Overview
For decades prior to its introduction into head and neck reconstruction, the gracilis muscle has been used as a local pedicled flap to restore sphincteric function in the anogenital area. Other uses of the gracilis have included coverage for moderately sized soft tissue defects in the upper and lower extremities, treatment of chronic osteomyelitis, and functional rehabilitation of the upper extremity. Although Freilinger was the first to use a free muscle graft for reanimation in facial paralysis, [1] Harii, in 1976, was the first to use the gracilis as a free tissue flap with microvascular anastomosis for facial rehabilitation. [2] In the head and neck, the main indication for gracilis free tissue transfer is for dynamic midfacial and lip reanimation for long-standing facial paralysis. [3, 4]
The gracilis muscle free flap is based on a single anatomically constant neurovascular pedicle. The muscle has an easily accessible donor site that allows a 2-team approach and has acceptable donor site morbidity. This muscle demonstrated reliable results for sustaining facial function after transfer. The gracilis muscle free flap is generally performed as a two-stage procedure: the cross-facial nerve graft is the first stage, and the second stage is the actual muscle transfer. In 1990, O'Brien was the first to report the use of the gracilis free flap for single-stage facial reanimation. [5] The revascularized gracilis muscle is one of the few muscles that has been used to attempt to achieve the most elusive of facial palsy rehabilitative goals: restoration of spontaneous emotional expression.
Indications
The gracilis free flap has been primarily used as a muscular, rather than a musculocutaneous, free flap because of the questionable consistency of musculocutaneous perforating vessels over the distal third of the muscle. The muscle itself has been shown to recover function within a few months. Concerns have been raised about the reliability of the overlying skin paddle. However, Yousif et al described excellent results in using the transverse gracilis myocutaneous flap in select patients. [6] They demonstrated that a large skin paddle can be harvested along with the muscle.
Moreover, in an anatomic study by Lykoudis et al, the cutaneous perforators were found to lie in the proximal third of the muscle. [7] In the clinical setting, Doppler is used to map the cutaneous perforators to ensure reliability. Alternatively, in settings in which cutaneous coverage is required, the gracilis free flap may serve as a recipient bed for skin grafts.
The most common indication for gracilis free flap in head and neck reconstruction is for dynamic reanimation of the midface and, occasionally, for the eye and forehead of the patient with permanent long-standing or congenital facial paralysis. [8] In these 2 types of facial paralysis, the native facial musculature is absent because of either severe atrophy or congenital causes. In cases of long-standing secondary facial paralysis, a branch of the contralateral normal facial nerve via a cross-facial nerve graft is used for neural input. [9] In cases of congenital facial paralysis, an alternative nerve graft (trigeminal or hypoglossal nerve) can be used for neural input. Less common indications include reconstruction of total or near-total glossectomy defect, repair of full-thickness scalp defects due to surgery or trauma, and soft tissue filling for surgical defects (eg, orbital exenteration). [10]
A retrospective study by Bhama et al indicated that facial reanimation of the smile can be successfully achieved using microvascular gracilis free flaps, with patients achieving improved excursion of the oral commissure and better facial symmetry, when the patient is smiling and when the mouth is at rest. The study involved 127 gracilis free flaps. [11]
A literature review by Roy et al reported that in patients with facial palsy who underwent free gracilis flap surgery, smile excursion improved by a mean change of 7.5 mm, with pooled proportions of flap failures being 2.9%. [12]
Another report by Roy et al, a retrospective study, determined that in 107 patients with Möbius syndrome who underwent free segmental gracilis muscle transfer for microneurovascular repair (197 procedures), surgeries involving innervation via the motor nerve branch to the masseter resulted in average commissure excursion improvements of 4.61 mm and 9.34 mm for bilateral and unilateral reconstructions, respectively. [13]
A retrospective study by Nicoli et al indicated that gracilis free flap procedures provide an effective means of reconstruction following orbital exenteration, offering a relatively large volume of well-vascularized tissue and better flexibility of placement. The study, which involved nine patients, found no morbidity at the donor or recipient sites over the mean 23.5-month follow-up period (although one patient died during follow-up as a result of cancer metastasis). The patients and surgeons considered the surgery’s cosmetic results acceptable. [14]
Anatomy
The gracilis is a long, thin, straplike muscle lying on the medial aspect of the thigh that measures approximately 25 cm in length and 6 cm proximally to 4 cm distally in width. The gracilis functions as a thigh adductor and hip flexor and is a superficially located muscle of the thigh adductor muscle group, situated just posteromedial to the adductor longus. The gracilis arises from the outer surface of the inferior ramus of the pubis and adjoining ischium and inserts into the medial surface of the tibia below the condyle, contributing to the tendinous pes anserinus. The muscle is innervated by a single motor nerve, the anterior branch of the obturator nerve, which measures up to 12 cm in length. This nerve often divides into superior and inferior segments before entering the muscle, making possible the dissection of functionally discrete units within the muscle. See the images below.
The vascular supply is via a single arterial branch and 2 venae comitantes arising from the adductor branch of the profunda femoris vessels or the medial circumflex femoral vessels. This vascular supply consistently enters the upper third of the muscle on its deep surface at 9 cm below the pubic tubercle after passing between the adductor longus and the adductor brevis muscles. The vascular pedicle ranges from 5-7 cm in length with an arterial diameter of 1.5-2.5 mm. In addition to the dominant vascular pedicle, the middle and lower thirds may receive contributions from small superficial femoral vessel branches. Contrast dye studies have shown that the dominant pedicle alone can supply the entire gracilis muscle.
The flap can also be harvested as a musculocutaneous tissue. Preoperative or intraoperative Doppler is used to mark the skin perforator. Usually, one or more cutaneous perforators are in the proximal region of the muscle. The skin paddle can be fashioned longitudinally along the muscle or transversely at the proximal third of the muscle. A skin paddle up to 20 cm X 10 cm can be used with this flap.
Preoperative Evaluation
A history is obtained with specific focus on whether any prior trauma or surgery related to the upper thigh occurred. When questionable, the main vascular pedicle can be evaluated with Doppler ultrasonography or angiography. In patients with no relevant past history, no investigation is required. If a skin paddle is to be used, then the perforator can be mapped preoperatively or intraoperatively. When used as part of a staged reanimation procedure, a positive Tinel sign can confirm cross-facial nerve growth through a previously placed reversed nerve graft. This sign is considered positive when paresthesias occur as a result of tapping the preauricular nerve graft stump. Cross-facial nerve regeneration typically takes 6-9 months.
Technique
The donor site is prepared and draped in the usual fashion. Draping should include the pubic symphysis and the medial condyle of the femur. Circumferential exposure of the thigh is desirable. Premarking the patient in the standing position can aid in identifying the muscle when supine. The patient is positioned supine with the hip externally rotated. The leg is abducted, and the knee is slightly flexed.
A 10-cm longitudinal incision is made on the posterior-medial thigh 10 cm below the pubic symphysis within a line drawn between the adductor tubercle and the medial condyle of the femur. The incision can also be made about 4-5 cm below the adductor line. Dissection is performed through subcutaneous tissue to expose the muscular fascia. The neurovascular pedicle is located at the upper part (anterior) of the upper third of the muscle. Neural and vascular pedicles are dissected approximately 10 and 6 cm, respectively. Blunt finger dissection is used to free the distal muscle. A second small incision approximately 10 cm above the knee is made, and the lower part of the muscle is bluntly dissected.
After the muscle is dissected, marking sutures are placed at 1-cm intervals along its length to aid in reestablishing normal resting length and tension after transfer. Reserve a minimum of 1 cm on each end for suture placement, thus making the harvested length 2 centimeters longer than the needed functional length. Suturing the ends of the muscle with an absorbable suture in a running fashion is also helpful to prevent anchoring sutures from pulling through.
After the distal portion of the muscle has been transected, the muscle is withdrawn through the subcutaneous tunnel, and the aponeurosal attachment to the pubis is separated. Hemostasis is achieved, and after assuring adequate pedicle length, the neurovascular pedicles are transected. The muscle can be divided into 2 functioning units, if desired, for eye and midfacial reanimation, although this practice is not recommended by most surgeons.
The upper third of the muscle (6-8 cm) is the part that is typically used for facial reanimation. In most cases, thinning of the muscle is necessary to avoid excessive bulk. One method of avoiding excessive bulk or skin tethering postoperatively is to use the anterior third to half of the muscle and preserve the investing layer of fascia. For forehead reanimation, removing the investing fascia and performing multiple partial cross cuts parallel to the direction of the muscle fibers (separating the muscle bundles) can accomplish the necessary thinning and broadening that is required.
A study by Braig et al indicated that in gracilis muscle transfer for facial reanimation surgery, the ideal weight of the muscle transferred depends on whether the tissue will be innervated by a cross-facial nerve graft or by the masseteric nerve. The investigators reported that in adults, the muscle weight should be lower when the masseteric nerve is used than when a cross-facial nerve graft is employed. [15]
If the gracilis is to be harvested as a myocutaneous tissue, the skin perforator is marked with a Doppler after the leg is prepared. The perforators are usually consistently present in the proximal portion of the muscle. The skin paddle can be fashioned in a longitudinal or transverse fashion and can be as large as 10 cm X 20 cm.
The wound is closed in the standard fashion. Even if the myocutaneous flap is used, the leg can be primarily closed. When muscle is transferred to the face, reestablishing normal resting muscle tension is important to ensure maximum muscle survivability and function. Position the neurovascular pedicle on the deep aspect to avoid damage if debulking procedures are required later. A nerve stimulator may be useful for estimating transferred muscle function in situ.
Complications and Donor Site Morbidity
The gracilis muscle is an expendable muscle whose absence rarely causes lower extremity weakness. In a 1995 study of 104 cases of gracilis free tissue transfer, Carr et al reported an in-hospital donor site complication rate of less than 10%. [2] Complications consisted of local wound problems (ie, pain, infection, bleeding) and a single case of temporary sciatic nerve palsy. Early complications were more commonly noted in the pediatric age group.
Long-term donor site issues related to scar characteristics (eg, pruritus, discoloration, width, sensitivity) were reported in approximately half of the cases. Other complications include tingling, pain, and hypesthesia. Functional difficulties were reported by 26% of patients, with 15% of them reporting temporary weakness lasting an average of 6 months. Six percent of participants reported persistent weakness that interfered with running, walking, or participation in sports. No differences were noted between partial and complete gracilis harvest.
A study by Purnell et al found similar rates of donor site wound healing complications between medial and lateral thigh-based flaps (17.4% and 21.3%, respectively) but did determine that wound healing complications are higher (25.9%) for gracilis flap donor sites when a skin paddle is included with the muscle. [16]
Concerns about donor site scarring led to the description of a minimally invasive technique for gracilis harvest using an endoscopic subcutaneous dissector. Reduction in scar length of over 50% can be achieved using such a method, although widespread acceptance and proven benefit has not been shown.
Results
Lack of a consistent grading system for facial rehabilitation makes comparison between studies difficult. Among the three primary donor muscles used for facial rehabilitation (ie, gracilis, latissimus dorsi, pectoralis minor), no conclusive evidence supports the superiority of one muscle over another. The gracilis offers excellent flap survival and an acceptable complication rate. Criticisms of the gracilis flap include excessive bulk and the lack of dual innervation. Dual innervation would have the theoretical potential of allowing independent movement of different facial regions (eg, eye and midface). [17]
A mini transfer of the muscle minimizes the bulk of the muscle that is transferred. Contractile force has been shown to be sufficient for facial movement with this selective muscle transfer. This ability to transfer only a part of the gracilis muscle is an advantage that the pectoralis minor and latissimus dorsi do not have. [18]
Using a nerve stimulator to divide the gracilis into functionally independent neuromuscular units has been described. This makes possible the establishment of 2 independently functioning neuromuscular units within the face (eg, eye and midface). This capability captures one of the primary perceived benefits of the pectoralis minor free flap. Although theoretically appealing and technically feasible, such an approach has not been proven superior to oculoplastic techniques in improving eye closure and preventing complications. Also, the use of a nerve stimulator also has not been proven clinically.
When used for facial reanimation, onset of function in the transplanted muscle depends primarily on the method used. When used as the effector organ for neural input through a previously placed cross-facial nerve graft, recovery times of up to 14 months can be expected before both stages are complete and facial function begins to return. After free tissue transfer at the second stage, muscle function begins to return 20-40 weeks postoperatively. Based on electromyographic studies, continued increases in innervation and activity are expected for several years.
Several studies have evaluated voluntary muscle activation with electromyography (EMG) after microvascular muscle transfer for facial paralysis. Gracilis, latissimus dorsi, and pectoralis minor (especially in children), have demonstrated similar findings in contractile force to mimic function. Moreover, a recent study by Yla-Kotola correlates the long-term function of the grafted muscles to MRI findings of normal muscle structure and volume. [19] In their study, they showed that the muscles retained about 20% of the original volume, enough for the voluntary function to be sufficient or satisfactory in two thirds of the patients with at least a House grade 3.
Gracilis free flap facial reanimation has been shown to be effective in children without secondary disfigurement as facial growth proceeds. Notably, several studies have shown trends toward younger patients reinnervating their muscle transplants more rapidly and achieving better ultimate appearance. Compliance with requisite postoperative rehabilitation efforts is adequate in the pediatric age group.
A retrospective study by Weiss et al indicated that in free gracilis muscle transfer for facial reanimation, the masseteric nerve can reliably serve as the donor nerve in patients of all ages. The study included 46 patients, who, based on their age at surgery (13-71 years), were divided into three cohorts. Flap innervation was successful in all but one patient, in the middle-aged cohort (31-51 years). The investigators found that facial symmetry outcomes at rest and when patients were smiling or laughing were similar for all cohorts. Scores for the Facial Clinimetric Examination (FaCE) scale, which measures facial movement, facial comfort, oral function, eye comfort, lacrimal control, and social function, were similar between the middle-aged and senior (52-71 years) cohorts. [20]
Another study by Weiss and colleagues reported that in two-stage free gracilis muscle transfer employing cross-facial nerve grafting, outcomes are age related. The pediatric (5-16 years) and young adult (17-30 years) cohorts demonstrated better postoperative static and dynamic facial symmetry than did the middle-aged group (31-51 years). A significant improvement in commissure excursion on laughing was seen only in the pediatric cohort. [21]
Although gracilis transfer for facial reanimation is usually performed as a two-stage procedure, a single-stage technique has been described in the literature. This technique uses a long neural pedicle with primary contralateral facial nerve anastomosis to reduce the function recovery time to 4 months. This decreases the period of rehabilitation by 10 months when compared with the two-stage procedure. The theoretical benefit of this technique is that it avoids sural nerve donor site morbidity, matches motor nerve input with motor nerve graft, requires neural ingrowth across 1 versus 2 anastomoses, and maintains vascularity of the nerve graft.
Functional results with single-stage methods that use ipsilateral facial nerve remnant, when available, are superior, implying that single-stage cross-facial methods may also be functionally superior. However, long-term benefits of the single-stage technique have yet to be evaluated. In fact, with the single-stage technique, more facial swelling and resting asymmetry may be noted.
Gracilis free tissue transfer carries a high survival rate (92-97%). Likewise, reinnervation rates with functional movement after transplant are high. Gracilis free tissue transfer can be expected to result in up to 3.5 cm (average 1-1.5 cm) of oral commissure movement with demonstrated consequent improvement in self-esteem. Improvement in functional problems such as speech, drooling, and drinking are also noted. Voluntary and independent movement of the transplanted side with symmetry at rest can be expected. Regaining involuntary spontaneous and emotional movement remains elusive. Latency of activity in the transplanted muscle may be present, such that rapid response during conversation or spontaneous movements is absent or inappropriate.
Although microneurovascular free tissue transfer for facial rehabilitation has been shown to result in a greater range of motion compared with temporalis transfer, this fact does not translate into better overall aesthetic appearance as judged by blinded reviewers. Criticisms of the gracilis free flap include excessive bulk and skin tethering. These detract from aesthetic appearance in up to 43% of patients. A second operative procedure may be required in up to 60% of cases. Conservative trimming during transplantation is important in order to preserve as much muscle function as possible. Note that with temporalis transfer; however, spontaneous emotional movement is extremely difficult, if not impossible.
Patient perception of improvement in facial reanimation after gracilis transfer is encouraging. In O'Brien's 1980 series, two thirds of patients felt that the results were excellent or good with only 9% reporting dissatisfaction. [22] Objective evaluation in his series demonstrated good-to-excellent symmetry at rest in 66% of cases, but only 25% of patients achieved good symmetry when smiling. In patients with split transfer to rehabilitate the eye, 40% of patients achieved acceptable eye closure. After his experience with free gracilis transfer to rehabilitate the eye, in 1990 O'Brien concluded that gold weight implants were superior for eye closure. [5] Overall, results were graded excellent-to-good in 51% of cases and fair in 40% of cases. A trend toward better results in cases of incomplete paralysis was statistically insignificant. Results tended to be better in the lower half of the face.
In another series of 63 patients who underwent gracilis transplantation, 94% of patients improved after free tissue facial reanimation; 80% achieved a moderate or better result overall.
A study by Park et al indicated that in patients who have undergone hemiglossectomy, tongue reconstruction using conjoined radial forearm/gracilis flaps is more effective than repair with radial forearm or gracilis flaps alone. Patients who received the conjoined flaps demonstrated better protrusion, elevation, and lateralization (on both sides) than did the gracilis-only patients, as well as greater elevation and defect-side lateralization than did the radial forearm–only patients. Moreover, in the conjoined-flap patients, articulation, intelligence, and dysphagia outcomes were superior to results in patients reconstructed with radial forearm or gracilis flaps only. [23]
Conclusion
The primary use of gracilis free tissue transfer in the head and neck region is in the form of a muscular free flap for the dynamic rehabilitation of long-standing permanent facial paralysis. When combined with cross-facial nerve grafting or used as a single-stage reconstruction, free tissue transfer offers the best prospect for restoring spontaneous emotional facial expression. Benefits of this muscle over other free flaps used for dynamic facial reanimation include consistent anatomy with large caliber vessels, ease of harvest, a 2-team approach, reliability, and acceptable donor site morbidity. Drawbacks include excessive bulk, skin tethering, and a donor site scar that may be minimized with minimally invasive techniques. Secondary procedures to refine the results are often necessary to achieve a good final result. Ultimately, the choice of muscle for dynamic facial reanimation depends on the surgeon's experience and comfort level.
-
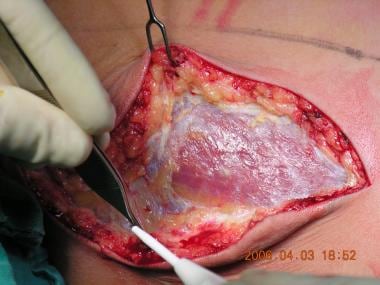
Subcutaneous dissection to the muscular fascia.
-
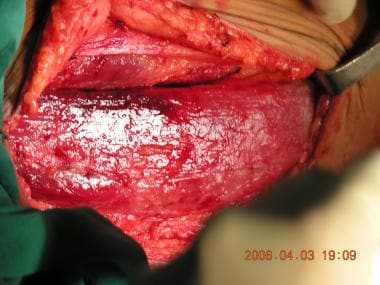
The gracilis muscle dissected; the proximal half is shown.
-
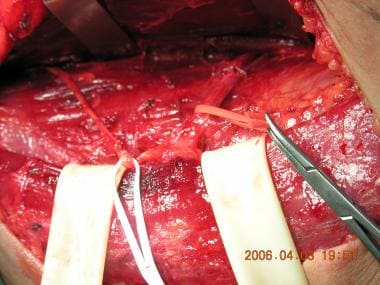
The neurovascular bundle is dissected.
-
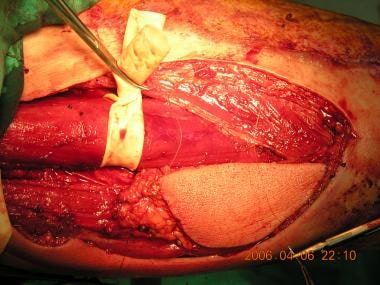
Skin paddle over the gracilis muscle is harvested to be used as a musculocutaneous flap.