3-dimensional bendable reconstruction plates. [28, 29, 30] The last 4 of these permit the best fixation and stabilization of the bone grafts and, thus, better healing with fewer complications. Vascularized bone grafts heal in much the same manner as mandible fractures. If rigid immobilization can be established, the bone flap has a greater potential for union. Rigid internal fixation using plates and screws accomplishes this goal of stabilization.
Options in plate selection include mandible reconstruction plates, miniplates, [31] and locking low-profile plates, all of which are available in titanium. Titanium plates offer the advantages of being much more biocompatible and mechanically similar to bone than other metals; thus, they do not have to be removed after the graft has healed.
Most reconstructive surgeons use 2- to 2.4-mm locking reconstruction or mandibular plating systems for rigid internal reconstruction fixation. These titanium reconstruction plates can be contoured easily prior to mandibular resection; this contouring allows for the maintenance of the occlusal plane and serves as a template for the bone graft. Gurtner and Evans used 2.0- or 2.4-mm reconstruction plates that are shaped and secured to the native mandible to create a template. [2] At least 3 bicortical screws are placed in the portions of the native mandibles that are not resected. These plates can be resterilized and then used as a template for the graft osteotomies at the harvest site, if desired.
Although many different techniques are used for joining the neomandible and the native mandible, rigid fixation with reconstruction plates allows rapid resumption of oral function and eliminates the need for maxillomandibular fixation (MMF). Still, some fundamental principles should be remembered to maximize reduction and stabilization. Haug evaluated titanium reconstruction plate use in mandible fractures and demonstrated that 3 bicortical screws in each reconstruction plate segment provided the maximum resistance to deformation.
These findings were supported by clinical information that Freitag and coworkers obtained. [32] They reviewed 52 patients treated with AO reconstruction plates. In 28 AO plates secured with 1-3 screws per segment, the following occurred: 5 infections, 2 lost plates, 2 loose screws, and 1 lost screw. In 24 AO plates secured with 4 or more screws per segment, no infections occurred, and no screws or plates were loosened or lost. Thus, if the native mandible offers enough length, this practice assists in both stabilization and load bearing potential.
Hidalgo (1989) recommends the use of miniplates for the fixation and contouring of vascularized bone grafts. [33] He used the Wurzburg titanium miniplate in a series of 27 patients who underwent mandibular reconstructions with radius, scapula, and fibular osteocutaneous free flaps. He reported no compromised flaps but did experience a high rate of complications, including nonunion in 2 of 107 osteotomy sites. Plate removal was necessary in 4 patients, 1 of whom had plate exposure in irradiated skin. A plate fracture occurred in 1 patient, leading to a nonunion, and an orocutaneous fistula occurred in 3 other patients. Hidalgo and others support the use of titanium miniplates because they are easy to use and allow exact fixation of each osteotomy site so that the graft can be more easily contoured. Also, the plates are easily molded, and few instruments and tools are needed for their application, especially when they are used with fluted screws.
A retrospective study by Knitschke et al indicated that in maxillary or mandibular reconstruction with a fibular free flap, incomplete bony fusion occurs at a significantly higher rate in persons in whom patient-specific implants (PSIs) are used than in those receiving conventional plates. While the PSIs in the study were inserted as continuous plates, the positioning of the conventional plates was segmental. Flaps were harvested for the PSI patients using cutting guides, with the guides also utilized to mark resection planes and drill holes at the recipient site. Flap harvesting and shaping in the conventional plate group were performed via freehand osteotomy. According to the investigators, the greater rate of incomplete osseous fusion in the PSI patients resulted from patient-specific osteosynthesis being too rigid. [34]
Locking reconstruction plates sized 2-2.4 mm have a low incidence of complications and can offer absolute stability in the functional restoration of mandibular defects. [35] Conventional bone and plate screw sets must be perfectly adjacent to the underlying bone to prevent movement of the underlying segments, thus affecting healing and occlusion. At times, contouring the plate to multiple osteotomy segments of the neomandible is difficult. Locking reconstruction plates and screws function as internal fixators; stability is attained by locking the screw to the plate. Thus, the plate may ride slightly off of the neomandible, in a low-profile position, while still applying rigid support. This slight bony separation also prevents disruption of the cortical bone blood supply. Locking reconstruction screws are also less likely to extrude, although this currently remains a potential complication in all plate and screw systems.
Newer locking bone plates allow for the use of both locking and nonlocking screws on a locking plate system (Locking Reconstruction Plate, Synthes Maxillofacial, Paoli, PA). Studies have shown their added benefit in the treatment of mandible fractures. [36, 37] Many dimensions of locking bone screws and plates are currently available from multiple companies that offer such devices. The need for expansion screws used in the older THORP system is eliminated because of a double thread that lies just below the screw head that engages the threads in the hole of the plate.
Immediate Reconstruction Versus Delayed Reconstruction
A long-standing controversy once existed between immediate and delayed reconstruction and was fueled by advances in surgical techniques, especially the increased use of VBFs. Proponents of delayed reconstruction stated that immediate reconstruction covers the primary site, decreasing the ability to detect recurrence. They also disfavored the extended length of surgery required for primary reconstruction, as well as the possibility of seeding cancer cells in newly dissected tissue planes and the presumed increased risk of infection from salivary contamination. [38] Proponents of delayed reconstruction also argued that one must ensure that the oncologic margins, especially the bony margins, are cleared by means of permanent sectioning before safe restoration can be achieved. Frozen sections are fairly reliable for soft tissues, but they are less suitable for mandibular margins.
Advances in frozen section analysis, especially of bony margins, the 2–surgical-team approach, and increased confidence in techniques of mandible reconstruction have all but vanquished much of the controversy that surrounds immediate reconstruction. Schusterman et al (1993) reviewed 182 patients who had undergone mandibulectomy at the M. D. Anderson Cancer Center, University of Texas, Houston. [39] Of 182 patients, 45% had involvement of the resected mandible on histologic examination; only 4 patients (2%) had positive margins.
Schusterman et al also performed 56 immediate mandibular reconstructions with free osteocutaneous flaps; in this series, the authors encountered no positive bone margins. They concluded that free-flap mandible reconstructions for tumor defects could be safely accomplished in the immediate setting. [39] Banis observed that the low rate of positive margins is unlikely a coincidence because extirpative surgeons have more confidence to extend resected margins when they are aware that reconstruction can be achieved with little regard to the size of the defect.
If a secondary reconstruction is to be performed, the primary surgery must include an attempt to stabilize soft tissue and bony remnants in a manner that decreases their displacement with use. Unfortunately, considerable scarring, soft tissue contracture, and fibrosis can precede delayed reconstruction and compromise functional and cosmetic restoration. [4]
In some areas of the body, functional and physiologic mechanisms are not hampered after the excision of large tumors if open wounds remain or if simple skin grafts are used. However, because of the physiologic, biologic, and functional characteristics of the oromandibular cavity, the treatment team is not afforded the luxury of delayed reconstruction. Immediate reconstruction is the most effective way to reconstruct these tumors technically. Additionally, compared with delayed reconstruction, immediate reconstruction results in better long-term functional outcomes. [39]
Over the past decade, primary reconstruction with fasciocutaneous flaps and osteocutaneous free flaps has greatly improved the quality of life for patients undergoing oromandibular resection. Markowitz and colleagues assessed functional and aesthetic results in patients undergoing composite reconstructions of the head and the neck with VBFs; they were unable to demonstrate any functional benefit in the delayed reconstruction group. [40] Other researchers have found that, compared with primary reconstruction, secondary reconstruction was associated with higher overall complication rates, longer hospital stays, and greater costs. [41]
When reconstructions are performed primarily, postoperative radiation therapy can be administered in a timely fashion. Secondary reconstruction can delay postoperative irradiation, which can increase both morbidity and recurrence. Thus, the advantages of primary reconstruction include a reduction in the number of surgical procedures and hospital stays, a shorter time during which the patient has deformity and morbidity from lack of function, the protection and preservation of vital structures, a reduced cost of treatment, and the rapid oral rehabilitation with a timely return to a normal social lifestyle. [4]
Reconstruction Materials
Mandibular reconstruction may involve the use of alloplastic materials, soft tissue coverage of mandibular reconstruction plates, nonvascularized bone grafts (NVBGs), free flaps and VBFs, fibular osteocutaneous free flaps (FOFFs), scapular osteocutaneous free flaps (SOFFs), iliac crest osteocutaneous free flaps (ICOFFs), radial forearm osteocutaneous free flaps (RFOFFs) and the latissimus-serratus-rib free flap (LSRFF). [42] Various characteristics of the free flaps are summarized in the Table.
Alloplastic materials
The potential for aesthetic reconstruction without donor site morbidity has led many on the search for suitable alloplastic materials. [4] In the past, implants were placed secondarily, months or years after the initial resection, to avoid possible problems from salivary contamination, subsequent infection, or tumor recurrence. Although these implants offered some restoration of continuity and bulk, overall success has been disappointing, especially when these devices are applied primarily in previously irradiated areas of the head and the neck. For these reasons, these devices are not currently favored and should be avoided, if possible.
Soft tissue coverage of mandibular reconstruction plates
An option for mandibular reconstruction includes the use of mandible reconstruction plates covered with soft tissue. However, the use of these plates may be restricted to locations where surgeons with microvascular expertise are unavailable or to patients who are at an increased risk with prolonged anesthesia during surgery. In rare cases, reconstruction plates and soft tissue may be used in patients with an extremely poor prognosis.
The pectoralis major myocutaneous flap is widely used to cover titanium plates to prevent extrusion. The deltopectoral myocutaneous flap and the trapezius myocutaneous flap are also used for the same purpose.
Cordeiro and Hidalgo conducted a retrospective review of 14 patients whose composite defects were reconstructed with a reconstruction plate and either a pectoralis major myocutaneous flap (9 patients) or soft tissue free flaps (5 patients). [43] Reconstruction plates extruded in 4 patients in the pectoralis group, compared with none in the free-flap group. The percentage of reoperations for the pectoralis group was significantly higher (88%) than that of the free-flap group (20%), and hospital stays were significantly longer in the pectoralis group as well. Wei (2003) evaluated 80 patients after mandibular reconstruction with a reconstruction plate and a soft tissue free-flap cover. [44] The average follow-up period was 22 months, and during that time 45 patients (69.2%) developed one or more complications.
The most common complication was plate exposure (46.2%), and 20 patients in the study went on to require a secondary, salvage fibular osteocutaneous free flap. Doty and colleagues measured forces that would lead to plate fatigue in an experimental mandible model with 2.4-mm and 3-mm reconstruction plates and showed that contralateral molar loading exerts a torsional force that is more likely to cause plate fracture. [45]
Although this was a small nonrandomized study, it did reveal some trends. Two of the 4 extrusions had been irradiated previously, and 3 of these had anterior defects. Other researchers have reported similar data, especially in regard to anterior defects treated with reconstruction plates and soft tissue coverage. [39]
Osteocutaneous free flaps offer a more vascularized alternative to regional flaps. The pectoralis flap frequently does not extend enough to provide secure coverage of a plate in anterior defects. Some defects may create undue tension on the suture line because of their distance from the pedicle. This tension can also stretch and compromise the vascular pedicle, causing decreased blood flow, especially in the distal suture line where blood flow is already low. Also, regional flaps can be bulky and difficult to wrap around reconstruction plates, adding to the traction of the shoulder-based pedicle. Lastly, regional flaps and plates offer poor cosmetic and functional alternatives to osteocutaneous free flaps. [46] They represent an alternative in patients who have lateral mandibular continuity defects with a poor prognosis, in whom dental rehabilitation is not desired or planned.
Internal distraction osteogenesis
Distraction devices applied to the mandible may be of some use in patients with lateral defects that will not undergo radiation therapy; however, most reports to date are experimental, have small patient numbers, or have low-to-moderate success rates. At this time, this therapy is not recommended, but may undergo advances in the near future with the use of bone and vascular modulators.
Nonvascularized bone grafts
Although both VBFs and NVBGs have been used to reconstruct mandibular defects, the indications for each remain somewhat ill defined. However, few proponents still support the use of NVBGs, especially in cases involving irradiated tissues. [47] Foster and associates evaluated 75 consecutive mandibular reconstructions, 26 of which involved NVBGs and 49 of which involved VBFs. Successful bony union occurred in 69% of NVBGs and 96% of VBFs (P< 0.001). NVBGs required 2.3 operations, as compared with 1.1 operations with VBFs (P< 0.001). Twenty-two patients had a total of 104 osseointegrated implants (8 patients receiving NVBGs with 33 implants and 14 patients receiving VBFs with 71 implants).
Overall implant success occurred in 82% of NVBGs and 99% of VBFs (P< 0.001). Also, a 100% success rate of osseointegrated implants was found in patients with VBFs who received irradiation as part of their therapy. Thus, although patients receiving VBFs were older, had larger defects, and were treated primarily for malignant disease with an associated higher incidence of radiation therapy, they had higher rates of bony union and implant success than those of patients receiving NVBGs. [47]
Free flaps and vascularized bone flaps
The successful transfer of free tissue with microvascular anastomoses depends on anastomotic patency, a functional end stage, aesthetic restoration, and adequate amounts of healthy soft and hard tissue for reconstruction of the defect. Khouri found that operative experience and sound judgment are the most critical factors related to improved success of free-flap transfers. Prevention of complications also comes from proper planning and awareness and meticulous technique with attention to details. [6]
Oral cavity tumors involving the mandible and primary tumors of the mandible require composite resections of bone; soft tissue; and often, facial skin. These defects can have profound aesthetic sequelae and are functionally hindering if not reconstructed. Mandibular reconstruction is difficult because of the contaminated wound bed, the frequent presence of an irradiated field, and the large compression and tensile forces created by the muscles of mastication. [2]
VBFs have revolutionized mandibular reconstruction. Even when these bone flaps are transferred from distant sites into areas of irradiation, compromised blood flow, and salivary contamination, the union of bone segments and the support of functional loads are the usual result. Osseointegrated implants also can be successfully placed within vascularized bone free flaps, contributing to rehabilitation and a stable dental arch. [28]
Ideal VBFs provide adequate shape, width, and length of vascular bone, while they produce minimal morbidity at the donor site. An ideal soft tissue source for oromandibular reconstruction is thin, moist, vascularized, and sensate; has a close cosmetic match to the facial skin; and has 3-dimensional versatility in reference to the bone graft. Unfortunately, the ideal vascularized bone graft for all oromandibular reconstructions does not exist; therefore, each patient and defect must be evaluated separately to determine the best surgical approach.
Fibular osteocutaneous free flaps
The FOFF has many proven advantages in mandibular reconstruction. It can provide bone as long as 25 cm, has a consistent shape throughout that length, and has a blood supply that parallels the course of the bone. [48, 49] The fibula can be transferred as either a free osseous flap or a free cutaneous flap. The flexor hallucis longus muscle also courses along the fibula; this largely expendable muscle is readily available to fill soft tissue defects, especially those inferior to the mandible and within the arch of the mandible. [50]
The variable vascular supply of the cutaneous portion of the FOFF occasionally limits its use in oromandibular reconstruction. However, the cutaneous portion of the flap is available in 91% of patients and can be harvested with enough surface area to cover the floor of the mouth, buccal mucosal loss, and intraoral and extraoral deficiencies. [48] Another advantage of the FOFF is the ability to use a 2-team approach, which is easily supported during creation of the FOFF because the fibula is located far from the head and neck region and patient positioning is rarely a problem. Lastly, the morbidity rate at the fibular donor site is low.
Distal to the lower border of the popliteus, the popliteal artery divides into the smaller anterior tibial artery and the larger posterior tibial artery. The FOFF is based on the peroneal artery, which is a branch of the posterior tibial artery. The peroneal artery arises from the posterior tibial artery within 2-3 cm of the popliteal bifurcation and descends toward the fibula. The peroneal artery lies medial to the fibula and passes between the tibialis posterior and the flexor hallucis longus.
The fibula is supplied with an endosteal circulation and a circular anastomosis of musculoperiosteal vessels, both of which arise from the peroneal artery. [6] Either of these circulations can support the osseous portion of the graft, but the musculoperiosteal vessels, which course within 1-10 mm of the cortex, allow graft survival when osteotomies are placed as far as 1 cm apart. [33, 51] Thus, the preservation of these musculoperiosteal vessels by including a protective cuff of muscle around the bone is clearly important. [52] The peroneal vascular pedicle is usually about 5-6 cm in length and about 2-3 mm in diameter. [51]
The blood supply to the lateral leg is either septocutaneous or musculocutaneous in origin. The septocutaneous branches course through the posterior crural septum and are dispersed in a segmental fashion every 2-6 cm, 15-27 cm distal to the fibular head. [6] These septocutaneous branches are responsible for about 30% of the lateral cutaneous circulation. [6] The other 70% of the lateral cutaneous circulation is derived from the musculocutaneous perforators, which arrive at the skin after penetrating the flexor hallucis longus, tibialis posterior, or soleus muscles. [53]
The osteocutaneous fibular free flap skin island can be raised solely on the basis of the septal perforators, without the incorporation of portions from the flexor hallucis longus or soleus muscles. [54] A skin island based on septal perforators alone must be designed over the distal third (at the junction between the middle and distal thirds) of the leg, and a Doppler study may be employed to map out the perforators in some patients. [54] Occasionally, septal perforators alone are not enough to supply the cutaneous portion of the flap. After evaluation of the perforators, musculocutaneous perforators must occasionally be traced through the soleus to the peroneal vessels. This is easily accomplished by identifying the perforator at the peroneal vessels and the septum and taking a small muscular cuff surrounding the vessel as it travels through the soleus.
Hayden (1991) and O'Leary (1994) described refinements in the fibular flap harvest, which includes the transfer of the cutaneous portion of the osteocutaneous flap as a sensate component using the lateral cutaneous sural nerve. [55, 56] Four principle sensory cutaneous nerves are derived from the lumbosacral plexus and supply the lower leg: the sural nerve (L1, L2), the saphenous nerve (L3, L4), the superficial peroneal nerve (L4, L5, S1), and the lateral cutaneous nerve of the calf (L5, S1, S2). The common peroneal nerve courses inferiorly in an oblique manner superficial to the lateral head of the gastrocnemius and toward the fibular head. It divides into the deep and superficial peroneal nerves after diving deep to the peroneus longus muscle. The common peroneal nerve can be easily palpated by rolling the nerve over the lateral head or neck of the fibula.
In the lateral aspect of the popliteal fossa, the common peroneal nerve sends a pair of cutaneous branches inferiorly; these are the sural communicating nerve (SCN) and the lateral cutaneous nerve to the calf (LCNC). The LCNC exits the common peroneal nerve inferior to the SCN. After piercing the deep fascia of the leg, the LCNC descends inferolaterally; the nerve then divides into fine sensory cutaneous components, supplying the posterolateral aspect of the calf. [56] These fibers are incorporated into the fibular free flap to produce a sensate component.
At transfer, the LCNC may be attached to the lingual nerve defect, and the SCN can be included as an interposition graft, bridging the defect created in the inferior alveolar nerve. The functional outcomes of nerve grafts in microvascular free tissue transfer have not been shown to be definitively better as compared with outcomes in patients without such adjunctive reconstruction.
Considerable variation has been noted in reference to the origin of the peroneal artery. These variations include those found by Hentz and Pearl, who noted a 5% incidence of a popliteal bifurcation in the absence of the posterior tibial artery. In this anatomical variation, the peroneal artery provides the dominant supply to the posterior muscular compartment. [57] If the peroneal vessels are sacrificed, the distal extremity may have ischemic complications.
The variations in vessel caliber, location, and zones of distribution of the lower extremity lead some to perform preoperative evaluations with either angiography or color Doppler studies. [6, 56] The authors still recommend obtaining an angiogram prior to the surgical date to properly evaluate ankle and foot blood flow because 10-20% of lower extremities show abnormal characteristics that can lead to significant postoperative morbidity. The role of preoperative angiography in a patient with a normal physical exam remains unclear; however, little controversy exists regarding the necessity of preoperative angiography when the history or physical examination suggests the possibility of lower extremity atherosclerosis, significant leg trauma, or prior lower extremity vascular reconstructive surgery. [58]
However, others argue that, even in the presence of known atherosclerotic disease and with the small percentage of vascular variability, little need for angiography exists because the yield does not outweigh the morbidity. [2, 50, 51, 59] The advent of magnetic resonance angiography (MRA) has allowed adequate vessel visualization without the need for invasive angiography, and as MRI continues to advance, these scans may become as comprehensible as standard lower extremity angiography.
Use of the FOFF began soon after the introduction of the free groin flap in 1973. Hidalgo (1989) was the first to report the use of the fibular free flap for mandibular defect reconstructions. [33] Hidalgo transferred these flaps to repair both anterior arch defects and hemimandible defects; the average defect size was 13.5 cm. He noted a 1-in-5 survival rate of his skin paddles. He concluded that large intraoral defects should not be restored with the FOFF.
Later, Hidalgo and Rekow reported on 60 reconstructions of the lateral portion, the anterior portion, or both portions of the mandible using the FOFF, in which 59 of the 60 flaps were successfully transferred. [50] A skin island was obtained in 85% of patients but was included in only 62% of the reconstructions. Ninety percent of all skin islands were viable. The average bone defect was 9.4 cm. Hidalgo reported 1 nonunion and 1 fibrous union.
At the conclusion of this follow-up study, Hidalgo and Rekow reconsidered the use of the FOFF for mandibular reconstructions, including cutaneous defects. Hidalgo advocated the use of ipsilateral fibular flaps for lateral mandibular defects because of the anatomical configuration and the adjacent soft tissues. [50] Hidalgo and Rekow concluded that the fibular free flap was an ideal choice unless the patient had a defect of the retromolar trigone or a lateral defect with a massive soft tissue component.
Wei et al used 27 fibular flaps for composite mandibular reconstructions with cutaneous defects in 25 patients. [44] The mandible defects were 6-14 cm in length. They reported 1 total flap failure for an overall 96.3% success rate and no isolated incidents of partial or total skin loss. All osteotomy sites healed primarily. Four patients had partial wound dehiscence, but no patients had significant gait disturbances. Wei advocated including the posterior crural septum and its perforators, located along the posterior margin of the fibula, into the flap. Unfortunately, the authors had difficulty harvesting enough skin to close some of the oromandibular defects; as a result, 2 simultaneous flaps were used for reconstruction.
Yim and Wei used 49 osteoseptocutaneous fibular free flaps to repair 47 composite mandibular defects that were 6-14 cm in length. [51] They reported 1 total (bone and skin flap) failure. Bony union occurred in all patients at 6-month follow-up. Minor recipient site complications, including partial wound dehiscence, hematoma, and superficial wound infections, were noted in a few patients; all of these resolved without sequelae.
Jones et al reported a 100% success rate in 34 mandible reconstructions using the fibular osteocutaneous flap based on the posterolateral intermuscular septum. [54] The skin paddles were 7-26 cm long and 4-15 cm wide. Mandible defect length ranged from 7-15 cm. Two of 5 patients who underwent primary closure required secondary skin grafts because of dehiscence. Although Jones et al reported good success, others still prefer to raise skin paddles with septomusculocutaneous perforators (including a cuff of flexor hallucis longus and soleus muscles). These surgeons also have favorable flap survival rates. [53, 39]
Overall complication rates and donor site morbidities remain low for the FOFF. However, adequate fibula must remain to support the knee joint, and 5-6 cm must remain to support the ankle mortise and the knee; otherwise, instability at these joints may occur. [51] Other morbidities include poor cosmetic outcome (as skin grafts generally should be used to close donor sites), especially if the width of the skin island exceeds 4-6 cm. [6, 53]
Some patients experience transient numbness over the dorsum of the foot and the first web space; this effect is secondary to intraoperative traction of the superficial and deep peroneal nerves. [51] With harvesting of the LCNC and the SCN for sensate fibular flaps, drop foot caused by the interruption of the vasa nervorum of the common peroneal nerve is possible. [56] Others have experienced interphalangeal joint weakness during extension and flexion of the great toe. [51] Daniels et al studied functional foot and ankle outcome after FOFF and found an 18% incidence of great-toe clawing, and 41% of patient were dissatisfied with their foot and ankle function at 3.1 years after the surgery. [60]
Scapular osteocutaneous free flaps
The SOFF is one of the more versatile free flaps used in head and neck reconstruction, yet it has some disadvantages, as well. This flap can be harvested with a variable amount of soft tissue, and it can be harvested with tissue islands, which may include the fasciocutaneous layer or simply the pliable fat and fascia after de-epithelization. Both internal and external orocutaneous defects may be corrected with the use of the large dual skin paddles. Two to 3 cm of vascular pedicle separates the skin flap from the bone. This unique vascular anatomy allows 3-dimensional independent positioning of the bone graft in reference to the skin paddles. [61, 62, 63] Multiple portions of well-vascularized corticocancellous bone also may be independently harvested because of the separate vascular supplies of the flap. The flap provides anatomically consistent vascular pedicles with large-bore vessels.
Additionally, 3-4 cm of bone can be extracted from the scapular angle to reconstruct the ascending ramus of the mandible. [62] The scapula can provide a bone graft about 3 cm in translated mandibular vertical height and 1.5 cm in translated mandibular thickness in men, but the graft can be considerably smaller in women. [64] Frodel et al demonstrated adequate bone stock for osteointegrated implants in men, but inadequate amounts were found in 14% (6/44) of women. Thus, most men may have adequate bone stock for both plating and osseointegrated dental implants; however, smaller women may need other reconstruction options.
The circumflex scapular artery (CSA) provides the blood supply to the skin overlying the posterior aspect of the scapula and the periosteum of the lateral border of the scapula. The CSA is a branch of the subscapular artery, which originates from the third portion of the axillary artery. The CSA courses through a muscular triangle created by the long head of the triceps laterally, the teres major inferiorly, and the teres minor superiorly. Before it exits this muscular triangle, the CSA sends several branches to the lateral scapular border and 2-3 branches to the teres minor. [65] After exiting the triangle, both vertical and horizontal branches are distributed to the fasciocutaneous area overlying the scapula, which can be as large as 14X21 cm. [62]
Flaps can be based on either the vertically oriented vessels (parascapular) or the horizontally oriented vessels (scapular). The vascular pedicles are 6-9 cm long and have vessel diameters of 2-3 mm. The flap is limited superiorly by the scapular spine, inferiorly about 3 cm above the inferior angle, medially 2 cm from the vertebral column, and laterally by the posterior axillary line. [65]
Unfortunately, the bone harvested from the SOFF lacks a segmental blood supply; therefore, one must be extremely mindful in the number of osteotomies made in the bone flap so that the distal bone segments are not jeopardized. [6, 64] To assist with this problem, Coleman and Sultan identified the consistent presence of the angular branch of the thoracodorsal artery, which serves as a vascular pedicle for the inferior pole of the scapula. Bone supplied by both the circumflex scapular artery and the angular branch can be harvested together or separately. As much as 8 cm of the inferior scapula supplied by the angular branch may be taken alone, independent of the CSA blood supply.
Swartz et al treated 26 patients with SOFF procedures; 21 of the 26 patients had composite mandibular defect reconstructions. [64] The average scapular bone was 10.7 cm long. They reported no flap failures and minimal donor site complications, especially after physical therapy. Various postoperative bony complications occurred in 19% of patients. This is high as compared with microvascular free tissue transfers.
Sullivan et al (1990) reviewed 31 SOFF procedures for reconstruction of soft tissue and bony reconstructions in the head and the neck. [63] Thirty of the 31 SOFF procedures involved mandibular reconstruction: 16 lateral mandibular, 8 anterior mandibular, and 6 anterolateral mandibular defects. The mean length of the vascularized scapula was 10 cm, and, in 2 patients, angle-to-angle reconstructions were performed using 14.5-cm bone grafts, although 1 failed. In 6 patients, the fasciocutaneous portion of the flap was used to replace soft tissue defects of both the oral cavity and the facial skin. The overall success rate of the SOFF procedures was 90%. Nonunion occurred in 2 instances, but both eventually healed with fibrous union. Minor complications occurred in 28% of patients.
Coleman et al (2000) conducted a retrospective review of over 8 years of head and neck operative cases involving free bone flaps at a single institution. [61] Of 64 bone flaps, 24 were SOFF procedures. Eighteen of the 24 SOFF procedures were performed for complex oromandibular reconstructions, 6 using a single skin paddle and 12 using double skin paddles. The mean cutaneous area harvested for all of the SOFF procedures was 110 cm2, compared with the cutaneous areas of 55.4 cm2 and 77.6 cm2 for the FOFFs and the ICOFFs, respectively. The mean bone flap lengths were 8.37 cm, 7.65 cm, and 10.1 cm for the SOFFs, FOFFs, and ICOFFs, respectively. The authors reported an overall donor site complication rate of 12.5% with no permanent adverse sequelae and only 1 flap failure. [61]
Limitations of SOFF procedures include decreased range of motion (ROM) of the shoulder and difficulty lifting objects above the head. Several upper-extremity muscle groups must be detached during the harvest of the SOFF. These detachments can cause weakness; however, to minimize these complications, some advocate immobilization of the shoulder for 5 days postoperatively, followed by rigorous exercise and physical therapy. [63, 64] Unfortunately, only 5 patients from Coleman et al's study (2000) participated in follow-up evaluations for ROM and strength. [61] Of those participating, most reported slightly limited or no limits in activities of daily living. The follow-up also demonstrated no constraints in ROM at the elbow or the arm, with no reduction in strength of the arm and the forearm.
Other surgical limitations of the SOFF procedures include difficulty in using a 2-team approach for flap harvesting and recipient site preparation; thus, intraoperative time may be increased significantly. If a 2-team approach is to be used, it can be technically challenging because of patient positioning. Some propose turning the patient 45° from the supine position or turning the patient into the lateral decubitus position with the support of a vacuum-controlled beanbag mattress. [62, 63, 66] The head and the neck are then supported with a Mayfield headrest. This technique allows ipsilateral exposure of the face, the neck, the back, and the shoulder; however, contralateral neck dissection becomes impossible. This is the key issue that has limited the broad application of this flap in clinical practice.
Iliac crest osteocutaneous free flap
The ICOFF offers a large curved piece of predominantly cancellous bone, measuring 6-16 cm in length, for reconstruction. [62, 67, 68] This largely cancellous bone, with its associated profuse blood supply, contributes to callus formation and has a favorable endosseous implant capability. [6] The crest of the bone has a natural contour that can be used to complement and repair mandibular defects, as it can be carved in a 3-dimensional manner to closely match the extracted bone.
However, the bone is not limited to carving alone; if the defect extends across the midline, osteotomies can be performed to re-create the anterior mandible and mentum. [67] The skin paddle is reliable when obtained in large enough quantities to include the larger myocutaneous perforating vessels, and an area as large as 10X20 cm may be taken for reconstruction. [67] Even with bulky tissue transfers, donor site cosmesis is extremely good in most patients.
Different shapes and orientations of the iliac bone may be harvested, and either crest may be used depending on the desired orientation of vessels in the flap and the recipient vessels. Hemimandibles, mandibular defects crossing the midline, anterior mandible defects, and lateral mandibular defects all may be corrected with careful construction of this flap. The classic Manchester design may be used to create a hemimandible from the ipsilateral anterior ilium, where the angle of the mandible is reformed by the anterior superior iliac spine (ASIS), the ramus from the bone between the ASIS and the anterior inferior iliac spine, and the body from the iliac crest. [69]
Four vascular pedicles supply the ilium: the superficial circumflex iliac artery (SCIA), the deep circumflex iliac artery (DCIA), the superior deep branch of the gluteal artery, and the ascending branch of the lateral circumflex artery. The 2 lateral vessels are inadequate for flap transfer. The SCIA supplies the dominant vasculature to the skin overlying the iliac crest, yet it has a variable anatomical distribution and only serves a small amount to the underlying bone, making it a poor choice for the basis of an osteomyocutaneous flap.
An iliac osteocutaneous flap based on the DCIA, which arises from the lateral aspect of the external iliac artery, is more ideal; the DCIA supplies the ilium with an endosteal and a periosteal blood and nutrient supply. [53] The DCIA also supplies the overlying skin with cutaneous perforators, which transverse the 3 muscle layers of the abdominal wall as it courses toward the ASIS. When the osteocutaneous flap is harvested, an obligatory cuff of these muscles must be included to protect the deep circumflex iliac vessels and the musculocutaneous perforators. [6] These perforators can be found as far as 8-9 cm posterolateral to the ASIS and within 2.5 cm superior to the inner table of the iliac crest. [6, 70, 53]
Ramasastry et al found a 1- to 2-mm diameter branch of the DCIA, called the ascending branch, to be the primary source to the internal oblique muscle, the endosteum, and the periosteum. This discovery made it possible to transfer 2 separate soft tissue flaps with the iliac bone and increased the overall maneuverability of the flap. [53] The diameter of the DCIA ranges from 2-3 mm, and the length of the DCIA from the ASIS to the junction at the external iliac artery is about 5-7 cm. [53] The vascular pedicle to the graft can be shorter; therefore, proper design of the bone graft is important, especially when only contralateral neck vessels are available. If the vascular pedicle is short, the contralateral iliac crest is more ideal because it places the vascular pedicle closer to the midline during reconstruction. [62]
Unfortunately, the iliac crest free flap has some drawbacks. The color match of groin skin is noticeably poor when it is compared with the face and the neck, and skin perfusion is poor when the skin paddle is positioned above the level of the oral commissure. [62, 67] Also, the iliac crest is not the ideal flap for angle-to-angle defects because the natural curvature makes lengthy contouring difficult, and the myocutaneous portion of the flap is bulky. [67]
The bulky soft tissue component also has limited 3-dimensional mobility in reference to the bone graft. The skin or muscle island may be rotated over the bone graft in small amounts, but the musculocutaneous perforators cannot tolerate extreme shearing and torsion, which may lead to flap necrosis. [62] Unfortunately, the skin portion is located outside of the iliac graft and must be turned to reconstruct intraoral defects. Yet, even with careful rotation over the mandible, the mere bulk of the flap can cause a poor aesthetic outcome in some patients. For all of these reasons, patient selection is extremely important. The iliac crest bone graft is better suited for younger, nonmuscular, nonobese patients with larger soft tissue requirements.
Harvesting of the iliac crest free flap involves extensive dissection, including the division of most of the abdominal wall muscles as well as the inguinal ligament. This extensive dissection, with the extraction of overlying tissue, increases the postoperative risk for groin and ventral hernia formation. The risk of hernia formation can be decreased with secure closure of the transversalis fascia and transverses abdominis muscle to the iliacus muscle; however, this closure is often difficult to achieve. Therefore, some advocate reinforcement with permanent sutures fastened through the iliac crest. [62] If the transversalis fascia is weak, Marlex mesh can be used as a reinforcing layer. The lateral cutaneous nerve of the thigh lies in the area of dissection, and hypesthesia of the nerve may be another complication of this extensive dissection.
Forrest et al examined 82 consecutive iliac donor sites for complications. Sensory disturbances were found in 27%; contour deformities, in 20%; unsightly scarring, in 12%; hernia formation, in 9.7%; and femoral nerve palsies, in 4.8%. In contrast, Urken et al (1994) found only 1 case of quadriceps muscle weakness because of an abdominal wall stitch causing compression along the femoral nerve; he reported only 15 donor site complications in 200 mandible reconstructions that used various free flaps, including 34 ICOFFs. [53]
Pain at the donor site can be a limiting factor in early mobilization, especially in elderly patients; thus, other flaps may be more appropriate for these patients. Occasionally, hip weakness is encountered for several weeks, but it generally subsides with time, and it may be a product of pain at the donor site. Urken et al (1995) favor progressive mobilization by the third or fourth postoperative day after osteomusculocutaneous or osteocutaneous flaps. [52] Patients usually begin assisted ambulation and passive and active ROM exercises by the seventh day. These exercises are conducted for 2 weeks, and stair climbing may be started within the third week.
Radial forearm osteocutaneous free flaps
The RFOFF is an important option in reconstructive surgery of the head and the neck because it is reliable, thin, pliable, and well vascularized and has sensate potential. The skin of almost the entire forearm, from the antecubital fossa to the flexor crease of the wrist, can be harvested. However, the RFOFF has been used with only moderate success when reconstruction of the mandible and large defects of the oral cavity is considered. The radius is thin and can provide only a short segment (8-12 cm) of monocortical bone that is easily devascularized with osteotomies. [43, 71]
The RFOFF is best suited for primary defects requiring a large amount of intraoral lining and a small lateral segment of mandibular reconstruction; these occur with defects of the ascending ramus, small lateral bone defects resected with a large amount of tonsil and pharyngeal mucosal, retromolar trigone defects, or portions of the floor of the mouth and the tongue. [43, 71, 72]
The RFOFF derives its blood supply from 9-17 fascial branches of the radial artery. The radial artery extends along the lateral intramuscular septum of the forearm, between the flexor carpi radialis and brachioradialis muscles. [53] Harvesting of the radial forearm free flap requires complete interruption of the radial artery; thus, the hand must totally rely on the ulnar artery. For this reason, an Allen test must be performed during the planning stages of surgery.
The lateral intermuscular septum is attached to the distal radius; the periosteum of the radius receives arterial branches through this connection, which allows for the harvest of vascularized bone. A segment of radius, limited proximally by the insertion of the pronator teres and distally by the insertion of the brachioradialis, can be harvested. However, the quantity of bone stock is restricted to 40% of the circumference of the bone. [53] A greater amount may be harvested if primary plate reconstruction of the donor radius is performed to help maintain support. The arterial pedicle length is limited by the radial recurrent artery, which is the first major division of the radial artery following its separation from the brachial artery. [53] In comparison, the cephalic vein offers much greater flexibility in length because it can be harvested throughout the entire course of the arm to the junction of the subclavian vein.
The cutaneous innervation of the forearm is derived from the medial, lateral, and posterior antebrachial cutaneous nerves. The lateral antebrachial cutaneous nerve is a continuation of the musculocutaneous nerve and acts as the primary sensory nerve of the forearm that is harvested with the flap. The nerve courses close to the cephalic vein in the proximal forearm but then continues into the hand near the thenar eminence.
Urken and Moscoso reported recovery of sensation in 80% of their 40 cases of sensate radial forearm flaps and noted that recipient nerves in the cortical distribution of the defects provided the most accurate and meaningful sensory information. Again, no meaningful data support the notion that neural reconstruction in these patients improves functional outcome in terms of mastication, deglutition, or oral competence. The radial nerve is a mixed motor and sensory nerve. The distal branches of the radial nerve supply the sensation to the dorsum of the hand, the thumb, the index finger, and the middle fingers. These branches are frequently encountered in the wrist when the RFOFF is elevated, but, with attentive technique, they can be preserved to maintain sensation. [53] Decreased sensation over the distribution of these nerves distal to the donor site has been reported in 5-17% of patients. [28]
Soutar and Widdowson successfully used the RFOFF in 12 of 14 patients who underwent oromandibular reconstruction. [73] They reported 1 flap failure and 1 postoperative death. Nine patients had noncomplicated osteotomies of the vascularized bone graft. Mounsey and Boyd presented findings in 4 patients who underwent reconstruction with the RFOFF and subsequent osseointegrated implant placement. [74] The radius was able to support osseointegrated implants in only 1 of the 4 cases. However, the authors noted successful denture use when defects were small and when the adjacent native mandible had osseointegrated implants placed to bridge the gap created by the vascularized graft.
Thoma et al reported on 60 consecutive oromandibular reconstructions using the RFOFF. Of the defects, 33% were lateral, 40% were lateral-central, and 27% were lateral-central-lateral. The mean skin flap size was 55 cm2, and the mean bone length was 9.4 cm. The authors noted a microvascular success rate of 98.3% and a radial forearm fracture rate of 15%. Nonunion of the mandible occurred in 5% of subjects, and hematomas occurred in 8.3% of subjects. The most common recipient site complication was plate removal, usually as a result of infected hardware. Bony union was assessed clinically and found in 95% of patients. [72]
The RFOFF is an attractive concept for reconstructing oromandibular defects; however, 2 major factors restrict its use for this purpose. The dimensions of the bone that can be safely harvested are limited by the requirement for structural integrity of the remaining radius. Other free flaps can provide much better bone stock with less morbidity. [52] The potential morbidity of the RFOFF resulting from pathologic fracture is about 23%. [75] These fractures severely limit supination and wrist flexion. However, some have found that prophylactic plating and keel-shaped harvesting osteotomies have led to a lower donor site morbidity rates with good functional lateral and posterior oromandibular outcomes. [76, 77]
The aesthetic deformity of the forearm after skin grafting is another major disadvantage of the RFOFF. [6, 52] The donor forearm must be immobilized in a full-length plaster cast for 6 weeks following surgery. Immobilization is important to prevent shearing of the muscles and the tendons underneath the skin graft. The initial volar dressing and the splint are applied in the operating room after the ulnar vascularity is confirmed; this is not removed for 7 days. Postoperative edema can be decreased by the elevation of the forearm. The ulnar vasculature should be intermittently evaluated during the postoperative period to ensure that edema and splint positioning do not compromise blood flow to the hand.
Latissimus-serratus-rib free flap
Peripheral vascular disease sometimes precludes the use of other flaps. Patients with head and neck cancer have a high prevalence rate of smoking, which increases the risk of peripheral vascular disease; this may preclude the use of extremity flaps. The latissimus-serratus-rib free flap (LSRFF) is an uncommon option used for bone and soft tissue reconstruction in the oral cavity. The combined free serratus anterior latissimus dorsi flap transferred on one vascular pedicle has been previously described.
The free serratus anterior-rib flap has been described for use in lower extremity reconstruction with good results. The flap has several advantages, including the ease of a 2-team approach, a large cutaneous paddle for reconstruction, possible orientation of the rib independent of the latissimus portion of the flap, low donor site morbidity, and a long vascular pedicle that contains large caliber vessels. The flap is easily harvested when other sites are plagued with vascular disease. It is best suited for lateral mandibular defects but can be used in a variety of situations. Unfortunately, it has a poor ability to maintain osseointegrated implants.
Cadaveric studies have been performed to show the reliability of the arterial and venous supply to the rib provided by the thoracodorsal artery. Hui et al performed arteriography through the thoracodorsal artery, followed by microscopic dissections at the rib periosteum. The sixth through ninth ribs showed consistent contrast enhancement of their respective intercostal vessels. The rib segments near the anterior axillary line contained the most abundant communicating vessels between the serratus muscle and the periosteum. Additional studies have shown that serratus muscle slips 5-9 are consistently supplied by a single dominant branch of the thoracodorsal artery. Each muscle slip has a dependable vascular pattern in which the serratus artery gives rise to common group of arteries, each of which supplies adjacent muscular portions.
The mean length of a muscle slip from its origin on the rib periosteum to the division of the common slip artery is 9.6 cm. These findings imply that the slips may be separated to the level of these common slip arteries, with up to 5 slips transferred on a single neurovascular pedicle and each slip oriented independently. The use of latissimus-serratus-rib flaps for oromandibular reconstruction was described originally in 1985. Netscher et al discussed the advantages of the composite latissimus-serratus-rib free flap. The cartilaginous portion of the rib is available to reconstruct the temporomandibular joint, the rib bone may be oriented independently of the soft tissue component of the flap, and a high volume of soft tissue is available for reconstruction of large defects. Further studies found the serratus-rib flap very reliable in oromandibular reconstruction in patients with osteoradionecrosis.
In this series of 29 reconstructions using the LSRFF, no complete flap failures were found; however, 48% of patients experienced early postoperative complications, and delayed wound-healing complications were seen in 14%. Most early postoperative complications were related to cardiopulmonary events and likely reflected preexisting medical comorbidities. Successful long-term reconstruction of jaw continuity was achieved in 27 of 29 patients, although delayed partial bone graft resorption was seen in one case of attempted maxillary reconstruction, and a case of bone graft nonunion was found in one mandibular reconstruction.
All 4 cases of delayed wound healing complications initially presented with hardware infection or extrusion. The cases of delayed partial maxillary bone graft resorption may have been elicited by the episode of hardware extrusion causing osteitis of the rib. In addition, the delayed bone graft resorption in one patient may have been related to the patient's underlying osteogenesis imperfecta, yet this patient had clinical and radiograph-revealed persistence of a serratus-rib graft used for mandibular reconstruction after a follow-up period of 45 months.
Additional advantages of the LSRFF include a long vascular pedicle that contains a large caliber of vessels rarely involved by peripheral vascular disease, the ability for primary closure of the donor defect with limited long-term donor site morbidity, the ability for a 2-team approach with no intraoperative repositioning, and the availability of a long bone graft that can span a large length of segmental mandibular resection. The size of the latissimus myocutaneous component can be tailored to match low-volume or high-volume soft tissue defects. Previous studies have demonstrated scapular winging; however, this has been minimally noticeable, with no patient attributing work disability to the donor site. The main disadvantage of the latissimus-serratus-rib flap is that it provides relatively thin bone with minimal cortical bone, making it ill-suited for placement of osteointegrated dental implants.
Flaps are harvested from the flank located ipsilaterally to the mandibular resection and cervical recipient blood vessels. The flaps are harvested by positioning the patient supine on a bean bag with slight elevation (10-20°) of the donor sight flank. This allows for simultaneous surgery at the head and neck and flank, and no intraoperative repositioning of the patient is required. An incision is made in the midaxillary line from the axilla in an inferior direction toward the ilium. Dissection is deepened onto the anterior border of the latissimus dorsi muscle. The loose areolar tissues between the latissimus dorsi and serratus anterior muscles are then dissected until the dominant branch of the thoracodorsal artery to the serratus anterior muscle is identified. The serratus anterior vascular branch is then dissected, and its branching pattern is determined.
The most inferior slip of serratus muscle found to be perfused by the serratus anterior branch of the thoracodorsal artery is selected to provide a periosteal blood supply to one of the ribs. This was usually the sixth or seventh rib. The intercostals muscles are divided and the parietal pleura is exposed above and below the identified serratus slip and rib. The long thoracic nerve is transected in the intercostal space immediately above the rib graft, leaving intact the motor innervation to the more cephalic slips of serratus anterior muscle. The posterior rib periosteum is elevated off the parietal pleura.
A cuff of soft tissues, including the intercostal blood vessels, is left attached to the rib bone graft. The rib is then divided anteriorly and posteriorly to complete elevation of the serratus-rib component of the flap. The length of the rib harvested is based on the anticipated length of the mandibular defect. The rib graft is centered at the anterior axillary line, as this is where the serratus anterior muscle has robust insertions into the rib periosteum.
After elevation of the serratus-rib component of the flap, the latissimus dorsi myocutaneous component is then designed and elevated according to the anticipated oral cavity soft tissue defect. The thoracodorsal pedicle is followed superiorly up to the take off of the subscapular vessels from the axillary vessels. Flap harvest is completed by ligating and transecting the subscapular artery and vein after completion of the oral cavity tumor resection.
The bone and soft tissues of the flap are trimmed to an appropriate size prior to completion of the flap insetting. The curvature of the rib graft is usually appropriate for reconstruction of hemimandibular defects without the need for osteotomies. For mandibular defects that crossed the midline, a single closing osteotomy can be made through the lingual cortex of the reconstructed mandibular symphysis. Microvascular anastomoses are created after insetting the flap.
Table. Characteristics of Osteocutaneous Free Flaps Used in Mandibular Reconstruction (Open Table in a new window)
Osteocutaneous Free Flap Qualities |
Fibular Free Flap |
Scapular Free Flap |
Iliac Crest Free Flap |
Radial Forearm Free Flap |
Latissimus, Serratus, Pedicled Rib Free Flap |
Vascular supply |
Peroneal artery and venae comitantes |
Circumflex artery and vein and angular branch of thoracodorsal artery |
Deep circumflex iliac artery and vein |
Radial artery and venae comitantes |
Thoracodorsal artery and venae comitantes |
Bone availability, cm |
As long as 25 |
6-15 |
6-16 |
8-12 |
8-12 |
Bone quality |
Good |
Good |
Excellent |
Fair |
Fair |
Bone shape |
Good |
Good |
Excellent |
Fair |
Fair |
Cutaneous 3-dimensional versatility |
Moderate |
Excellent |
Fair |
Fair |
Moderate |
Skin paddle size, cm |
10 x 20 |
14 x 21 |
10 x 20 |
8 x 15 |
25 x 40 |
Tissue match |
Moderate |
Excellent |
Fair |
Good |
Fair |
Osseointegratability |
Good |
Good |
Excellent |
Poor |
Poor |
Pedicle length, cm |
4-8 |
4-9 |
5-7 |
Variable |
6-16.5 |
Pedicle diameter, mm |
1.8-3 |
2-6 |
2-3 |
2-3 |
1.5-4 |
Neurosensory potential |
Yes |
No |
No |
Yes |
Yes |
Two-team approach |
Yes |
Difficult |
Yes |
Yes |
Yes |
Donor site morbidity |
Skin graft if flap > 6 cm, potential for joint instability |
Limited ROM and strength of shoulder, aesthetically displeasing scar |
Contour hip deformity, postoperative pain, gait disturbance, hernia |
Radius fracture; need for prolonged use of long arm cast; decreased sensation in the dorsum of the hand, the thumb, the index finger, and the middle fingers |
Limited ROM and strength of shoulder, aesthetically displeasing scar |
Reconstruction of the Ramus and Condylar Defects
Defects of the mandible can include those of the posterior mandible and the condyle. These deficiencies present a unique challenge. The temporomandibular joint (TMJ) is a complex joint on which the condyle hinges, and it has the ability to translate anteriorly, posteriorly, and laterally. For many years, the routine treatment method ignored the condyle after resectioning and resulted in a free-floating ramus. Although the interincisal opening is adequate in many patients, deviation of the mandible and altered functional occlusion are common. With closing, tangential rather than vertical striking occurs at the occlusal surfaces, causing difficulty with mastication. Tangential striking can cause undue wear on the teeth, which accelerates the formation of dental caries, especially in patients treated with irradiation. [48] If the ascending ramus is also deficient, the face can appear concave.
The goals of condylar reconstruction are to preserve an adequate interincisal opening and to preserve the dual joint function of the mandible to stabilize the muscles of mastication and to maintain preoperative occlusion.
Several options have been discussed in the literature about reconstruction of condylar defects. Incomplete reconstruction of the condyle does not allow the ramus to extend to the glenoid fossa. This method relies on the contralateral joint to maintain adequate stabilization and ROM; however, as discussed previously, this rarely occurs, and malocclusion is the end result. An alternative is to use a prosthetic condyle and attach it to the end of the bone flap. The condyle has a unique shape, which varies widely in size among individuals; therefore, matching a prosthetic condyle with the native condyle may be difficult in most patients. These variations in size and shape may also affect functional results.
The long-term safety results of a titanium prosthesis within the glenoid fossa are also unknown. However, some have successfully used these prostheses and noted no erosion of the glenoid fossa. [48] This has fallen to disfavor by many because of long-term implant-related complications of extrusion and infection. A study by Patel and Maisel commented on 4 patients who underwent reconstruction with condylar prostheses after hemimandibulectomy. The authors noted extrusion and exposure of devices as well as migration into the epitympanum of one patient with associated cochlear damage and hearing loss. [78]
However, Daniel (2004) reported on 6 patients who underwent mandibulectomy, including the condyle, and were primarily reconstructed with a titanium condylar prosthesis. [60] These researchers were able to preserve the TMJ cartilage and joint capsule and applied purse-string sutures to hold the prosthesis in position. The plate was also covered with muscular flaps to help prevent extrusion. Follow-up ranged from 7 months to 6 years and no plates were exposed, although only 3 patients were alive at the end of the study. Five of the 6 patients underwent perioperative radiation therapy.
Another option for condylar reconstruction involves shaping the end of the free flap to function as a simulated condyle, while others use a small segment of carved rib attached to the end of the bone flap. Although accurate shaping is difficult to achieve, the functional result is quite reasonable. [48] The Food and Drug Administration has also approved the use of a total joint prosthesis, which can be manufactured in accordance with the specific defect. While this device has served many patients with TMJ reconstruction, no studies are currently investigating the utility of this device for reconstruction on patients with cancer.
Hidalgo (1994) discussed the use of condyle autotransplantation after hemimandible resection in 14 patients. [48] Before resection, mandibular height from the top of the condyle to the inferior border of the angle is measured. This measurement should be the same after the condyle is reattached. Once the harvested condyle is verified free of disease with frozen sections, it can be mounted on the osseous portion of the free flap with miniplates as a nonvascularized graft. The condyle and the free flap are then set at MMF. The capsule is then closed around the condylar head with a 3-0 polyprolene permanent suture. Hidalgo maintained MMF for 10-14 days postoperatively. The authors have placed their patients in arch bars and guiding elastics for 2-3 weeks postoperatively and found that this works well.
Hidalgo reported no wound infections, no ankylosis or trismus, and no grafts requiring removal. The interincisal opening averaged 28.75 mm in patients receiving postoperative radiation therapy, compared with 43.37 mm in patients who did not receive radiation (P =0.007). Preoperative occlusion was unchanged in 13 patients and slightly altered in 1 patient. Hidalgo concluded that condyle transplantation, as a nonvascularized bone graft to the osseous portion of a free flap, was effective and safe.
A problem exists with this method in that accurate frozen section clearance of bony margins is notoriously difficult. Thus, this method may not be recommended in patients who have overt mandibular involvement of the immediate subcondylar region. However, Weisenberger et al introduced a fixation method using a conventional microwave that would shorten the time it takes to prepare bone margins for observation by a pathologist. [79] Slides were made suitable for histopathologic review within 2-3 hours after extraction, allowing margins to be evaluated within the intraoperative period. In the 10 patients studied, the microwaved specimens had a 100% correlation with slides that were prepared over the course of 7 days using the standard decalcification technique.
Subsequent advances produced a simple and reliable assessment of bony margins at the time of the operation. Following resection of the specimen, a 4- to 5-mm sharpened curved osteotome is used to obtain thin sections of the cortical mandible at its surgical margin. The osteotome can be used on both cortical and cancellous bone. On cortical bone, the osteotome is directed parallel to the cut surface of the bone to produce thin bony sections. Cortical specimens are cut thin enough to be translucent. The cortical bony margins are then processed by the surgical pathology department in the routine method used for soft tissue frozen sections. A standard cryostat and microtome can be used to prepare slides for staining. Standard hematoxylin and eosin staining is performed. This method showed that 21 of 22 specimens were confirmed positive on frozen section and reconfirmed by decalcified permanent specimens.
Osseointegrated Dental Implants
Functional dental rehabilitation is an important portion of total restoration. Interest in the use of osseointegrated implants in VBFs has increased. Branemark and colleagues developed the use of osseointegrated implants, and they are now used worldwide. These implants are used to support implant-borne dentures, facilitating postreconstruction dental rehabilitation, including speech, mastication, occlusion, and cosmesis. Osseointegrated dental implants can be placed in a 1- or 2-stage procedure. During the 2-stage procedure, initial fixtures are placed in the neomandible, allowing at least 12 weeks of healing after their placement. The implants are then uncovered and fitted with transmucosal abutments. Final soft tissue modifications are then performed to create an optimal platform for the dental prosthesis and to allow for proper hygiene.
Good candidates for osseointegrated implants include patients that have the following:
-
Absence of systemic morbidities that might compromise osseointegration
-
Favorable tumor prognosis based on stage and grade
-
Positive attitude and realistic expectations for prosthesis
-
Good oral hygiene
-
Lack of trismus and good tongue function
-
Suitable maxillomandibular relationship
-
Sufficient bone quality and quantity
Many discussions have taken place concerning which osteomyocutaneous free flaps best support osseointegrated dental implants. Frodel et al compared the bone availability in cadavers by using the fibula, the ilium, the scapula, and the radius with respect to free flaps. [80] A vertical height of 7-10 mm is commonly quoted as the minimum length of bone used for implant placement. Frodel et al chose a minimum height requirement of 10 mm and a minimum width of 5.75 mm. Iliac, scapular, fibular, and radial osseous flaps were harvested from 22 cadavers. Nearly all of the iliac crest and fibula bone flaps had adequate dimensions for implantation.
The fibula had the added advantage of bicortical purchase on the implants, enhancing stabilization. Most scapula flaps had adequate bone dimensions for implantation; those lacking proper dimensions were from female patients. The radius had the largest number of areas that were inadequate for implantation, showing insufficient bone stock at 2 of the 3 points of measurement in 29% of the flaps. As with the scapula, all of these deficient specimens were from female cadavers.
The authors concluded that iliac and fibular flaps consistently had bone dimensions that were adequate to receive osseointegrated implants; also, while the radius and the scapula can consistently be used in men, these may be poor choices for elderly women. [80] Moscoso and associates (1994) published similar results using a 5-mm width cutoff, and they found that the iliac crest is the most consistent implantable donor site, followed by the scapula, the fibula, and the radius. [28]
Osseointegrated implants can be placed at the time of primary reconstruction or at a delayed procedure. Proponents of the delayed placement present several arguments, including the following: (1) Patients with oral cancer have a poor prognosis, and the technique should be reserved for long-term survivors. (2) Implants may compromise the circulation to the newly placed osteocutaneous free flap. (3) Immediate placement of dental implants permits less precise implant placement. [81] Gurlek et al placed 60 implants in the neomandibles of 20 patients who had not received radiation.
These implants were placed at a mean of 15.5 months from the time of primary mandibular reconstruction. The authors reported 5 failures, with a 91.6% success rate. Teoh et al (2005) placed 100 implants in 24 patients 4-6 months after mandibular reconstruction with fibular free flaps; 7 of these patients underwent radiation therapy. These researchers found a 5- and 10-year success rate of 97% and 79.9%, respectively. The implant survival rate was worse (67%) when the implants were placed in a previously irradiated mandible. Older patients (≥ 61 y), anterior implants, and implants with a diameter of 3.75 mm or less. [82]
Others believe that implants should be placed at the time of primary reconstruction. Urken points out that a 6-week lag time usually occurs between surgery and radiation treatment and that a 6-week lag time also occurs between the beginning of radiation treatment and the onset of its effects on bone. Thus, the osseointegration process has 12 weeks to work. He also believes that implant placement with primary surgery is superior to later placement because of increased exposure.
In Urken's series, 81 of 360 dental implants were exposed to postoperative adjuvant radiotherapy. Urken reported a success rate of 86% in radiated implants versus 95% in implants that were not exposed to radiation. He also reported the poor success of osseointegration in bone that was previously exposed to radiation. [81] Sclaroff et al (1994) had similar results. [83] They reported an 88% success rate in 11 patients who received radiation therapy after reconstruction and the placement of 4-pin fixed mandible staple implants.
McFadden currently recommends that patients who have undergone irradiation treatment receive 20 dives in a hyperbaric oxygen chamber before 1-stage implants are placed. He then has his patients follow with an additional 10 dives (oral communication, 2001).
Roumanas et al analyzed 46 subjects who underwent oral cavity reconstruction and placement of implants and prosthesis for oral rehabilitation. [84] Of these patients, 23 completed conventional prosthesis (CP) treatment and evaluation, and, of these, 15 completed treatment and evaluation with an implant prosthesis (IP).
The defect and nondefect chewing sides were evaluated with standardized tests of mastication of peanuts. Swallowing tests were also administered. Masticatory function of the patients prior to surgery was markedly compromised. The performances worsened following ablative and reconstructive surgery (PS) on both the defect and nondefect sides. Restoration with a CP and an IP significantly improved performance over the PS interval and did not differ from performances at entry prior to surgery (P >0.05). In addition, the performance on the defect side with the IP was significantly greater than the performance with the CP.
Summary
Within the last 30 years, vast advances have been made in the area of microvascular and reconstructive surgery. However, mandibular defects remain a difficult challenge for reconstructive surgeons. The challenge is to restore airway support, oral competence, verbalization, mastication, deglutination, and acceptable aesthetics, allowing the patient to return to society.
Osteocutaneous free flaps allow for the transfer of bone and soft tissue with a rich vascular supply and preserved osteogenic potential; thus, the effects of infection from oral flora and postoperative radiation are well tolerated, and the neomandible can play an active role in osteosynthesis. Currently, osteocutaneous free flaps used for mandibular reconstruction are predominantly harvested from the fibula, the ilium, the scapula, and the radius. The fibular flap represents the workhorse of mandibular reconstruction at most institutions. Osteointegrated implants also can be successfully placed in vascularized bone free flaps before radiation to assist in the functional rehabilitation of patients with such flaps. The careful selection of osteocutaneous free-flap donor sites for these reconstructions allows the optimal restoration of both function and cosmesis.
-
A Synthes 2.0-mm locking mandibular plate with interpositional bone graft.
-
A Synthes 2.4-mm locking reconstruction plate used for a comminuted mandible fracture repair.
-
A 2.4-mm locking mandibular reconstruction plate.
-
Locking reconstruction plate with an osseous interposition graft.
-
A 2.0-mm mandible locking plate system.
-
A 2.4-mm locking mandibular reconstruction plate.
-
A threaded plate hole accepts both nonlocking and locking 2.0-mm screws. Nonlocking screws permit greater angulation.
-
A 2.0-mm locking screw.
-
Comparison of the heads of locking and nonlocking 2.0-mm screws.
-
Large 2.0-mm mandible locking plates.
-
Mini 2.0-mm mandible locking plates.
-
Various profiles and plates of the locking 2.0 variety.
-
Mandibular locking plates, 2.4 mm.
-
Three-dimensional fashioning of the reconstruction plate.
-
Three-dimensional fashioning of the reconstruction plate.
-
Three-dimensional fashioning of the reconstruction plate.
-
Cutting the reconstruction plate.
-
Locking reconstruction plate cutters.
-
Forming and cutting instrumentation for 2.0-mm locking plates.
-
Bending instrumentation for 2.4-mm locking reconstruction plates.
-
Various profiles and plates of the locking 2.0-mm variety.
-
Postoperative view of mandibular reconstruction with an osteocutaneous flap without osseointegrated dental implants.
-
Postoperative view of mandibular reconstruction with an osteocutaneous flap without osseointegrated dental implants.
-
Postoperative view of mandibular reconstruction with an osteocutaneous flap without osseointegrated dental implants.
-
Postoperative intraoral view of an osteocutaneous free flap without osseointegrated implants.
-
Postoperative Panorex image of an osteocutaneous free flap without osseointegrated implants.
-
Intraoperative intraoral view of delayed placement of osseointegrated implants into an osteocutaneous free flap.
-
Postoperative intraoral view of osseointegrated implants in an osteocutaneous free flap.
-
Inferior view of prosthesis that is seated onto the osseointegrated implants.
-
Panorex image after placement of prosthesis onto the osseointegrated implants.
-
Intraoral view of the osteocutaneous free flap with osseointegrated implants and seated prosthesis.
-
Postoperative view of mandibular reconstruction with an osteocutaneous flap with osseointegrated dental implants.
-
Postoperative view of mandibular reconstruction with an osteocutaneous flap with osseointegrated dental implants.
-
Intraoral view of osteointegrated implants placed after an osteocutaneous free flap was created.
-
Intraoral view of osseointegrated implants placed after an osteocutaneous free flap was created, with prosthesis in place.
-
Osseointegrated implants and the mechanism by which they integrate with their bony surroundings.
-
Preoperative view of patient with floor of mouth squamous cell carcinoma invading the mandible.
-
Preoperative view of patient with floor of mouth squamous cell carcinoma invading the mandible.
-
Preoperative intraoral view of patient with floor of mouth squamous cell carcinoma invading the mandible.
-
Intraoperative view of patient with floor of mouth squamous cell carcinoma with markings for identification of the iliac crest free flap. ASIS indicates anterior superior iliac spine, IL indicates inguinal ligament, and FA indicates femoral artery.
-
The vascular and muscular anatomy of the left iliac crest region for purposes of harvesting an iliac crest free flap.
-
A right iliac crest osseous free flap (shown here) is used to reconstruct a right hemimandibular defect.
-
A left iliac crest osseous free flap (shown here) is used to reconstruct a right hemimandibular defect.
-
A right iliac crest osseous free flap (shown here) is used to reconstruct a left hemimandibular defect.
-
This diagram shows the versatility of the iliac crest osteomyocutaneous free flap in reconstructing lateral mandibular defects.
-
This diagram shows the versatility of the iliac crest osteomyocutaneous free flap in reconstructing lateral mandibular defects.
-
Postoperative view of patient with floor of mouth squamous cell carcinoma with fibular free flap reconstruction and 2.4-mm locking reconstruction plate.
-
Postoperative view of patient with floor of mouth squamous cell carcinoma with fibular free flap reconstruction and 2.4-mm locking reconstruction plate.
-
Postoperative intraoral view of patient with floor of mouth squamous cell carcinoma with fibular free flap reconstruction and 2.4-mm locking reconstruction plate.
-
Postoperative view of failed iliac crest free flap in same patient with floor of mouth squamous cell carcinoma and exposed 2.4-mm locking reconstruction plate.
-
Intraoperative view of same patient as in Image 46 with floor of mouth defects of the bilateral anterior bodies and symphysis of the mandible, the floor of mouth, and the anterior tongue.
-
Surgical planning of incisions for a fibular osteomyocutaneous free flap.
-
Surgical planning of incisions for a fibular osteomyocutaneous free flap.
-
The vascular anatomy of the lower leg demonstrating the peroneal artery and its origin as well as its supply to the fibula.
-
The lateral muscular anatomy of the lower leg as encountered in harvesting a fibular free flap.
-
The muscular anatomy of the lateral lower leg as encountered in harvesting a fibular free flap.
-
Intraoperative view of patient with floor of mouth squamous cell carcinoma with fibular free flap reconstruction showing harvest of the left fibular free flap with bone and skin paddles.
-
Anatomical axial view of the left lower leg demonstrating muscles, bones, and vascular supply.
-
Anatomical axial view of a harvested left fibular free flap. This flap uses the myocutaneous perforators to the skin paddle by including a cuff of flexor hallucis longus and soleus within the flap.
-
The harvest of a cuff of soleus and flexor hallucis longus for musculocutaneous perforators versus septocutaneous perforators supplying the skin paddle of a fibular free flap.
-
Posterior superficial anatomical view demonstrating the origin of the lateral sural cutaneous nerve. Hayden (1991) and O'Leary (1994) described the transfer of the cutaneous portion of the osteocutaneous flap as a sensate component using the lateral cutaneous sural nerve.
-
Intraoperative view showing a split thickness skin graft covering a fibular free flap harvest site.
-
Hidalgo used miniplates on osseous free flaps to secure bone segments.
-
A more traditional approach to plating fibular segments is the use of a titanium locking or nonlocking mandibular plate. Note that the periosteum is left intact on the neomandible segments. This image also shows the placement of an osseointegrated implant at the time of surgery.
-
Intraoperative view of patient with floor of mouth squamous cell carcinoma with fibular free flap reconstruction and 2.4-mm locking reconstruction plate. Notice the excellent mandibular height that is maintained.
-
Postoperative Panorex of patient with floor of mouth squamous cell carcinoma with fibular free flap reconstruction and 2.4-mm locking reconstruction plate.
-
Preoperative view of patient with floor of mouth squamous cell carcinoma.
-
Preoperative coronal CT scan of patient with floor of mouth squamous cell carcinoma.
-
Preoperative coronal CT scan of patient with floor of mouth squamous cell carcinoma.
-
Preoperative axial CT scan of patient with floor of mouth squamous cell carcinoma.
-
Preoperative axial CT scan of patient with floor of mouth squamous cell carcinoma.
-
Partial glossectomy, mandibulectomy, and neck dissection from patient with squamous cell carcinoma.
-
Intraoperative view of patient with floor of mouth squamous cell carcinoma, showing resection and tongue, jaw, and floor of mouth defects.
-
Intraoperative view of patient with fibular free flap reconstruction using a 2.4-mm locking reconstruction plate. This image shows the fitted fibula to the reconstruction plate prior to flap harvesting.
-
Intraoperative view of patient with floor of mouth squamous cell carcinoma, showing resection and fibular free flap reconstruction using a 2.4-mm locking reconstruction plate.
-
Immediate postoperative view of patient with floor of mouth squamous cell carcinoma, showing resection and fibular free flap reconstruction using a 2.4-mm locking reconstruction plate.
-
Immediate postoperative view of patient with floor of mouth squamous cell carcinoma, showing resection and fibular free flap reconstruction using a 2.4-mm locking reconstruction plate.
-
Preoperative Panorex of patient with floor of mouth squamous cell carcinoma.
-
Postoperative Panorex of patient with floor of mouth squamous cell carcinoma, showing resection and fibular free flap reconstruction using a 2.4-mm locking reconstruction plate.
-
Left forearm demonstrating the potential cutaneous area available for a radial forearm free flap.
-
The nervous and venous anatomy of the forearm. The lateral antebrachial cutaneous nerve can be used to make a sensate flap.
-
This image shows the blood supply to the forearm and the hand.
-
Anatomical axial view of the left forearm. The osteocutaneous radial forearm free flap is shown with the integration of the antebrachial cutaneous nerve, the radial artery, and the cephalic vein.
-
The vascular supply of the posterior scapula. The circumflex scapular artery (CSA) provides the blood supply to the skin overlying the posterior aspect of the scapula and the periosteum of the lateral border of the scapula. The angular branch can be included in the flap to supply the inferior 2-3 cm of the scapula.
-
The vascular supply of the posterior scapula. Note that the thoracodorsal artery can be included in the flap to gain more latissimus dorsi and skin paddle for reconstruction.
-
The immense versatility of skin paddle designs when planning reconstructions with a scapular free flap.
-
The immense versatility of skin paddle designs when planning reconstructions with a scapular free flap.
-
Left scapular free flap dissection showing osseous perforators and their relationship to the teres musculature. The dotted lines demonstrate the osteotomy sites for this flap.
-
Harvested right scapular free flap with blood supply and overlying cutaneous paddle.
-
Three-dimensional stereolithography design of a reconstruction plate, specific for a defect and to add height to the mandibular reconstruction. Courtesy of Stryker CMF (Craniomaxillofacial) (http://www.stryker.com/en-us/products/Craniomaxillofacial/index.htm).
-
Use of computer programing in conjunction with system engineers to achieve 3-dimensional stereolithographic models prior to surgery for patient-specific plating. Courtesy of Stryker CMF (Craniomaxillofacial) (http://www.stryker.com/en-us/products/Craniomaxillofacial/index.htm).