Overview
Overview
Nasal physiology is greatly dependent on the physical structure of the nose. Seemingly individual aspects of the nasal cavity collectively affect nasal function. This article reviews pertinent basic nasal anatomy, nasal physiology, and objective measurements of nasal airflow (see image below). An overview of topics relevant to nasal airflow when performing rhinoplasty is also included.
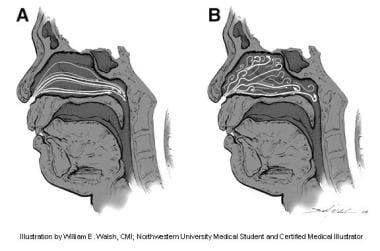 (A) Laminar nasal airflow at a low inspiratory flow rate. (B) Turbulent nasal airflow at a higher inspiratory flow rate. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
(A) Laminar nasal airflow at a low inspiratory flow rate. (B) Turbulent nasal airflow at a higher inspiratory flow rate. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
Anatomy
External nasal anatomy can best be considered in structural thirds. The upper third includes the paired nasal bones. The middle third is composed of the stiff paired upper lateral cartilages fused to the septal cartilage in the midline. The lower third of the nose consists of softer lower lateral cartilages. From a functional standpoint, the lower and middle thirds of the nose play an important role in the nasal valve. The external nasal valve is defined laterally by the nasal ala and medially by the septum, whereas the internal valve is defined by the attachment of the upper lateral cartilage to the septum, which forms an angle of approximately 15°. [1] The anterior face of the inferior turbinate defines the lower boundary of the internal nasal valve. The entrance of the nasal cavity consists of the piriform aperture, which is defined as the frontal process of the maxillary bone, floor of the nose, and lateral fibrofatty tissue.
Physiologic function of the nose
The nasal physiologic functions, such as warming and humidification, are vital for upper airway function. An adult inspires up to an estimated 10,000 liters of air daily. [1] Filtration of environmental particles occurs first in the nasal cavity. The largest particles are filtered by vibrissae. Nasal resistance is an important factor in considering airway resistance, contributing up to half of the total airway resistance observed in adults. [1] Through heat exchange, the nasal mucosa maintains the nasal cavity at a range of 31–37° C. [1]
One theory regarding the efficiency of heat exchange relates to the location of the sphenopalatine artery. This vessel courses anteriorly in the nasal cavity over the turbinates, whereas air flows in a posterior direction, forming a countercurrent exchange. [1] Thus, the two opposing motions create a more efficient heat exchange process. (Lending further support to this theory, computational simulation of gradual inferior turbinate resection demonstrates a stepwise reduction in air-warming capacity. [2] ) However, this process remains imperfect, and as much as 10% of heat loss occurs in the nose.
Humidification is another important process of nasal physiology. [1] Vascular mucosa increases relative humidity to 95% before air reaches the nasopharynx. Physiologic nasal fluids and ciliary function are vital to maintain immune defense through normal mucociliary flow. A number of nasal neurovascular reflexes occur as well. The nasopulmonary reflex suggests that pressure on one nasal sidewall causes ipsilateral pulmonary congestion. [1]
The nose may serve as a contributing factor in voice modification. Previous authors have noted that nasal aerodynamics may have a role in modifying high-frequency sounds and consonants. [1] Such aerodynamics also contribute to the olfactory system. [3] The active process of sniffing allows environmental particles to reach the olfactory system, which is located at the skull base. Moreover, in-vivo models have demonstrated that the frequency of sniffing can attenuate scents, acting as a rheostat to modulate smell. [4]
A study by Li et al indicated that a larger airflow vortex—its size likely influenced by the width-to-height ratio of the external nose and the nasal vestibule notch index—improves olfactory sensitivity to odors with high mucosal solubility. In addition, according to the report, a narrower vestibule region appears to intensify the airflow vortex toward the olfactory region, further contributing to such sensitivity. [3]
Ciliary flow is a vital component of normal sinonasal function. [5] The ciliary structure in the nose, consisting of two layers, provides an important immunologic defense mechanism. Resting on a pseudostratified ciliated cell layer, mucociliary flow occurs throughout the nose and paranasal sinuses. [6]
The nasal cycle is an additional feature of normal nasal physiology. This cycle causes turbinate hypertrophy to periodically alternate airflow between the two sides of the nose, resulting in intermittent unilateral obstruction approximately every 3 hours. Age, sleep, and posture are among the many physiologic factors that have been shown to modify the nasal cycle. [7] A computational nasal air-conditioning model developed by White et al indicated that during the nasal cycle, the airway that conducts most of the airflow, and with it, most of the transfer of heat and water mass, undergoes some airway surface liquid dehydration, while the other airway maintains enough hydration to allow continuous mucociliary clearance. [8]
Nasal Resistance
Nasal airway resistance accounts for more than 50% of total airway resistance. [6] The nasal cavity has been modeled as 2 resistors in parallel. [1, 9] The 3 components of nasal resistance are as follows: the nasal vestibule, nasal valve, and nasal cavum. [6]
The term nasal valve most often refers to the internal valve, which is the limiting region of airflow. The nasal valve is defined as the lower edge of upper lateral cartilages incorporating the anterior ends of the inferior turbinates adjacent to the nasal septum. [6] The angle between the septum and the upper lateral cartilage is 10-15°. [10] The nasal valve is usually located less than 2 cm distal in the nasal passageway, approximately 1.3 cm from the naris. The average cross-sectional area is 0.73 cm2. [6] Nasal resistance is composed of two structural elements; the first layer is composed of underlying bone, cartilage, and muscle, while the second layer consists of the overlying mucosa.
Both environmental and intrinsic factors affect nasal resistance. Factors decreasing resistance include exercise, sympathomimetics, rebreathing, atrophic rhinitis, and erect posture. [1, 11] Exercise causes sympathetic vasoconstriction and contraction of the alae nasi, increasing the capacity of the nasal passages. Rebreathing has been shown to increase arterial carbon dioxide levels, causing nasal vasoconstriction and a reduction in nasal resistance. [11] Going from a supine to an upright position decreases jugulovenous distention and nasal airway resistance. [11]
Causes of increased nasal resistance include infective rhinitis, allergic rhinitis, vasomotor rhinitis, hyperventilation, supine posture, alcohol, aspirin, and cold air. [12] In vasomotor rhinitis, vagal overactivity causes increased resistance. Nasal resistance increases markedly in the first 2-3 cm of the nasal airway. [1]
The nasal vestibule is the first component of nasal resistance. The nasal vestibule is composed of compliant walls that are liable to collapse from the negative pressures generated during inspiration. [1] The vestibule has been termed the external nasal valve. Studies have shown 30 L/min is the limiting flow during inspiration at which nasal airway collapse occurs in this area. [1] The nasal vestibule is primarily supported by alar cartilage and musculofibrous attachments. Despite the tendency, airway collapse is prevented by activation of the dilator naris muscles during inspiration. During expiration, positive pressure is the driving force for nasal vestibule dilation.
A study by Silva demonstrated that cephalic malposition of the lower lateral nasal cartilages is closely associated with external nasal valve insufficiency, finding 17 patients who presented with insufficiency among 23 cases of cephalic malposition (74%). [13]
Facial nerve paralysis can cause loss of active contraction and contribute to airway obstruction. In suspected facial nerve damage, activity of the alae nasi muscle may be tested. [14] Loss of innervation can result in alar collapse even in quiet respiration. The voluntary flaring of the naris has been attributed to a possible 20% reduction of nasal resistance, a product of facial nerve contribution to nasal airway resistance. [1] In patients with nasal alar collapse, the size of the external nasal valve can, in comparison with controls, shrink by over 40% during forced inspiration, significantly impeding nasal airflow. [15] Active dilation of the dilator naris occurs during exercise, reducing airway resistance. [16]
A major area of resistance occurs at the anterior tip of the inferior turbinate at the entrance to the piriform aperture. This important area is called the internal nasal valve. The nasal valve represents the narrowest segment of the airway. [1] In total, the valve area includes the distal end of the upper lateral cartilage, the head of the inferior turbinate, the caudal septum, the floor of the nose, the frontal process of the maxilla, the lateral fibrofatty tissue, and the piriform aperture. [9] The nasal valve area is considered as a region rather than an oblique cross-sectional area of the nasal passageway. In 1983, Haight and Cole demonstrated that the anterior end of the inferior turbinate could advance as much as 5 mm with the administration of histamine. [12]
Bachmann and Legler (1972) stated that the nasal valve area occurs at the entrance of the pyriform aperture, which corresponds to a major site of resistance anterior to the tip of the inferior turbinate. [17] The valve area is dynamic; venous erectile tissue of the turbinate and septum can cause marked obstruction. The clinical relevance of the nasal valve relates to its location. Treatment directed at the inferior turbinate will have marked effects on nasal airway resistance; trimming of the septum posterior to the valve area has fewer effects on resistance. A positive Cottle test result may signify resting narrowing of the nasal valve. A Cottle test result is considered positive if the soft tissue and nasal vestibule are lateralized, increasing the valve angle and airflow.
The nasal cavum is located posterior to the piriform aperture. Its overall contribution to total airway resistance is small. The component of nasal cavum resistance is determined by degree of vascular engorgement of tissues. Acoustic rhinometry demonstrates that the tip of the inferior turbinate narrows the airway immediately posterior to the nasal valve, while the turbinated regions of the nasal passage have relatively large cross-sectional areas. [1]
Plotting the nasal cycle, Tan et al used unilateral peak nasal inspiratory flow (UPNIF) and unilateral minimal cross-sectional area (UMCA) readings to determine airflow rates and resistance values, respectively. With the ratios between right and left UPNIF and UMCA calculated, the investigators found a directly proportional relationship between 1/resistance ratio and airflow rate ratio. The study indicated that in persons with a normal nasal cycle, data points are situated close to the regressed line, with the points occurring a significant distance from the line in patients with nasal dysfunction. [18]
A prospective cohort study by Kaura et al indicated that employment of the Nasal Obstruction Balance Index (NOBI) leads to better correlation of scores for the unilateral PNIF, acoustic rhinometry, and visual analog scale for nasal obstruction (VAS-NO). The NOBI was derived by dividing the difference between left and right nasal airway measurements by the maximum unilateral measurement. It was found to aid in predicting septal deviation by helping to identify the more obstructed side of the nose, revealing this, for example, in 92.4% of cases when used with the PNIF, post decongestant. [19]
Clinical Evaluation of the Nose
Evaluation of nasal obstruction may consist of a physical examination, rhinoscopy with adequate light and a nasal speculum, chemical analysis, imaging studies, and rhinomanometry. Chemical analysis may include neutrophil counts relating to the presence of infection, eosinophil counts in allergic conditions, and mast cell counts in food allergy. Imaging studies include CT scans and MRI. Rhinomanometric techniques include passive, active, and acoustic methods.
Flow Mechanics of Airflow
Briefly, the biomechanics of nasal resistance relate to the study of turbulent flow. Resistance is pressure (P) divided by flow (Q). Based on principles from the Poiseuille equation for laminar flow equations, a decrease in radius (for example, of the nasal airway) causes a four-fold decrease in flow (where L is length, r is radius, η is viscosity, and ρ is density). [6]
Q = (Δ P π r4)/(8 η L)
Reynolds number = 2rQ ρ/η
Mathematically, a Reynolds number greater than 2000 is equated with turbulent flow. [1] The presence of laminar or turbulent flow in the nasal passageway is pertinent to the physiology of air exchange (see image below). Inspiratory flow is generally considered as laminar flow. Inspiration lasts approximately 2 seconds and ranges from 12-18 m/s at the nasal valve area during quiet respiration. [1] Comparatively, expiratory flow has more components of turbulent flow. By disrupting the boundary between the laminar airflow and the mucosa, [20] turbulent flow facilitates the exchange of heat and moisture. Turbulent flow occurs when transnasal pressures exceed 40-80 Pa. Expiration lasts approximately 3 seconds. [1] As the turbulent air passes over the inferior and middle turbinates during expiration, there is greater contact with the nasal mucosa for heat recovery. [21] Turbulent flow requires more energy expenditure but results in better mixing, which contributes to nasal function. Turbulent flow can prevent clearance of air, which can cause a sensation of obstruction regardless of nasal passage patency.
 (A) Laminar nasal airflow at a low inspiratory flow rate. (B) Turbulent nasal airflow at a higher inspiratory flow rate. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
(A) Laminar nasal airflow at a low inspiratory flow rate. (B) Turbulent nasal airflow at a higher inspiratory flow rate. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
Nasal resistance is important for respiratory physiology. Automatic positive end-expiratory pressure (Auto-PEEP) occurs from the work that is involved in overcoming resistance during expiration. In postlaryngectomy patients, alveolar collapse is imminent with the loss of nasal airway resistance and Auto-PEEP. The glottis acts as an internal valve to regulate expiratory airflow, thus allowing alveoli to stay open longer during expiration and allowing continued gas exchange.
Rhinomanometry
Rhinomanometry has evolved in recent years as an objective measurement of airway resistance. One of the earliest methods of measuring nasal airflow consisted of moving a pressure cannula along the nasal passage to measure pressure-flow relationships. [22] Active rhinomanometry refers to flow measurement from the respiratory cycle. The 3 types of active rhinomanometry include anterior, postnasal, and posterior rhinomanometry. Passive rhinomanometry uses airflow from an extrinsic source, such as an air pump. [1]
Anterior rhinomanometry measures unilateral airflow. Both nasal passages may be measured separately. [6] Anterior rhinomanometry and acoustic rhinomanometry are among the most common methods of clinical measurement of airflow. [9]
Posterior rhinomanometry, another form of measurement, utilizes a pressure sensor in the mouth. In this method, total flow from both nasal passages can be measured together, or each nasal passage can be measured separately. One disadvantage of posterior rhinomanometry is that not all patients are able to relax the soft palate sufficiently. In addition, an oral pressure sensor may be prone to artifact due to movement in a patient's mouth. [6] A significant number of patients seem to be unable to be studied using rhinomanometry for various reasons.
The use of a facemask during rhinomanometry rather than a nasal cannula may be more accurate, as nasal cannulae may erroneously dilate the nasal airway.
Passive rhinomanometry relies on the production of airflow from an external source. With a constant pressure, the flow is measured from the nasal mask. However, it has been noted that external introduction of airflow causes "reflex-evoked changes of the thickness of nasal mucosa." [9] Accordingly, this method has found little clinical application.
Anterior rhinomanometry measures the transnasal pressure (see image below), which is the difference in pressure from the naris to the nasopharynx. In this method, a pressure probe is placed at the opening of the nostril not being tested. [9] The nasal passage acts as an extended tube and assumes the airway pressure of the nasopharynx equals the pressure at the naris of the nontested side. Total resistance can then be calculated from 2 unilateral measurements. However, anterior rhinomanometry cannot be used to measure airway resistance in patients with septal perforations.
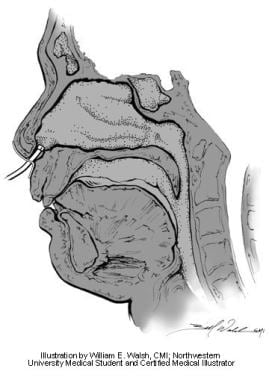 Anterior rhinomanometry. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
Anterior rhinomanometry. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
Postnasal rhinomanometry uses a pressure sensor placed along the floor of the nose into the nasopharynx (see image below). A separate pressure transducer is located at the entrance of the nasal cavity. [9] Transnasal pressure differences are then measured.
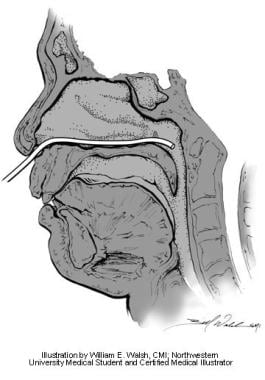 Postnasal rhinomanometry. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
Postnasal rhinomanometry. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
Posterior rhinomanometry involves placing a pressure sensor transorally into the posterior pharynx (see image below). Pressure differences from the nares to the nasopharynx are then measured. Total resistance is measured directly. In this method, patients must be coached to keep the intraoral tube in place. [6]
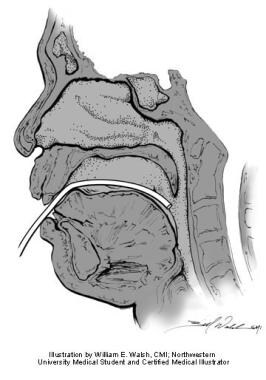 Posterior rhinomanometry. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
Posterior rhinomanometry. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
Rhinomanometry yields flow-pressure curves. Laminar flow is based on a linear relationship between flow and pressure. Although airflow increases with increased transnasal pressure, higher pressures may yield more turbulent flow secondary to the effects of airway resistance. Turbulent flow results in a limitation of flow generated despite greater transnasal pressure differences. [9] As nasal obstruction occurs, the amount of flow that can be generated plateaus sooner as a result of turbulence, despite greater pressure changes.
The clinical value of rhinomanometry relates to degree of obstruction. The 2 major types of obstruction are mucosal hypertrophy and structural deformity. Rhinomanometry is performed with and without decongestion and total resistance is calculated. Resistance above 0.3 Pa/mL/s is usually symptomatic. [6] The one caveat of rhinomanometry is that no resistance can be measured when the nasal passage is completely obstructed.
Decongestion is used to determine a mucosal cause of nasal resistance. Marked reduction in resistance with decongestion suggests mucosal disease. Decongestion causing less than a 35% decrease in resistance suggests a structural cause of nasal obstruction rather than a mucosal cause. [6]
Acoustic rhinomanometry serves as a diagnostic tool that, based on the analysis of a sound pulse generated into the nasal passages, quantifiably measures the length and area of the nasal cavity. The sound reflected from the nasal airway is transformed into an area-distance plot. The average distance of maximum constriction is within 2 cm. [9] Hilberg in 1989 and Grymer in 1991 demonstrated that cross-sectional area measurements correlated well with radiologic measurements of nasal airway constriction. [23, 24]
Acoustic rhinomanometry has been considered more accurate near the nasal valve area than areas more posterior in the nasal passage. [25] One advantage of acoustic rhinomanometry is that it provides measures of cross-sectional area along the length of the nasal passage. In contrast, rhinomanometry is based on measurements of the narrowest segment of airway. The normal average minimum cross-sectional area is 0.7 cm2 and ranges from 0.3-1.2 cm2. With decongestion, the average cross-sectional area may increase to 0.9 cm2. [1] Using acoustic rhinomanometry, the average distance from the naris to the anterior portion of the inferior turbinate is 3.3 cm, and the average distance from the naris to the posterior portion is 6.4 cm. [9]
The main use of acoustic rhinomanometry is its ability to localize areas of constriction. However, technical challenges exist. Malpositioning of the nasal tube can lead to distortion. Essentially, conventional rhinomanometry determines nasal patency, or an individual's ability to breathe. Acoustic rhinomanometry may be used for states of changing musculovascular conditions or changes in nasal valve dimensions. [9] However, the clinical usage of acoustic rhinomanometry remains guarded. Tomkinson and Eccles stated that the amount of change in cross-sectional area compared with decongestants did not correlate with change in symptom scores. [9]
Clinical Causes of Nasal Obstruction
Causes of nasal obstruction [6]
-
Turbinate hypertrophy
-
Rhinoplasty
-
Septal perforation
-
Valvular collapse
-
Neoplasm
-
Polyposis [26]
-
Septal hematoma
-
Rhinitis medicamentosa
-
Vasomotor rhinitis
Mucosal vasodilation from pathogen-induced histamine release may cause allergic nasal obstruction. Inflammation and drainage from sinusitis may contribute to obstruction. Septal deviation is a common cause of obstruction. The relative size of turbinate hypertrophy is important because 50% of airflow is in the middle portion of the airway. Turbinates adjacent to a septal perforation may hypertrophy secondary to nasal airway turbulence, causing further airway resistance. Valvular collapse secondary to insufficient cartilaginous support may cause nasal obstruction. In addition, rhinoplasty may be the significant factor in iatrogenic nasal obstruction.
Rhinoplasty and Nasal Aerodynamics
As many as 10% of patients have been identified with nasal obstruction following rhinoplasty. [28] A study by Yu et al indicated that more than 60% of individuals seeking revision rhinoplasty experience concerns regarding nasal obstruction and mouth breathing. [29] Obstructive symptoms may be due to nasal valve contraction caused by improper placement of intercartilaginous incisions. [28] Others suggest that inaccurate trimming of the upper lateral cartilage can cause protrusion of the cartilage into the nasal airway, resulting in nasal obstruction. [30] The in-office Cottle maneuver tests lateral displacement of the cheek and can help to diagnose nasal valve obstructions. Improvement of nasal airflow, a “positive” Cottle test, may suggest collapse or obstruction of the nasal valve post-rhinoplasty.
A successful rhinoplasty is both aesthetically and functionally sound. The success of a postoperative external nose is diminished when the patient is unable to breathe through his or her nose. A narrowed supratip area due to overresection of the upper lateral cartilages may cause significant nasal obstruction. [31] The periosteum of the bony nasal vault serves to hold the nasal bones in place after osteotomy. Sachs states that getting the elevation of the periosteum to be exact is crucial. Periosteal elevation should begin 2 mm above the caudal edge of the nasal bones and proceed laterally to the extent that is half the distance of the width of the remaining nasal bones after hump removal.
Osteotomies may be the most destructive portion of the rhinoplasty, so careful planning is crucial. One approach to osteotomy first focuses on the glabellar area. Sachs believes that most glabellar regions do not need narrowing; thus, osteotomies do not have to be carried to their most superior extent in the nasal-frontal suture line. Sachs also states that periosteal elevation should be minimized. Insertion of the osteotome is also important to prevent the formation of scarring of the anterior vestibule; a nasal speculum should be used to retract the nasal vestibule as laterally as possible before insertion of the osteotome along the piriform aperture. As the literature has indicated, osteotomies are relatively contraindicated in patients with short or extremely thin nasal bones. [32]
Helal et al studied the effects of internal and external osteotomies on the internal nasal valve. [33] The authors found that both types of osteotomies cause narrowing of the internal nasal valve, but neither type of osteotomy caused more narrowing than the other.
The nasal valve is an important consideration in rhinoplasty. In particular, 2 incisions have great potential to change the dynamics of the valve. The intercartilaginous incision and the disarticulation of the upper lateral cartilage from the septum have the potential to increase nasal resistance. If the upper lateral cartilages are excessively medialized, narrowing of the middle nasal vault obstructs the internal nasal valve. [34] The caudal aspect of the upper lateral cartilage should not be excessively trimmed. The nasal valve functions by moving into the vestibule as airway resistance increases. This movement is hampered when insufficient mucosa remains for coverage of the caudal aspect of the upper lateral cartilages. During alteration of the lower lateral cartilages, care should be taken not to resect too much of the most caudal aspect of the cartilage. [31] Overresection in this area leads to alar collapse and nasal obstruction.
Consistent with maintaining lower lateral cartilage support, the nasal tip may be modified by trimming the cephalic borders of the cartilage and inserting tip grafts superficial to the cartilage or by rotating the domes by lateral trimming of tip cartilages. [31] Moreover, during rotation of the dome by lateral trimming of tip cartilage, internal vestibular skin must also be trimmed appropriately to avoid airflow obstruction. The feet of the medial crura are additional factors. Sachs states that protrusion of the medial crura into the nasal airway may contribute to obstruction. [31] In addition, excessive cephalic trimming can further weaken the lateral crura and result in alar rim collapse and obstruction. [32]
Sachs writes that an important area of the septum to consider is the upper bony septum in the area of the perpendicular plate of the ethmoid. [31] Sachs states that this area may cause nasal obstruction and prevent inward displacement of the nasal bones during osteotomy. Correction of the inferior portion of the septum is also important. Rhinoplasty that includes alar base resection moves the sides of the nose medially, and the turbinates can be in contact with previous septal deflections.
Hypertrophic turbinates may also contribute to nasal obstruction after rhinoplasty; resection of the inferior turbinate may be necessary. [32, 35] While outfracture is a potential solution, submucosal turbinate resection with volume reduction is often favored. [35, 36] Septal deformities that affect the nasal valve and narrow the angle between the septum and upper lateral cartilage may cause nasal obstruction.
In addition to noted cosmetic benefits, septoplasty and septal surgery for the treatment of septal deviation are shown to produce objective benefits for nasal patency. [37] It should be noted, however, that otherwise asymptomatic septal deviations may become problematic for patients after rhinoplasty. [21] Kim and Papel note that separation of the upper lateral cartilage and septum during dorsal hump reduction may cause a destabilization of the upper lateral cartilages and increase obstruction at the internal nasal valve. [38] Tardy suggests that the upper lateral cartilages (unless deformed or asymmetrical) should be left attached to the septum in the vast majority of patients and that damage to the internal valve commonly results from separating the upper lateral cartilages from the septum. [39]
Tardy states that manipulating the lateral-most extent of the alar cartilages is seldom necessary; removal of cartilage in this area may cause dimpling and varying amounts of nasal obstruction. [39] Inaccurate lateral osteotomy and infracture can cause significant narrowing of the nasal valve. [40] Webster describes a modified approach to lateral osteotomy that preserved a portion of bone at the frontal process of the maxilla at the piriform aperture. [41]
Correction of nasal valve obstruction following rhinoplasty may include inferior turbinectomy, septoplasty, spreader or alar grafts, and nasal valvuloplasty. [42, 43] Scar excision, skin grafting, or Z-plasty may be performed to treat scarring of the nasal valve. [25] Many cases of nasal obstruction following septorhinoplasty relate to previously unrecognized structural problems, such as septal deflections, deviation of the ethmoid bone, and hypertrophied turbinates, as well as allergies. [44, 45] Patient assessments and evaluations are critical for appropriate appraisal of preoperative nasal pathology.
Prospective analyses of patients undergoing functional septorhinoplasty report significant improvement in obstructive symptoms. [46, 47, 48] Similarly, aesthetic rhinoplasty may routinely improve respiratory function and does not necessarily have deleterious effects on the nasal airway. [49]
In conclusion, the ability to alter the physical structure of the nose has notable potential for changing a patient's nasal airflow. Care must be taken to consider the dynamics of the nasal airway and preserve its function.
Questions & Answers
Overview
What is significance of nasal aerodynamics?
What is external nasal anatomy?
What are the physiologic functions of the nose?
What is the role of airway resistance in nasal aerodynamics?
How is nasal obstruction evaluated?
What are the flow mechanics of nasal aerodynamics?
What is the clinical value of rhinomanometry?
What is acoustic rhinomanometry?
What causes of nasal obstruction?
What causes nasal obstruction following rhinoplasty?
What is the role of the nasal valve in nasal aerodynamics?
What is the role of the nasal tip in nasal aerodynamics?
What is the role of the septum in nasal aerodynamics?
How is nasal obstruction caused by rhinoplasty corrected?
-
(A) Laminar nasal airflow at a low inspiratory flow rate. (B) Turbulent nasal airflow at a higher inspiratory flow rate. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
-
Anterior rhinomanometry. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
-
Postnasal rhinomanometry. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.
-
Posterior rhinomanometry. Illustration by William E. Walsh, CMI; Northwestern University medical student and certified medical illustrator.







