Brief Overview of Molecular Biology and Cancer
The discovery of the structure of DNA by Francis Crick and James Watson in the early 1950s ushered in a new era in molecular biologic science. This technologic and biologic revolution continued through the 1960s to today, and the explosion in technology has fueled the current expansion of knowledge into the working of the human cell. By 2003, 99% of the chemical base pairs that make up human DNA had been sequenced to within 99.99% accuracy (with the sequencing reported to have reached 100% by 2021). In turn, as a result of research into the abnormal cancer cell, the basic understanding of the cell has greatly improved. Successful translational research has allowed gene therapy trials to proceed.
Work across many laboratories is geared toward elucidating the genetics behind cancer, discovering cellular mechanisms that lead to cancer, and elucidating intracellular and intercellular interactions that allow this progression. [1] Identifying candidate precursors or enabling genes may pave the way for cancer screening, as with the ret proto-oncogene and medullary thyroid carcinoma. One technique that is garnering wide attention in this realm is the DNA array, in which a number of different genes from an individual can be processed to look at certain genotypes. Eventually, new treatments will develop from these efforts.
A few gene therapy trials now target head and neck cancer, which makes up only 4% of all cancers but has a dismal prognosis in advanced stages. [2, 3, 4, 5] Of the estimated 41,000 patients in the United States who develop this cancer each year, 13,000 are believed to die from the disease. In other parts of the world, head and neck cancer is much more prevalent. The male-to-female ratio in head and neck cancer is 2:1.
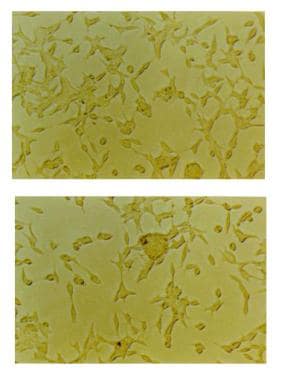
An image depicting head and neck squamous cell carcinoma in vitro can be seen below.
Cancer Model
Cancer is characterized by uncontrolled growth and division of a cell, with extension beyond the normally limiting basement membrane and through the boundaries of normal cells. This, in turn, creates a clonal population of a single abnormal cell. Because of its mutated aggressive genetics, this cell has a selective growth advantage over its neighbors. Different theorists have argued how this mutation occurs.
Multihit theory
A reasonable model is based on colon cancer development. This is the multihit theory of tumorigenesis, in which a series of multiple triggering events in the genetic and cellular makeup of a cell ultimately cause cancer. Some modeling analyses suggest 5 separate events are required in colon cancer. [6] These events lead the cancer cell to escape normal cell growth and control mechanisms, to avoid system control mechanisms (ie, immunologic surveillance), and to establish a nutrient supply.
The multihit model postulates that several unique genetic mutations combine to cause cancer. However, which mutations are most important, which events must occur, and if a specific order is involved in molecular tumorigenesis is unclear. In colorectal cancer, multiple mutations are present. For example, in the hereditary cancer syndromes familial adenomatous polyposis or hereditary nonpolyposis colorectal cancer, mutations have been discovered in the APC gene and DNA mismatch repair genes. Although the exact process has not been elucidated, the sum of these mutations is believed to lead to cancer development in this model.
In head and neck cancer, the histologic progression of severity from hyperplasia, dysplasia, and carcinoma in situ to invasion provides a framework to understand the genetic progression of cancer. Early clonal genetic events in benign hyperplastic lesions show losses at chromosomes 9p21 and 3p. p21 and other tumor suppressor genes' inactivation are therefore most likely involved in the first steps of the cancer model, with later stages possibly involving protooncogene activation and inflammation. One model postulates 6-10 genetic steps in the pathway from premalignant lesion to frank malignancy. Time and cumulative exposure to carcinogens are believed to greatly modify this risk and development.
External Influences and Molecular Mechanisms
Among the risk factors for the development of head and neck cancer are alcohol and tobacco abuse, viral infection, and sun exposure. Each of these external factors has been studied in some detail.
Tobacco abuse
Tobacco has been determined to be carcinogenic in animal models. The effects of tobacco are broad and affect the entire mucosa of the upper aerodigestive tract. It may induce changes in a number of different cellular sites within the pharynx and larynx. This ability has led to one particular model of oral cancer development, field cancerization. In this model, not only is the local cancer a threat, but much of the surrounding tissue is also likely to become cancerous. This has necessitated resection with wide margins in the treatment of oral carcinoma. It also explains the high prevalence of second primary tumors in these locations, especially in the esophagus. Many of these second primary tumors, although separate in time and space, can be traced to a common clonal origin, with similar early genetic alterations leading to the pathway of cancer.
Clinical data have shed further light on the mechanisms of tobacco-induced neoplasms. Certain individuals appear to have greater susceptibility to cancer caused by tobacco because of their genotypic makeup. Specifically, whether or not wild-type p53 is present normally or whether it is mutated, missing, or nonfunctional may affect one's risk of cancer. If p53 is mutated, missing, or nonfunctional, risk of cancer from tobacco use may be increased.
Tobacco is known to have various mutagenic effects through the formation of free radicals and epoxides. Investigators have studied the body's response to these effects via detoxifying enzymes. One example, the glutathione S-transferase enzyme, which has numerous polymorphisms, may be related to the development of cancer in certain predisposed individuals. Another molecular scavenger, uridine 5'-diphospho (UDP)-glucuronosyltransferase, may increase risk of larynx cancer 3.7 times if present with low activity.
Tobacco and alcohol synergy
Tobacco and alcohol use have a synergistic effect that increases the rate of cancer causation. This effect is not merely additive but also geometric. The risk of head and neck cancer in persons who smoke and drink is up to 17 times greater than the risk in persons who neither smoke nor drink.
Viral infections
Viral infections have long been known to cause certain cancers and to be strongly linked to others. Well-known examples include human T-cell lymphoma virus (HTLV) in T-cell lymphoma and human immunodeficiency virus (HIV) in Kaposi sarcoma. In the head and neck, Epstein-Barr virus (EBV) has been implicated in the development of nasopharyngeal carcinoma, antibodies to which may help determine the prognosis.
Human papillomavirus (HPV) DNA, RNA, or both have been found in several types of head and neck cancer. HPV has an affinity for epithelial cells. The E6 protein of HPV binds to RB, a tumor suppressor gene, inactivating it with the potential for unchecked cell growth. One study has shown that HPV-16 DNA detected in the lymph nodes of patients affected with HPV-16–positive oropharyngeal cancer indicates metastatic involvement. Patients with tumor-free lymph nodes but with a high viral load value may have developing lymph node metastasis, and this finding may serve as a marker. [7]
HPV types 16 and 18 have been implicated in cervical cancer. Another tumorigenic mechanism is by E6 protein–mediated pathways that lead to p53 degradation. Research has shown HPV DNA in a high proportion of oropharyngeal head and neck cancer. An inverse relation of HPV positivity in these cases with p53 has also been shown. This suggests a causal mechanism for cell proliferation. The link between HPV and other head and neck subsites, however, is unclear.
A study by Scott-Wittenborn et al indicated that in some US populations, more than 90% of oropharyngeal squamous cell carcinomas are associated with HPV. Looking at HPV biomarkers in patients with oropharyngeal squamous cell carcinoma at tertiary care centers between 1995 and 2019, the investigators found that the prevalence of these increased over time. Overall, the rates at which p16 and oncogenic HPV in situ hybridization occurred rose from 44% to 91% and from 39% to 92%, respectively. More specifically, rates reached 92% and 94%, respectively, in White patients; 72% and 67%, respectively, in Black patients; and 100% and 100%, respectively, in Hispanic patients. [8]
Sun exposure
Sun exposure is causally linked to squamous cell carcinoma of the skin, as well as to melanoma and basal cell carcinoma. Radiation causes DNA damage in the following 3 ways: directly (breaks DNA), indirectly through changes in oxidative enzyme systems (buildup of free radicals damages DNA), and indirectly to DNA repair mechanisms (causes faulty DNA). Similarly, irradiation has been shown to increase the risk for radiation-related sarcomas and thyroid cancer.
Drug and environmental factors
Certain drugs may predispose one to cancer formation. For instance, immunosuppressive regimens may predispose patients to transplant-associated tumors. Other environmental agents associated with head and neck malignancy are the chewing of betel nuts in Asia (oral cancer) and nickel and wood dust exposure (sinus carcinoma).
Systemic and Local Factors in Cancer
In addition to outward influences, intrinsic systems are at work in cancer development.
Systemic factors
For a cancer to grow, it must be able to avoid the body's immunologic cancer surveillance system. This system relies on major histocompatibility (MHC) proteins to recognize self from nonself. It depends on the interrelationships between antigen-presenting cells (APCs) and dendritic cells to effector cells, such as lymphocytes and monocytes, and the humoral immune system. Despite intensive research aimed at identifying cancer-specific antigens for use in directed therapy and vaccines, the apparent lack of specific cancer antigens has complicated this effort. In addition, the cancer state causes widespread immune system suppression, affecting dendritic cells and their production of lymphokines and monokines, cell-signaling molecules. In patients with head and neck cancer, the native output of thymic T-cells is less than that in healthy controls.
Certain lymphocytes are known to target tumor cells, with these tumor-infiltrating lymphocytes (TILs) being found in a number of solid tumors. A study by Ngamphaiboon et al indicated that in patients with head and neck squamous cell carcinoma, a high CD8+ TIL score (6 or greater) is associated with significantly longer overall survival. A correlation was also found between the high score and patients with a status of never having smoked, the presence of oral cavity cancer, and presentation with stage M0. [9]
Similarly, a study by Shimizu et al indicated that in patients with oral squamous cell carcinoma, an association exists between a high CD8+ tumor-infiltrating T-cell density in the parenchyma at the tumor’s invading edge and improved overall and disease-specific survival. The investigators also reported a correlation between a high stromal density of these T-cells at the tumor’s periphery and improved recurrence-free survival. [10]
One current area of work uses TILs to target tumors by sensitizing them to tumor antigens outside the body and then reintroducing them, making TILs home in on the neoplasm. Patients with head and neck cancer who have a high percentage of apoptotic TILs have lower 5-year survival rates.
Other immune modifications include packaging genes with TIL, such as cytokine tumor necrosis factor (TNF), which causes local tumor cell lysis without systemic side effects. Class I MHC markers such as leukocyte antigen human leukocyte antigen (HLA)-B7 likewise could be activated into populations of tumor cells, leading to expression on the tumor cell surface with subsequent rejection and destruction by the immune apparatus.
Local factors
In addition to systemic factors, local factors allow the maintenance of the cancer state. In squamous cell carcinoma of the head and neck, along with most other cancer conditions, the tumor secretes angiogenic growth factors that promote vascular proliferation. This enables the tumor to grow, because nutrient constraints would eventually limit the tumor's volume unless a new source of nutrition were found. This fact has remarkable implications for newer antiangiogenesis drugs that fight cancer.
The latest antineoplastic drugs include this class of antiangiogenesis compounds. Bevacizumab, a monoclonal antibody that targets this phenomenon of angiogenesis, has shown promise in clinical trials of breast, lung, and colorectal cancer treatment, prolonging survival.
Matrix metalloproteinases (MMP) are a family of proteins involved in degrading the extracellular matrix (ECM) framework of cells, including collagen. Attention has been focused on these molecules because of the potential of metastasis in cells that have this ability to invade the basement membrane. In head and neck cancer cell lines, tumor, and primary larynx cancer, levels of MMP were indeed found to be elevated. Part of this elevation may be in relation to epidermal growth factor (EGF).
Cellular and Molecular Interactions
Overall, the genetics behind carcinoma in the head and neck region are complex [11] ; environmental influences play a significant role. This is in contrast to some other cancers, such as retinoblastoma, which have a simple Mendelian inheritance pattern.
Perhaps the greatest growth in our understanding of cancer is at the cellular level. Methods that elucidate genomic cancer hotspots include evaluation of chromosomal loss of heterozygosity. Besides gene mutations, other mechanisms of loss of function of an allele may have the same effect. For example, a genetic change that affects a homozygous deletion of a tumor suppressor gene may be a sentinel cancer event. Maternal and paternal alleles in normal tissue are compared with the same in tumor tissue, and the areas of loss or gain are identified. This is aided by analysis of microsatellite markers, which are tandem repeats of the genetic code in mostly noncoding areas. By amplifying these flanking areas around genes through the polymerase chain reaction (PCR), specific genes of interest that correspond to the loss or gain are identified. [12, 13] In this fashion, a map of where to search for candidate genes involved in molecular oncogenesis is created.
Cell cycle
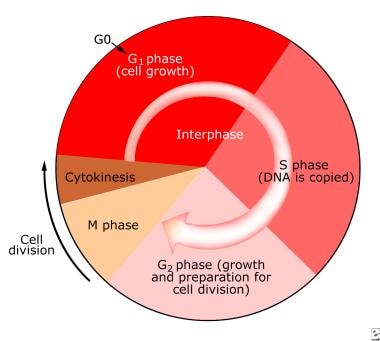
In the normal noncancer state, the cell cycle is tightly regulated (see the following image); only a few tissue types have cells that are constantly dividing (eg, skin, mucosa). Other cells go into a state of senescence, whereby they no longer grow and divide. In vitro, cells in culture, such as fibroblasts, divide for only a limited number of cycles. Also, as the cells grow and multiply, they respect the boundaries of neighboring cells. In the cancer cell, however, both of these usual mechanisms are lost; this is abundantly clear in culture.
Cancer cells may be continually regenerated and, unabated, will grow in a disorganized manner. Once normal cells enter the quiescent phase of cells leaving the mitotic cycle (G0), or cell senescence, they do not reenter the cycle of growth, synthesis of proteins and DNA, and mitotic division. The gate of entry back into the replicative cycle is the domain of oncogenes, which facilitate entry, and tumor suppressor genes, which block the pathway.
Oncogenes
Among the more well-studied oncogenes is the cyclin family. The cyclins are significant in the entry pathway from the G0 state to the presynthetic gap, or phase of cells before DNA synthesis (G1), growth phase and thereby entry into the cell cycle. In the normal state, cyclins respond to growth factors and are otherwise quickly degraded; however, buildup of cyclin D1 protein, a kinase, leads to the phosphorylation of the Rb protein—a tumor suppressor. This inactivation of Rb allows the cell to progress into division mode. [14, 15]
Cyclin D1 has been shown to be amplified in head and neck squamous cell carcinoma. It resides on chromosome 11q13, which is amplified in up to one third of head and neck cancer cases. [16] Interestingly, most of these cases were from the pharynx. This may suggest a complementary mechanism for p53 inactivation along with human papillomavirus (HPV) oncogenic integration in this cancer subsite. [17] A study by Dhingra et al indicated that in head and neck squamous cell carcinoma, an association exists between greater expression of cyclin D1 and higher tumor stage and lymph node metastasis. [18]
Other oncogenes operative in head and neck cancer resemble this model of tyrosine kinase phosphorylation of key molecules that leads to cell division (eg, human epidermal growth factor receptor [EGFR], the STAT3 protein, myc, ERBB2).
Epidermal growth factor receptor
Cetuximab, a monoclonal antibody against EGFR, has been approved for use in human head and neck cancer chemotherapy. Its action is to bind EGFR, thereby preventing epidermal growth factor (EGF) from binding to the cell surface and stimulating cell growth. EGFR overexpression is believed to be an early event in carcinogenesis. Its expression is increased with increased severity of premalignant lesions. In larynx cancer, levels of EGFR have been found to correlate with metastasis-free survival and overall survival.
Downstream from EGFR, the signal transducing and activators of transcription (STATs) are phosphorylated and activated by the tyrosine kinase region of EGFR. They then bind to DNA promoters and effect gene transcription. It may have an antiapoptotic role and has also been found to be a likely early event in the progression to head and neck cancer.
Tumor suppressor genes
Tumor suppressor genes work in much the opposite fashion. In its hypophosphorylated state, Rb binds and inactivates a transcription factor, EF1, which is responsible for entry into the cell cycle. RB resides on chromosome 13q, and, although loss in this region has been found in head and neck cancer, RB gene mutation has been inconsistently identified.
The p53 protein product is perhaps the most widespread cancer mutation, germane to discussion of head and neck cancer as well. It acts slightly upstream of Rb in the cycle, at the G1/synthesis (S) checkpoint. Normally, p53 is upregulated in response to cellular injury and DNA damage, arresting the cell at this checkpoint. [19] Apoptosis may begin if the damage is irrevocable. With mutant p53, however, this important check is lost, and the cell proceeds with DNA synthesis and cycling. p53 is located on chromosome 17p, another area identified as abnormal in human head and neck squamous cell cancer.
Studies that examine the relation of p53 to head and neck cancer have found mutations in 33-59% of tumors. However, clinical correlations of this significance in prognosis have been equivocal. One study has shown an increasing prevalence of p53 mutation with increasing histologic severity in invasive head and neck cancer.
Another well worked-out pathway of tumor suppressors concern the p family of proteins (p16, p14, p21, p27). These molecules prevent the phosphorylation of Rb and, therefore, the cell's continuation in the cycle as well.
p16 has been studied in some detail. It binds to and inactivates the cyclin-dependent kinases CDK4 and CDK6, preventing the phosphorylation of Rb; thus, the cell's continuation through the G1/S growth checkpoint. The method of faulty p16 production appears to be via hypermethylation of its promoter, a form of gene silencing that does not involve sequence mutation. The p16 gene resides on chromosome 9p21, an area previously shown to be marked by allelic imbalance and loss.
Nuclear Factors in Head and Neck Squamous Cell Carcinoma
In the nucleus of the cell, DNA replication occurs during the synthesis phase (see the image below). In the normal state, this process is highly accurate, with the frequency of an error occurring estimated at 1 in a billion base pairs per cell generation. Nonetheless, however precise, this system has potential for intrinsic mutations with each passage of the cell through the cycle of division.
DNA repair
To combat potential intrinsic mutations, numerous DNA repair enzymes help to ensure the fidelity of the genetic code; however, even these systems are imperfect and may allow genetic change or mutation. The importance of these repair enzymes is clearly evident in the disease xeroderma pigmentosum, in which a defect in these systems allows unchecked DNA damage from ultraviolet light. Persons with this disorder develop squamous cell carcinoma, basal cell carcinoma, and melanoma of the skin, often at a very early age.
Apoptosis
Another mechanism to ensure the appropriateness of the cell is apoptosis. As discussed earlier, p53 protein promotes apoptosis in response to DNA damage. A different protein, Bcl-2, blocks this apoptosis and, in so doing, allows the continuation of the cell through the cycle. In essence, Bcl-2—which has been shown to be overexpressed in human squamous cell carcinoma as well as in its namesake, B-cell lymphoma—prolongs cellular survival.
Another enzyme that may do the same but in a different fashion is telomerase. Telomeres are the ends of DNA strands. They are normally not well replicated and thereby shorten with each replication cycle. By doing so, telomeres function as cellular clocks, keeping track of how many times a cell has divided, until it ultimately remains in a quiet state. Telomerase is an enzyme that is able to lengthen these shortened ends and thus prolong the period in which the cell may divide. It is normally found in germ cells and at low levels in regenerating tissues. Research has highlighted this molecule in human cancers, showing it to be present in up to 90% of all cancers, including head and neck cancer.
A study by Liu et al found that survivin, which belongs to the inhibitor of apoptosis protein family, is not only present in higher quantities in oral squamous cell carcinoma but exists in the nucleus of the carcinoma cells. This contrasts with most normal oral tissue cells, where survivin is either absent or exists in the cytoplasm but not the nucleus. Moreover, statistical analysis demonstrated a correlation between nuclear survivin and the carcinoma’s TNM stage and differentiation grade. [20]
Nuclear factor kappa B
Nuclear factor kappa B (NFkB), a cytosolic protein, is normally bound to inhibitor factor kappa B (IkB). IkB is degraded in response to inflammatory cytokines. NFkB also has binding sites for cytokines such as interleukin (IL)-1 and then translocates to the nucleus, where it functions as a transcription factor, having either inhibitory or activating function. Possible roles include immune modulation, inflammation, apoptosis, and cell division. Previously, a mouse model has shown NFkB to have a role in malignant transformation. In head and neck cancer cell lines, inhibition of NFkB led to decreased growth. Multiple molecular targets in the signaling pathway of NFkB have also been shown to be altered in neoplastic transformation.
Cyclooxygenase inhibitors
The cellular inflammatory response may thus be an important source for propagation of cancer. Cyclooxygenase (COX)-1 and -2 upregulation leads to increased levels of prostaglandins in head and neck cancer. They may also play a part in decreasing apoptosis and increasing angiogenesis through an increase in vascular endothelial growth factor. Clinical studies are ongoing to see if COX inhibitors may be useful as chemoprevention in head and neck cancer, as it has been found for colon cancer.
A study that looked at a COX-2 inhibitor, celecoxib, in chemoprevention of oral precancerous lesions showed that COX-2 is overexpressed in a number of premalignant and malignant oral lesions. [21] This is linked to inhibition of apoptosis and metastasizing potential. Administration of celecoxib in a mouse model of oral cancer delayed the onset of early lesions and slowed the growth of established oral tumors. The effect was dose dependent.
Retinoid agents
Chemopreventive measures that involve the administration of retinoid agents have also shown promise in in vivo studies and premalignant head and neck lesions. The retinoic acid receptor family comprises nuclear receptors similar to thyroid and steroid hormones. They function as ligand-dependent transcription factors and are involved in cellular growth, as well as in angiogenesis.
Gene Therapy and the Newest Frontiers
It is probable that no one pathway alone is responsible for squamous cell cancer; rather, like the colon cancer model, many pathways converge to cause this cancer. Multiple mitigating and inciting influences also exist. Advanced age, for example, with the accumulation of genetic changes over time and possible immune suppression, adds to the increased risk of head and neck carcinoma.
A report by van der Kamp et al proposed the establishment of a new subcategory of patients with head and neck squamous cell carcinoma, namely, elderly individuals who are HPV-negative and lack a history of alcohol and tobacco consumption. Carcinogenesis in this group, according to the investigators, may involve a multistep process associated with genomic instability, immunosenescence, cell cycle disruption, and telomere shortening. [22]
A study by Kalfert et al suggested that differences in the microRNA (miR) profile influence the pathobiology of head and neck squamous cell carcinoma of the oropharynx and larynx. The investigators found a correlation between oropharyngeal origin of p16-positive squamous cell carcinoma and tumor levels of miR-34a. The study also noted significant differences in the levels of let-7a, miR-200c, and miR-34a levels between oropharyngeal and laryngeal squamous cell carcinomas, while, compared with normal squamous epithelium, the squamous cell carcinomas showed upregulation of miR-21, miR-200c, and miR-34a and down-regulation of miR-375. [23]
Chromosomal imbalance
The genetic changes, aside from the specific mutations discussed, can be grouped into a larger category of genetic change: chromosomal imbalance. Chromosomal imbalance includes chromosomal instability and break points, translocation, amplification and deletion, oncogene activation, and methylation. The complex external milieu of the cell, with cancer promoting angiogenesis, and the body's natural homeostasis, which favors a steady state, are also factors. Ultimately, both environment and genetics underlie cancer development in susceptible individuals.
Targeted gene therapy
The next frontier in research for squamous cell cancer of the head and neck is in the evolution of newer and better treatments. Because so much has been learned about the genetics of cancer, targeted therapies are possible. Gene therapy has gone from theory to practicality in the form of a number of current research trials that target head and neck cancer. [24, 25, 26, 27, 28, 29, 30, 31] This modality makes sense in the head and neck region, because this region is readily accessible for treatment and injection and is easily monitored; [32] furthermore, the current disease management has much morbidity and potential for disfigurement, as well as continuing poor prognosis in the later stages.
In vitro, antisense DNA technology against the cyclin family of oncogenes has been used with some success. Human trials have included a marginally successful attempt to replace the wild-type p53 protein in tumors. [33] Human trials have also been undertaken using adenovirus without the E1B region, which normally inactivates p53, with the goal of the virus targeting cells with abnormal p53, such as cancer cells. [34] Some subjects’ disease partially responded to this treatment. Another approach has been to inject the class I major histocompatability complex (MHC) molecule into tumors to incite a brisk immune response. This, too, has succeeded in only a few cases.
Vaccines
Other technology that derives from our understanding of the molecular basis for human squamous cell carcinoma includes vaccines. In advanced melanoma, vaccines are made using patients' own immune cells that are selected and sensitized to the tumor and then replaced. This technique has proved promising in a number of patients. Use of various chemokines such as interleukin and tumor necrosis factor (TNF) has been attempted with some response but at the expense of often burdensome adverse effects. One other area in which multiple drugs are being developed is monoclonal antibodies. Blocking antibodies against the epidermal growth factor (EGF) receptor have been used, again, with variable effect.
Future prospects
Clearly then, the clinical translational arm of current research is in its nascent stage. As the growth in knowledge of the fundamentals of cell biology continues, understanding of head and neck squamous cell carcinoma, a complex disease, will progress. Future research will focus on deciphering how molecules and molecular events affect staging and prognosis. New tumor markers have yet to be defined. The ultimate goals include the reduction and prevention of head and neck cancer, which is possible with awareness and tobacco cessation programs, and a simple effective treatment that will lead to a favorable prognosis.
-
Head and neck squamous cell carcinoma in vitro (cell culture).
-
The cell cycle.
Tables
What would you like to print?
- Brief Overview of Molecular Biology and Cancer
- Cancer Model
- External Influences and Molecular Mechanisms
- Systemic and Local Factors in Cancer
- Cellular and Molecular Interactions
- Nuclear Factors in Head and Neck Squamous Cell Carcinoma
- Gene Therapy and the Newest Frontiers
- Show All
- Media Gallery
- References