Overview
Perineural spread of a tumor, or spread of tumor along a nerve, is one of the more insidious forms of tumor growth. [1] This form of spread is more commonly found in malignant rather than benign lesions, and its presence is considered a marker for poor prognosis and decreased survival rates.
Malignancies can disseminate to distant sites via several different routes. The 2 most common routes are via lymphatic and venous structures. Although our understanding of the pathogenesis of perineural spread has evolved over the last century, the exact mechanism of perineural spread is still poorly defined. The process was initially thought to occur via spread through lymphatics within the nerve sheath, but this concept was rejected after the discovery that lymphatic channels do not penetrate the epineurium. The most accepted current theory is that nerves provide a pathway of least resistance for tumor growth.
Current research suggests that tumors play a more active role in perineural invasion than previously thought. The neural cell adhesion molecule (NCAM) is an immunoglobulin that has several functions (including adhesion, proliferation, and migration of neural cells) and is thought to play a role in perineural invasion. [2] Several studies have shown that the NCAM is expressed in a large percentage of patients with adenoid cystic carcinoma, although its role in perineural invasion associated with squamous cell carcinoma (SCC) is not as well defined. Other studies have shown that a wide variety of neurotrophic growth factors and matrix metalloproteinases are also expressed in cancers that exhibit perineural spread.
A case study by Fukai et al of a patient with perineural spread of adenoid cystic carcinoma along the mandibular nerve suggested that progression of this lesion is associated with elevated expression of ephrin type-A receptor 2 and a transition of the tumor cells from an epithelial to a mesenchymal phenotype. [3]
Perineural spread is a well-recognized phenomenon in head and neck cancers. SCCs are the most frequent neoplasms to exhibit this behavior, followed by adenoid cystic carcinoma (ACC), lymphoma, and rhabdomyosarcoma. Because of their extensive and intricate network of nerve fibers within the head and neck, the trigeminal and facial nerves are the nerves most commonly affected. In addition, these nerves have various interconnections between them that serve as a mechanism for widespread dissemination.
The image below depicts the pertinent nerve branches related to the trigeminal nerve and the pterygopalatine fossa.
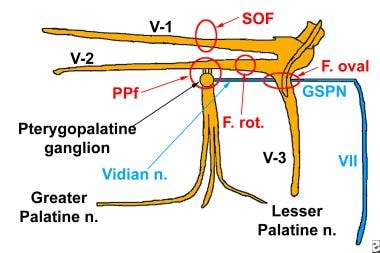 Perineural spread of tumor along the fifth and seventh cranial nerves. This pictorial diagram lists the pertinent nerve branches related to the trigeminal nerve and the pterygopalatine fossa, as well as the facial nerve and its relation to the pterygopalatine fossa.
Perineural spread of tumor along the fifth and seventh cranial nerves. This pictorial diagram lists the pertinent nerve branches related to the trigeminal nerve and the pterygopalatine fossa, as well as the facial nerve and its relation to the pterygopalatine fossa.
Perineural spread has a wide variety of clinical manifestations. Patients can experience pain, burning, or dysethetic sensations following the course of the nerve involved. With advanced disease, complete denervation can lead to muscle atrophy. However, note that up to 40% of patients with radiographic or histological perineural invasion are completely asymptomatic. This aspect of the disease highlights the need for appropriate imaging studies in the management of head and neck cancer.
Computed tomography (CT) scanning and magnetic resonance imaging (MRI) play complementary roles in assessment of perineural tumor spread. CT scans are superior to MRI for evaluating bony changes, which is important because a substantial portion of the trigeminal and facial nerves are surrounded by, or contained within, bony structures. Bony destruction may be inferred from MRI by soft tissue tumor within the expected areas of bony canals or channels. MRI is superior to CT scanning in evaluation of soft tissue tumors and in its ability to discriminate between normal and tumor tissue. MRI also provides a more accurate assessment of intracranial spread to the Meckel cave, the cavernous sinus, the cisternal portion of the trigeminal nerve, and the facial nerve in the internal auditory canal (IAC) or cerebellopontine angle (CPA).
Treatment guidelines for patients with perineural spread have not been established. Patients typically undergo surgery followed by radiation therapy (RT). Most of the work in this field has been done studying squamous cell carcinoma (SCC) of the skin. One large series of 135 patients with skin cancer in the head and neck divided patients into those with incidental finding of perineural invasion, versus those with clinical findings. After treatment with surgery and radiation, local control rates were 87% for those with an incidental finding and 55% for those with clinical symptoms.
In a study of 45 patients with perineural spread of head and neck cutaneous SCC, Phung et al found the estimated 5-year overall and disease-free survival rates to be 45% and 26%, respectively. Median overall, disease-specific, and disease-free survival periods were 4.5 years, 5.1 years, and 1.7 years, respectively. In 66.7% of cases, spread occurred along the trigeminal nerve alone, most commonly the maxillary branch. The next-most-frequent number of cases involved the facial nerve alone (28.9%), with two patients (4.4%) demonstrating spread along both the trigeminal and facial nerves. [4]
Another issue to consider is the treatment of uninvolved nerves in patients with confirmed perineural invasion. One study pointed out that in its series of 12 patients, 11 patients had concomitant disease in cranial nerve V and cranial nerve VII. This was a major reason for recurrence. More work needs to be done to elucidate the most common patterns of spread to maximize treatment outcomes while minimizing the effects of radiation.
Anatomy
Pterygopalatine fossa
The pterygopalatine fossa is a small space just behind the maxillary sinus and in front of the pterygoid plates of the sphenoid bone. The fossa is bounded anteriorly by the infratemporal surface of the maxillary sinus and posteriorly by the base of the pterygoid process and the anterior surface of the great wing of the sphenoid bone. The superior boundary is made up of the inferior surface of the body of the sphenoid and the orbital process of the palatine bone. The medial border is the vertical aspect of the palatine bone and the lateral border is the pterygomaxillary fissure. [5]
An important feature of the pterygopalatine fossa is the many connections that it makes to other areas of the cranium. The fossa communicates with the orbit, nasal cavity, and infratemporal fossa via the inferior orbital fissure, sphenopalatine foramen, and pterygomaxillary fissure, respectively. In addition, 5 foramen exist: the sphenopalatine canal medially, the pterygopalatine canal inferiorly, and 3 from the posterior wall. The posterior foramens are the foramen rotundum, pterygoid canal, and pharyngeal canal. The former is unique in that it cannot be visualized with either CT or MRI.
The maxillary nerve, which is the second branch of the trigeminal nerve, traverses the fossa and sends many branches through its foramen. Also within the fossa is the sphenopalatine ganglion, or ganglion of Meckel, and the third section of the maxillary artery.
Trigeminal nerve
The trigeminal nerve is the largest cranial nerve and has 3 major branches: the ophthalmic, the maxillary, and the mandibular nerves.
The ophthalmic nerve exits from the trigeminal ganglion, which is contained within the Meckel cave, and continues forward within the walls of the cavernous sinus. From the walls of the cavernous sinus, the ophthalmic nerve exits through the superior orbital fissure along the roof of the orbit as the supraorbital nerve. It then exits from a notch along the superior orbital rim to supply sensation to the skin of the forehead.
The second division of the trigeminal nerve, the maxillary nerve, also exits from the Meckel cave to travel within the walls of the cavernous sinus just below the first division. From the cavernous sinus, the maxillary nerve exits the skull base through the foramen rotundum, which courses along the inferior edge of the lateral walls of the sphenoid sinus and joins the pterygopalatine fossa along its posterior margin.
The infraorbital nerve exits from the infraorbital canal just below the infraorbital rim to supply sensation to the skin of the cheek, lower eyelid, upper lip, and side of the nose. The sphenopalatine ganglion gives off the lesser and greater palatine nerves, which traverse similarly named foramen, and the nasopalatine nerve that exits via the sphenopalatine foramen. The greater palatine nerve supplies the hard palate, and the lesser palatine nerve supplies the soft palate, uvula, and tonsil. The posterior superior alveolar nerve leaves the maxillary nerve just before it enters the infraorbital groove and courses through the lateral wall of the maxillary sinus.
The third division of the trigeminal nerve, the mandibular nerve, has both motor and sensory divisions. It exits directly from the floor of the Meckel cave through the foramen ovale and then travels along the medial aspect of the masticator space giving off branches to the masseter, temporalis, medial, and lateral pterygoid, tensor veli palatini, and tensor tympani muscles. The mandibular division also gives off the auriculotemporal nerve, which passes through the parotid gland to supply sensation to the temporal scalp, and the lingual nerve, which courses to the tongue to supply sensation. The mandibular division continues as the inferior alveolar nerve and enters the mandibular canal through the mandibular foramen. It continues in the mandibular canal to the mental foramen, where branches exit to supply sensation to the skin of the chin and the lower lip.
Facial nerve
The seventh cranial nerve has several functions in the head and neck. It provides innervation to the muscles of facial expression, general sensory innervation to the ear, special sensory innervation to the tongue, and autonomic innervation of the salivary glands. After the facial nerve exits from the pons in the cerebellopontine angle, it enters the internal auditory canal (IAC). From the IAC, the facial nerve courses forward and in a lateral direction through the fallopian canal until it joins the geniculate ganglion, located along the anterior petrosal ridge near the dehiscence for the greater superficial petrosal nerve. From the geniculate ganglion, the facial nerve takes a hairpin turn and extends in a posterior direction to course posteriorly along the medial wall of the middle ear cavity just above the oval window.
At the posterior end of the middle ear cavity, the facial nerve takes another right-angle turn to descend in the vertical facial canal to exit eventually at the stylomastoid foramen at the skull base. From the stylomastoid foramen, the facial nerve descends into and splits the parotid gland along its long axis gland into a deep and superficial portion. All of the major trunks of the facial nerve branch out within the substance of the parotid gland.
Along its course, the facial nerve gives off several branches. The greater superficial petrosal nerve exits from the geniculate ganglion and courses along the floor of the temporal fossa in a groove extending forward below the Meckel cave and lateral to the carotid canal. The sympathetic plexus surrounding the carotid artery gives off a branch, the deep petrosal nerve, which then joins the greater superficial petrosal nerve to form the Vidian nerve, which then enters the Vidian canal. The Vidian canal is located inferior and medial to the foramen rotundum. If the skull base is extensively pneumatized, the Vidian canal may appear as a bony ridge floating up from the floor of the sphenoid sinus. The Vidian nerve terminates at the sphenopalatine ganglion in the pterygopalatine fossa.
In the middle ear cavity, the facial nerve gives off a small branch to the stapedius muscle, and within the vertical facial canal, the chorda tympani. The chorda tympani then ascends to the middle ear cavity to exit via the petrotympanic fissure and descends in the masticator space to join the lingual nerve.
Despite the numerous superficial branches of the facial nerve to the face, no reports exist to date of facial nerve perineural tumor spread from superficial skin cancers accessing the numerous branches of the muscles of facial expression. Therefore, this article does not discuss the superficial branches distal to the parotid gland.
Communications between the nerves
The trigeminal and facial nerves directly communicate in 3 locations. First, the sphenopalatine ganglion forms a junction between the Vidian nerve and branches of the maxillary nerve. The Vidian is derived from the greater superficial petrosal nerve, which is a branch of the facial nerve. Another branch of the facial nerve, the chorda tympani, directly joins the lingual nerve, a division of the mandibular nerve. Third, the auriculotemporal branch of the mandibular nerve crosses through the body of the parotid gland at right angles to the facial nerve and, here, usually has direct communications with the facial nerve.
Additionally, the bony channels and fissures that surround the branches of the trigeminal nerve communicate and allow spread of neoplasm from one division of the trigeminal nerve to another. Potential communication exists between the first and second divisions of the trigeminal nerve at the orbital apex, where they are in close proximity to each other after passing through the superior orbital fissure. The inferior orbital fissure joins the pterygopalatine fossa and allows potential spread of tumor from the ophthalmic nerve in the orbit to the maxillary nerve in the pterygopalatine fossa. The orbit also communicates with the masticator space through the inferior orbital fissure. Thus, a lesion from the ophthalmic nerve or the maxillary nerve may spread directly to the mandibular nerve. The direct lateral communication of the pterygopalatine fossa through the pterygomaxillary fissure to the masticator space allows potential spread of neoplasm between the maxillary and mandibular nerves.
All of these connections are important because they serve as channels for perineural tumor spread. Once tumor has reached the pterygopalatine fossa or the foramen rotundum, it can spread into the cavernous sinus, into the Meckel cave, and eventually along the cisternal portion of the trigeminal ganglion into the lateral aspect of the pons. Likewise, from the pterygopalatine fossa through the Vidian canal, tumor can spread to the petrous bone to involve the facial nerve. Knowledge of these connections allows physicians to predict where tumor may eventually spread.
Tumor Spread Along the Trigeminal Nerve
Several different pathways exist which allow tumors to spread from one branch of the trigeminal nerve to the other. As mentioned above, tumors that gain access to the pterygopalatine fossa can spread to different branches of the trigeminal nerve and even the facial nerve.
Cutaneous malignancies of the face can spread centrally via sensory branches of the trigeminal nerves. Superficial lesions of the forehead that access the supraorbital nerve can affect the ophthalmic division of the trigeminal nerve. Tumors originating in the ethmoid and frontal sinuses, along with those from the lacrimal gland, can also spread via the ophthalmic nerve.
The maxillary branch of the trigeminal is primarily affected by tumors originating in the nasopharyngeal region. Tumor can access the sphenopalatine foramen located posterior to the nasal cavity near the roof of the nasopharynx and close to the sphenoethmoid recess. From the sphenopalatine foramen, tumor can spread to the pterygopalatine fossa. Theoretically, oropharyngeal tumor can access the pharyngeal canal, which joins directly to the pterygopalatine fossa. However, to date, no reports of perineural tumor spread by this route exist. Oropharyngeal tumors can also spread in the superficial mucosal space to the nasopharynx and again access the pterygopalatine fossa via the sphenopalatine foramen.
Squamous cell carcinoma (SCC) of the maxillary sinus has multiple routes that allow cancers to access to the pterygopalatine fossa. Invasion of the lateral walls allow access to the superior alveolar nerve, which originates from pterygopalatine ganglion in the pterygopalatine fossa. The most direct route would be penetration of the posterior wall, which would place the cancer directly within the pterygopalatine fossa. In addition, tumor from the maxillary sinus can exit via various draining ostia into the nasal cavity and then spread to the nasopharynx. From the nasopharynx, tumor can then enter the pterygopalatine fossa via the sphenopalatine foramen.
The mandibular division is often affected by masses in the masticator space. Lesions found here are usually primary tumors from another location, most commonly nasopharyngeal tumors. Access is from the pterygomaxillary fissure. However, primary tumors of the muscle, such as rhabdomyosarcoma, lymphoma, and (rarely) metastatic tumor, can arise within the masticator space. Orbital tumors can also directly spread to the masticator space via the inferior orbital fissure. Because the masticator space contains the main portion of the mandibular nerve, which ascends along the medial margins of the masticator space, tumors can ascend to the foramen ovale, then to the Meckel cave, and then along the cisternal portion of the trigeminal ganglion into the lateral aspect of the pons. Tumors from the lower lip, floor of mouth, and chin region can spread along the inferior alveolar nerve.
Lesions in the parotid gland can spread via the auriculotemporal nerve to the mandibular nerve and thus up to the foramen ovale. [6] Cancers that invade the mandible can spread via the inferior alveolar nerve to the foramen ovale. Tumors of the hard and soft palate can access the greater and lesser palatine nerves. From these nerves, tumor can ascend to the pterygopalatine fossa.
Peripheral spread is less common. Lesions that involve the pterygopalatine fossa can spread into the cheek region along the infraorbital groove or into the orbit via the inferior orbital fissure. Tumors such as meningioma may involve the cisternal portion of the trigeminal nerve and may spread antegrade into the Meckel cave and then to either the pterygopalatine fossa via the Vidian canal or downward through the foramen ovale to the masticator space. Tumors may exit the pterygopalatine fossa to the cavernous sinus via the foramen rotundum or to the greater superficial petrosal nerve via the Vidian canal.
From the cavernous sinus, tumors can spread to the Meckel cave and then along the cisternal portion of the trigeminal nerve to the lateral aspect of the pons or the trigeminal nuclei. From the greater superficial petrosal nerve and geniculate ganglion, tumors can spread either to the IAC/CPA region or into the middle ear cavity and eventually down the vertical facial nerve canal to the stylomastoid foramen. To date, no report exists of antegrade spread down the pharyngeal canal to the oropharynx.
Tumor Spread Along the Facial Nerve
The primary access point to the facial nerve is at the stylomastoid foramen at the skull base. Thus, the most common tumors to access the facial nerve are squamous cell carcinomas (SCCs) of the external ear/skin or tumors of the parotid gland. The most common lesion of the parotid gland to spread in a perineural fashion is adenoid cystic carcinoma, followed by acinic and mucoepidermoid carcinoma. Once in the vertical facial canal, tumor can then ascend to the middle ear cavity and exit either via the eustachian tube or out from the geniculate ganglion along the greater superficial petrosal nerve, which becomes the Vidian nerve. Thus, tumor accessing the facial nerve could spread to the pterygopalatine fossa along the Vidian nerve and thus the pterygopalatine ganglion of the maxillary nerve.
Tumors can also follow the course of the facial nerve past the geniculate ganglion to the fallopian canal and exit from the IAC into the CPA region and eventually into the cranial nuclei of the facial nerve within the medulla. Very rarely, tumor within the temporal fossa can access the geniculate ganglion through the hiatus along the anterior petrosal ridge from which the greater superficial nerve exits. From the geniculate ganglion, tumor can then access the middle ear cavity and even extend down the vertical portion of the facial canal.
Tumors arising within the petrous bone involving the facial nerve can spread antegrade along the greater superficial petrosal nerve and then the Vidian nerve to the pterygopalatine fossa and its ganglion.
Adenoid cystic carcinoma (ACC) is a relatively uncommon malignancy in the head and neck but has a well-documented propensity for perineural invasion. [7] It generally has a very slow growing, indolent course with frequent recurrences and late metastasis. It is found more often in minor salivary glands than major, although the latter has a higher association with perineural invasion. This has been attributed to proximity of nerves to the major salivary glands. Interestingly, studies have shown that perineural invasion does not always portend a worse prognosis in ACC. It did, however, correlate with positive margin status, and therefore higher recurrence rates. Controversy also exists regarding the correlation of perineural invasion and distant metastasis. ACC in the nasosinal region has the worst prognosis due to late presentation and difficult surgical anatomy.
Depending on the reported series, the facial nerve will be involved in 50% of the cases when the pterygopalatine fossa is involved by tumor. Again, the 2 most important connections between the trigeminal and facial nerves are the Vidian nerve joining the second division of the trigeminal nerve to the greater superficial petrosal nerve of the facial nerve and the parotid gland joining the third division of the trigeminal nerve to the facial nerve. Another potential communication could be from the facial nerve via the chorda tympani branch of the facial nerve to the lingual nerve, which arises from the third division of the trigeminal nerve. Therefore, if perineural tumor spread involving the trigeminal nerve is noted, careful examination of the facial nerve should be performed for signs of tumor spread.
Imaging Approach to the Evaluation of Perineural Tumor Spread
Perineural invasion is often asymptomatic, heralding the importance of careful radiologic evaluation in cases in which it is suspected. [8] A recent study that retrospectively analyzed films of patients with histologically confirmed cases of perineural invasion highlighted this concept. Of the 38 patients included in the study, only one report specifically mentioned perineural invasion, with an additional 4 documenting signs of perineural invasion without specifically mentioning it. Imaging from 30 (78%) patients in whom invasion could be seen on retrospective analysis had no mention in preoperative reports. Also of note, the remaining 8 patients had no evidence of perineural invasion on imaging, even with histological conformation.
CT and MRI play complementary roles in the evaluation of perineural spread, but MRI has become the modality of choice for detection because of its accuracy in differentiating normal tissue from tumor. The sensitivity of MRI in detecting perineural spread has been reported to be 95%. Postcontrast T1-weighted images provide valuation information regarding the presence or absence of fat surrounding the foramen. T2-weighted images are also useful in differentiating inflammatory changes verses bony destruction by a mass, as the former will light up on T2. For example, perineural tumor spread to the mastoid may be confused with benign inflammatory changes that may occur if the eustachian tube is obstructed. In this situation, MRI would be useful in differentiating inflammatory changes from neoplasm.
Examples of direct evidence of perineural spread on MRI would be enlargement and enhancement along the course of a cranial nerve. As mentioned above, fat is generally present just after a nerve exits a foramen. Obliteration of these fat pads is a key element in detecting perineural spread. Evaluation of certain foramen and areas should be routine in evaluating head and neck malignancies. These include the supraorbital foramen, pterygopalatine fossa, foramen rotundum, Vidian canal, palatine foramen, foramen ovale, mandibular foramen, and stylomastoid foramen. Another radiographic finding is the replacement of CSF in the Meckel cave with enhancing soft tissue. Tumors in the lateral aspect of the pons should also raise suspicion of perineural tumor spread.
Indirect findings can also be detected on MRI images. As mentioned above, muscle denervation can occur in advance cases. This presents as increased signal uptake on T2-weighted images, as well as contrast enhancement. Denervated muscles can also appear swollen, and if longstanding, muscle can be replaced by fatty tissue.
A final nuance in the evaluation for perineural spread on is the presence of a venous plexus along the course of the facial nerve, specifically at the anterior genu, tympanic, and mastoid segments. This is important as it can often be mistaken for neoplasm or perineural invasion.
Although MRI has become the standard for evaluation of perineural invasion, CT has a role as well. CT is less expensive, and is less dependent on patient cooperation. Evidence of perineural invasion on CT is largely dependent on its superior detail of bony structures. Examples of findings include bony expansion of canals, fissures, and foramina or even frank destruction of these structures by soft tissue mass.
-
Perineural spread of tumor along the fifth and seventh cranial nerves. This pictorial diagram lists the pertinent nerve branches related to the trigeminal nerve and the pterygopalatine fossa, as well as the facial nerve and its relation to the pterygopalatine fossa.
-
Perineural spread of tumor along the fifth and seventh cranial nerves. This diagram depicts the bony channels that allow communication with the pterygopalatine fossa.
-
Perineural spread of tumor along the fifth and seventh cranial nerves. This diagram shows that the pterygopalatine fossa is a very small space located posterior to the maxillary sinus and anterior to the pterygoid plates.
-
Perineural spread of tumor along the fifth and seventh cranial nerves (CNs). This diagram demonstrates the communication of the pterygopalatine fossa with the masticator space through the pterygomaxillary fissure (PTMf) in axial projection as on a CT scan. The relations of the branches of CN V with the adjacent visceral spaces in the face region are also shown.
-
Perineural spread of tumor along the fifth and seventh cranial nerves. This is an axial CT scan of a patient with biopsy-proven squamous cell carcinoma of the left nasopharynx. The soft tissue tumor within the left sphenopalatine foramen (SPf) is enlarging it as well as enlarging the adjacent pterygopalatine fossa (PPf).
-
Perineural spread of tumor along the fifth and seventh cranial nerves. This patient has squamous cell carcinoma (SCC) of the nasopharynx that has accessed the pterygopalatine fossa (PPf) via the sphenopalatine foramen (SPf). The neoplasm has spread to the left masticator space through the pterygopalatine fossa and along the Vidian canal into the left temporal bone to involve the facial nerve. This contrast-enhanced tumor is of low signal on T2-weighted images (not shown), confirming that this is SCC rather than secondary mastoid inflammatory changes due to obstruction of the eustachian tube. Spread of tumor has also occurred, with destruction of the left half of the clivus. Compare the normal right pterygopalatine fossa, which is a thin space behind the maxillary sinus and is filled with fatty tissue. Note that the entire left temporal fossa exhibits abnormal contrast-enhanced tumor. This is most likely due to tumor accessing the foramen spinosum along with the middle meningeal artery, which is located in its entirety lateral to the foramen ovale. This is not perineural tumor spread, but it does result in involvement of the entire inferior temporal fossa dura. Axial postcontrast T1-weighted image.
-
Perineural spread of tumor along the fifth and seventh cranial nerves. This is the same patient as in Image 6 but at a higher level of a T1-weighted postcontrast MRI. Tumor has spread from the highest portion of the pterygopalatine fossa into the cavernous sinus on the left side, which is quite enlarged compared to the normal right side. From the cavernous sinus, the tumor has spread along the cisternal portion of the trigeminal ganglion into the lateral pons. This spread along the cisternal portion is true perineural tumor spread, whereas that within the pterygopalatine fossa is also perineural tumor spread but could be a pathway of least resistance in a bony canal.
-
Perineural spread of tumor along the fifth and seventh cranial nerves (CNs). This depicts the same patient as in Image 6. This is a sagittal T1-weighted postcontrast MRI through the temporal bone showing perineural tumor spread from the nasopharynx to the pterygopalatine fossa and to the Vidian canal. Further spread of tumor is along the horizontal and vertical portions of the facial nerve (CN VII)—the so-called walking cane appearance. In addition, tumor is also present within the left mastoid and temporal bones.
-
Bilateral nasopharynx squamous cell carcinoma with invasion of the left half of the clivus appears on this axial T1-weighted postcontrast MRI. There is also soft tissue tumor within the bilateral Meckel cave, which normally are of cerebrospinal fluid signal rather than this soft-tissue signal. There is also perineural tumor spread along the right Vidian nerve (short yellow arrows) back to the geniculate ganglion of the petrosal portion of the facial nerve (CN VII).
-
Perineural spread of tumor along the fifth and seventh cranial nerves (CNs). This is an axial T1-weighted postcontrast MRI of a patient with known squamous cell carcinoma (SCC) of the right ear which has accessed the right facial nerve (CN VII) via the stylomastoid foramen at the skull base and has spread along the vertical segment of the facial nerve up to the petrous apex and into the middle ear cavity and anterior to the geniculate ganglion with spread beyond the anterior margin of the left petrous ridge to the Vidian nerve.






