Practice Essentials
Sleep-disordered breathing (SDB) encompasses a spectrum of disorders with implications in many fields of medicine. The spectrum itself (in order of increasing significance) includes primary snoring, upper airway resistance syndrome (UARS), and obstructive sleep apnea hypopnea syndrome (OSAH). Further advancements in the study of this disease spectrum have blurred the distinctions between the different syndromes.
Apnea is defined as a cessation of breathing for 10 seconds. In obstructive sleep apnea (OSA), the apnea is accompanied by observed ventilatory effort (ie, a chest rise/fall). [1]
In its most recognizable and ubiquitous form, SDB is represented by snoring. On occasion, snoring is totally benign, a consequence of a removable stimulus (ie, nasal congestion, excess fatigue, central nervous system [CNS] depressants, abnormal sleep positions), or both. [2, 3]
More strikingly, snoring indicates an underlying pathologic change that is more significant than the auditory annoyance of one's bed partner.
The purpose of this article is to provide a concise but thorough review of SDB (most notably OSA), including its pathogenesis and sequelae and current recommendations for diagnostic evaluation and treatment. OSA is a major health issue in the Western Hemisphere, and its symptoms and pathology have a wide range of consequences. For example, daytime somnolence, the cardinal symptom of OSA, can result in everything from minor reductions in daytime functioning to fatal motor vehicle accidents. In addition, owing to the increased morbidity and mortality in patients with OSA and concurrent cardiovascular disease, researchers have focused on the association of cardiovascular disease and OSA. [4, 5, 6]
A study by Pinilla et al indicated that in persons under age 50 years, OSA produces physiologic effects normally associated with aging. Specifically, the investigators reported that the apnea-hypopnea index (AHI), the arousal index, and nighttime oxygen saturation below 90% had a dose-response relationship with “the following aging hallmarks: alteration of cellular communication, deregulation of nutrient sensing, mitochondrial dysfunction and genomic instability.” No such relationship was found in patients aged 50 years or older. [7]
OSA is ubiquitous and has variable consequences; thus, physicians must have a cursory understanding of its disease process.
The image below details an algorithm for the treatment of snoring and obstructive sleep apnea syndrome.
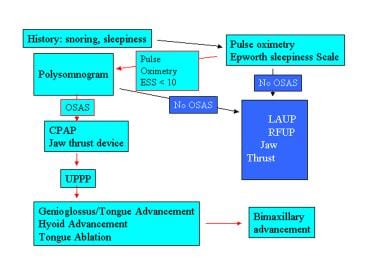 Snoring and obstructive sleep apnea syndrome (OSAS) treatment algorithm. ESS - Epworth sleepiness scale; LAUP – laser-assisted uvuloplasty; RFUP - radio frequency uvuloplasty; CPAP - continuous positive airway pressure; UPPP - uvulopalatopharyngoplasty.
Snoring and obstructive sleep apnea syndrome (OSAS) treatment algorithm. ESS - Epworth sleepiness scale; LAUP – laser-assisted uvuloplasty; RFUP - radio frequency uvuloplasty; CPAP - continuous positive airway pressure; UPPP - uvulopalatopharyngoplasty.
Go to Obstructive Sleep Apnea (OSA), Childhood Sleep Apnea, Surgical Approach to Snoring and Obstructive Sleep Apnea, Obstructive Sleep Apnea and Home Sleep Monitoring, Oral Appliances in Snoring and Obstructive Sleep Apnea, Upper Airway Evaluation in Snoring and Obstructive Sleep Apnea, and Sleep-Disordered Breathing and CPAP for more information of these topics.
Workup in obstructive sleep apnea
Workup in OSA can include use of the following:
-
Full-night polysomnography (PSG) - The criterion standard for diagnosing OSA [8]
-
Pulse oximetry - When combined with the Epworth sleepiness scale, pulse oximetry is a very good screening tool for deciding which patients should undergo PSG
-
Epworth sleepiness scale - A commonly used and statistically validated questionnaire for daytime sleepiness
-
Mallampati score of the oropharynx - Used to predict the severity of OSA or to select patients for specific OSA procedures by describing the tongue size in relation to the oropharynx
-
Friedman classification - Developed to predict the successful outcome of surgery, specifically uvulopalatopharyngoplasty (UPPP), for patients with OSA
-
Multiple Sleep Latency Test (MSLT) - A good measure of narcolepsy and rapid eye movement (REM) sleep deprivation, which may be present in severe OSA
Management of obstructive sleep apnea
Continuous positive airway pressure
Continuous positive airway pressure (CPAP) is the criterion standard of therapy for obstructive sleep apnea (OSA) and is the most commonly used initial treatment.
Oral appliances
Dental devices are simple, nonsurgical alternatives that are generally effective for simple snoring and mild sleep apnea. These devices fall under the categories of mandibular advancement devices (MAD) and tongue-retaining devices. [9]
Surgical treatment
Surgical procedures include the following:
-
Tracheostomy - The creation of a direct opening of the trachea (via tracheotomy) into which a tube is placed to bypass a potential upper airway obstruction
-
LAUP, RFUP, and UPPP - Soft-palate surgery is the most common type of attempted surgical correction of OSA; laser-assisted uvuloplasty (LAUP), radiofrequency uvuloplasty (RFUP), and UPPP have been used as the first step in surgical intervention
-
Advancement procedures - Genioglossus, hyoid, and suture tongue advancement are common advancement procedures; bimaxillary advancement is used when all other attempts have failed
-
Nasal and tongue surgery - Nasal procedures are often used as an adjunct to make CPAP available in the postoperative period; tongue resection and submucosal radiofrequency surgery have been shown to be efficacious in patients whose OSA does not respond to soft-palate procedures [10]
-
Multistep procedures - Surgical treatment for OSA is often managed with a multistep approach
Terminology in Sleep-Disordered Breathing
Snoring, upper airway resistance syndrome (UARS), and sleep apnea are generally regarded as stages in a single process of airway obstruction. Sleep-disordered breathing (SDB) is a generic term that classifies these stages of disease on a spectrum, and these stages differ only in severity and presentation.
Physicians should familiarize themselves with the terminology and definitions used in the specific sleep laboratory of their patients. As delineated below, definitions for specific indices may vary from laboratory to laboratory. Efforts are being made to standardize such measurements, but ubiquity has not been established.
Snoring
Snoring is a respiratory sound, typically occurring during inspiration or expiration, that is generated in the upper airway during sleep. Simple snoring, usually noted by a patient’s bed partner, has no clinical sequelae. In this scenario, patients do not experience daytime somnolence (see The Epworth Sleepiness Scale) and usually present to the doctor only at the urging of their bed partner or family member. Suggestions to reduce snoring include weight reduction, [11] positional modification during sleep, or an oral appliance.
If a patient presents with snoring and comorbid hypertension or daytime somnolence, he or she should be further evaluated. Respiratory effort–related arousal (RERA) occurs when upper airway narrowing has led to an increased respiratory effort. As noted on the electroencephalogram (EEG) of a polysomnogram (PSG), this extra effort could stimulate arousal. The clinical relevance of RERA is not as well documented as the apnea-hypopnea index (AHI), but RERA is assumed to impact daytime somnolence. When patients are noted to have several RERAs but have a clinically insignificant AHI, they are often thought to have UARS.
Patients with UARS often present with daytime somnolence and snoring. These patients may display anthropomorphic abnormalities and decreased posterior airspace with retrodisplacement of the tongue. Airflow is limited, but any subsequent apnea or hypopnea is minor enough to cause no concomitant oxygen desaturation. This condition is associated with esophageal pressure changes as noted during a sleep study conducted with an esophageal pressure monitor. Daytime somnolence is a consequence of disturbed, but not apneic, sleep.
Apnea
Apnea is defined as a cessation of breathing for 10 seconds. In obstructive sleep apnea (OSA), the apnea is accompanied by observed ventilatory effort (ie, a chest rise/fall). [1] In central apnea, no ventilatory effort is seen. Pure central apnea is rare. A mixed apnea is a disordered breathing event that begins as a central apnea and ends as an obstructive one. A hypopnea episode is a partial reduction in ventilation with continued effort for at least 10 seconds. Although the criteria for hypopnea may vary from laboratory to laboratory, a generally accepted definition is a 30% reduction in airflow from baseline level plus a 4% or greater decrease in oxygen saturation.
The AHI is the cornerstone of placing a specific patient on the sleep apnea spectrum. AHI is the sum of apneas plus hypopneas in 1 hour of sleep. Although the severity of apnea is not measured by AHI alone (other factors include clinical presentation [daytime somnolence], hypoxemia, sleep fragmentation, and presence of arrhythmias), the following is a simple classification system of OSA severity [2] :
-
An AHI of less than 5 is classified as normal.
-
An AHI of 5-14 is classified as mild apnea.
-
An AHI of more than 30 is classified as severe apnea.
The respiratory disturbance index (RDI) is similar to the AHI except for a few nuances. AHI measures total sleep time and averages the number of apneas and hypopneas per hour. RDI uses total recording time (this includes awake time in the sleep laboratory). RDI may also include RERA, which AHI strictly excludes. Although no superior index for measuring SDB currently exists, AHI is not without its shortcomings. For example, AHI does not factor in the degree of oxygen desaturation or the number of arousals throughout the night. Both of these could be indices for daytime symptoms and pathogenic sequelae of the disorder.
Obstructive sleep apnea syndrome
Obstructive sleep apnea syndrome (OSAS) is defined as a chronic respiratory sleep disorder typified by recurrent episodes of partial or complete upper airway obstruction during sleep that cause cessation of airflow in the presence of respiratory effort. These episodes cause repeated arousals and fragmented sleep and are due to the various anatomic and physiologic dysfunctions.
Clinicians should be aware of all of symptoms associated with OSAS. Although experiencing all of them is unusual for any individual patient, any one of the symptoms may be the first indication of OSAS in a patient who presents with symptoms other than snoring or tiredness.
Nocturnal symptoms include the following:
-
Restless sleep and snoring (observed in 90% of patients)
-
Sleep disruptions
-
Choking
-
Esophageal reflux
-
Nocturia
-
Heavy sweating
Daytime symptoms include the following:
-
Excessive daytime somnolence: This symptom is observed in 70% of patients. Motor vehicle accidents are a significant concern in patients with OSA.
-
Morning headaches
-
Mood alterations
-
Sexual dysfunction
-
Hearing loss
-
Automatic behavior
-
Short-term memory loss
-
Hypnogenic hallucinations
Sleep Studies and Stages
Sleep studies are the cornerstone of diagnosis and evaluation of obstructive sleep apnea (OSA). Polysomnography (PSG), for example, is an overnight study that records a multitude of physiologic factors, including brain waves, muscle tone, eye movements, respiratory effort, and oxygen saturation (see below). [12] Individual-patient studies are often performed in a sleep laboratory, where the state, quality of sleep in each stage, and the apnea-hypopnea index (AHI) can be determined. [13]
The following studies are used to evaluate and diagnose OSA:
-
Electroencephalography (EEG) – Assesses brain waves
-
Electromyography (EMG) – Assesses muscle tone
-
Electrooculography ((EOG) – Assesses eye movements
-
Measurement of airflow, chest, and abdominal efforts – Assesses respiratory effort
-
Electrocardiography (ECG) – Assesses heart rate, rhythm, and activity
-
Pulse oximetry – Assesses oxygen saturation
Sleep is broadly divided into rapid eye movement (REM) sleep and non-REM (NREM) sleep. Based on differences noted by PSG, NREM sleep is further subdivided into 4 stages. The 5 stages of REM and NREM constitute 1 sleep cycle, which typically lasts about 90 minutes. A person has 3-4 sleep cycles during an average night of sleep.
NREM sleep is loosely associated with a quiet brain in a movable body and constitutes about 75-80% of sleep. It begins with stage I sleep, and the EEG distinguishes sleep from wakefulness. Below, Table 1 outlines the sleep stages, percentage of total sleep, characteristic EEG waves, and defining traits. [14] REM sleep is often called paradoxical sleep. Despite significant brain activity, the patient is difficult to arouse and there is a loss of postural motor tone. Dreaming also occurs during REM sleep.
Table 1. Stages of Sleep (Open Table in a new window)
Stage |
Total Sleep, % |
EEG Wave |
Defining Traits |
I |
2-5% |
Theta wave |
Light sleep, hypnic jerks, conscious awareness |
II |
45-55% |
Sleep spindles and K complexes |
Consolidated sleep, loss of conscious awareness, slowed heart rate, decreased body temperature |
III |
3-8% |
Delta/slow wave |
Deep/restorative sleep, difficult to arouse |
REM |
20-25% |
Alpha (wakefulness) |
Dream sleep, paradoxical sleep, paralysis, high cortical activity |
EEG = electroencephalogram; REM = rapid eye movement. |
|||
Transitions between NREM and REM sleep are often accompanied by brief movements, arousals, or both. These movements and arousals are physiologic and are not considered abnormal or sleep disruptions. NREM sleep is dominant in the first one third of the total sleep episode. REM sleep increases in percentage of the sleep cycle as the sleep episode progresses and dominates the last one third of the total sleep episode.
REM sleep accounts for approximately 50% of total sleep in the neonatal period, 20-30% of total sleep in childhood, and 20% of total sleep throughout the rest of life. Delta sleep decreases steadily from childhood to old age, but REM sleep remains fairly constant. The continuity of sleep declines with age. The sleep of the elderly is often disrupted by many arousals (sometimes hundreds) and may lead to symptoms of poor sleep quality. The need for sleep does not decrease with age.
Understanding the normal sleep cycle is helpful in evaluating the effects of sleep-disordered breathing (SDB). A common sign of severely disrupted sleep is an increase in the percentage of stage I sleep, resulting in poor sleep quality and excessive daytime sleepiness (EDS).
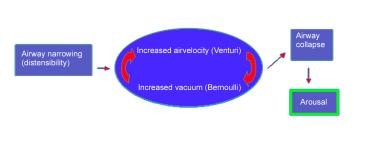
Sleep apnea often worsens during REM sleep. Muscle tone of the hypopharyngeal and oropharyngeal musculature decreases, resulting in upper airway collapse, as depicted in the image below. Airway obstruction, oxygen desaturation, and (in severe cases) retention of carbon dioxide cause the characteristic arousals from sleep that are associated with sleep apnea.
A study by Kulkas et al indicated that in episodes of OSA lasting 30-45 seconds, peripheral oxygen desaturation tends to be more severe in females than in males, suggesting that obstructive apnea episodes of over 30 seconds pose greater harm to women than men. [15]
Sleep Physiology
The physiologic changes of sleep affect multiple systems, including the cardiovascular, central nervous, pulmonary, gastrointestinal, thermoregulatory, and endocrine systems.
Cardiovascular system
The cardiovascular system is controlled by the autonomic nervous system (ANS). Generally, vagal tone increases and sympathetic input decreases throughout the night, leading to the following cardiovascular changes:
-
Heart rate (HR) and blood pressure (BP) decrease during NREM sleep.
-
Further decreases in HR and BP occur during tonic REM.
-
HR and BP increase during phasic REM.
-
Some cardiac dysrhythmias may diminish or disappear during sleep, but premature ventricular contractions (PVCs) increase during REM sleep.
Central nervous system
Changes in the central nervous system (CNS) during sleep include the following features:
-
The blood flow to the brain increases during sleep and is higher during REM sleep.
-
Intracranial pressure and temperature increase during REM sleep and decrease during NREM sleep. These differences may indicate variations in the metabolic activity of the brain.
Pulmonary system
Pulmonary changes also occur during sleep. Respiration becomes completely involuntary. Specifically, ventilatory control becomes mainly metabolic in that breathing is triggered by carbon dioxide (CO2) levels. Also, the sensitivity of the respiratory chemoreceptors decreases during sleep; thus, a higher concentration of CO2 is needed to stimulate ventilation. The partial pressure of CO2 (PCO2) typically rises by 4 mm Hg during sleep. In a healthy person, this increase is physiologic; however, in a patient with lung disease, this increase may result in significant oxygen desaturation. Chemical responses to hypoxia and hypercapnia decrease in NREM and decrease further in REM. This is another reason why OSA is most severe during REM.
Other pulmonary changes include the following:
-
A decrease in tidal volume leads to an overall decrease in ventilation.
-
In NREM sleep, respiratory rate, tidal volume, and minute ventilation decrease, leading to an increase in end-tidal CO2 and a decrease in oxygen (O2) saturation.
-
During REM sleep, respiration may be rapid and irregular.
-
Upper airway resistance increases up to 7 times that of waking levels. Muscle tone is lost, especially in the intercostal and pharyngeal muscles; however, the diaphragm maintains its tone.
-
Mucociliary clearance, alveolar oxygen tension (PAO2), and arterial oxygen tension (PaO2) decrease. The drop in PaO2 is normally less than 2%.
-
Patients with SDB experience arousals due to labored breathing. The exact stimulus for arousals is unknown, but mechanoreceptors in the upper or lower airway or the diaphragm may be responsible.
Gastrointestinal system
Gastrointestinal changes during sleep include the following features:
-
Gastric acid secretion generally increases during sleep and peaks in REM sleep.
-
Swallowing and esophageal motility decrease during sleep.
-
Other effects have been minimally studied and have yielded conflicting results.
Thermoregulatory system
Changes in thermoregulation during sleep include the following:
-
During REM sleep, thermoregulation and perspiration are absent; the body becomes poikilothermic (cold blooded).
-
Overall body temperature decreases during sleep.
Endocrine system
Sleep also affects the endocrine system and includes the following effects:
-
Growth hormone levels peak during the early hours of sleep and gradually decline.
-
Prolactin (PL) levels are affected by sleep and increase during both nocturnal and daytime sleep.
-
Thyroid-stimulating hormone (TSH) levels tend to decrease during sleep, which may coincide with the decreased metabolic needs during sleep.
-
Melatonin and cortisol levels are affected by circadian rhythms but not by sleep itself.
Relevant Anatomic Features in OSA
In order to understand obstructive sleep apnea (OSA), it is necessary to review the features of the oropharynx and soft palate and their muscles, cephalometric findings, and anthropomorphic characteristics.
Oropharynx and soft palate
The airway is passively opened without direct muscular action. In contrast, closure is active and is accomplished by the muscles of the oropharynx. Intrinsic muscular tone keeps the airway in its normal, patent configuration.
Muscular anatomy
The levator palatini muscles originate at the apex of the petrous bone and the cartilaginous portion of the eustachian tubes. These muscles obliquely extend into the posterior superior palate, where they blend at the midline into the palatal aponeurosis.
If the tensor palatini muscles are relaxed during the contraction of levator palatini muscles, 2 dimples appear on the oral surface of the palate. If the tensor stiffens the palate, the palate raises without altering its shape. In either case, the nasopharynx is effectively shut off by the valve action of the levator palatini.
The soft palate superiorly elevates and comes into contact with the Passavant ridge at the anterior part of the atlas. The palatoglossus originates at the anterior palatal aponeurosis and inferiorly extends to the tongue. The palatoglossus comprises the anterior pillar, and its function is to raise the tongue and narrow the oropharynx.
The palatopharyngeus originates as 2 heads at the palatal aponeurosis and the hard palate with the insertion of the levator palatini. The 2 heads join to form the posterior tonsillar pillar and extend to insert into the larynx at the thyroid cartilage near the base of the superior cornu. This muscle elevates the larynx, depresses the soft palate, and constricts the pharynx. Some anatomic descriptions indicate that the fibers from the palatopharyngeus originate at the hard palate and posteriorly extend inside the fibers of the superior constrictor to form the Passavant ridge. According to other descriptions, the Passavant ridge is formed only by the superior constrictor.
The musculus uvulae, which raises the uvula, originates at the palatine spine and inserts into the uvula. The tensor veli palatini arises from the pterygoid plate, the sphenoid bone, and the cartilaginous eustachian tube. The tensor veli palatini inferiorly extends, passes under the hamulus, medially progresses, and then inserts into the palatal aponeurosis. It principally tenses the soft palate.
The 10th cranial nerve (CN X) innervates all the oropharyngeal muscles except the tensor veli palatine. This muscle is innervated by the fifth cranial nerve (CN V).
Craniofacial features
Jamieson et al used cephalometric roentgenograms to evaluate 155 nonselected patients with OAS, of whom only 2 patients with sleep apnea had normal cephalometric findings. [16] The remaining 150 patients had at least 2 landmarks that significantly differed from the normative data. The most common findings were retropositioned mandible, a nasion-sella-basion angle that was more acute than expected, a lower-than-expected displacement of the hyoid bone, and a normal position of the maxilla. [16]
Mouth breathing may result in worsening apnea. An interincisor opening of 1.5 cm causes an associated 1-cm dorsal displacement of the mandibular angle. Assuming that the genioglossus retains its shape, this action results in a 1-cm decrease in the oropharyngeal diameter. Additionally, muscular relaxation during rapid eye movement (REM) sleep results in worsening tongue retrodisplacement. The combination of a decreased diameter of the airway and the decreased muscle tone contributes to airway obstruction.
Both bony and soft-tissue abnormalities within the proximity of the upper aerodigestive tract can also contribute to OSA in patients.
Anthropomorphic measurements
Almost all anthropomorphic measurements have been associated with the apnea-hypopnea index (AHI), but neck circumference may be the most important factor. [17] Neck circumference has been reported to be equivalent in its predictive effect to a combination of other factors, such as body mass index (BMI), age, or sex. [17] Flemons et al studied multiple body parameters for their predictive values and documented that 26 anthropomorphic measurements were significantly associated with the AHI. [17] Only height and thigh circumference were not associated with the AHI. The investigators concluded that 4 parameters were the most important and approximated the more complex models for OSA: neck circumference, hypertension, habitual snoring, and gasping or choking. [17]
None of these parameters is perfect, but they are used to identify which patients are appropriate candidates for polysomnography (PSG). Most importantly, none of these parameters are indicated to be predictive of treatment success.
A study by Kwon et al indicated that women with moderate to severe OSA tend to have a larger ascending aortic diameter than do females without the condition, with the adjusted mean difference in the report being 0.12 cm. No such relationship between aortic diameter and OSA was seen in men. [18]
Risk Factors for Sleep Apnea
The following conditions are risk factors for sleep apnea:
-
Obesity (body mass index [BMI] >30 kg/m2) is a primary risk factor for obstructive sleep apnea syndrome (OSAS). According to the Wisconsin Cohort Sleep study, a 10% increase in weight is associated with a 6-fold risk of developing sleep-disordered breathing (SDB). [19] About two thirds of the patients with OSAS are more than 20% above their ideal body weight.
-
Sex: Obstructive sleep apnea (OSA) affects up to 4% of men and 2% of women. The incidence in women increases after menopause.
-
Obstruction: The upper airway may be obstructed at any level. Some physicians may use the Mallampati classification scale to help communicate this idea of airway obstruction (see Mallampati Classification).
-
Associated anthropomorphic measurements (especially the neck circumference): A neck circumference of more than 43 cm (17 in) is a predictor for an increased apnea-hypopnea index (AHI). [17]
-
Pulmonary disease: Chronic obstructive pulmonary disease (COPD) and restrictive or neuromuscular diseases of the lungs can cause airway problems.
-
Central nervous system (CNS) depressants: Alcohol and sleeping pills cause a more relaxed airway, which results in its collapse.
-
Tobacco use
-
Acromegaly (when associated with macroglossia)
-
Supine position during sleep
-
Craniofacial anomalies and previous trauma/surgery that affects upper airway function or patency
Pathophysiology of Airway Obstruction
Anatomic and physiologic factors combine to cause the pathognomonic pharyngeal collapse associated with sleep apnea. An imbalance between forces that promote airway dilation and forces that promote collapse is associated with sleep apnea. In addition, the long-term cardiovascular consequences of obstructive sleep apnea (OSA) are of major concern in sleep medicine today. To date, no definitive association has been made, and numerous studies have yielded conflicting results; however, a strong association undoubtedly exists between OSA and cardiovascular or cerebrovascular disease.
Airflow
The following factors promote airway collapse:
-
Small airway size
-
Upper airway resistance
-
Negative inspiratory pressure
-
Extraluminal tissue pressure
-
Small mandible
-
Supine position
-
Upper airway inflammation
The following factors promote airway patency:
-
Pharyngeal dilator muscles
-
Larger airway and mandible
-
Higher lung volume
If the physical and spatial relationship of the facial skeleton creates a large airway, the patient can tolerate excessive soft tissue (such as adipose tissue seen in obese patients). Otherwise, the flow of air may be impeded, resulting in snoring and obstructive sleep apnea syndrome (OSAS). If a patient has a congenitally narrower airway, excessive soft tissue, increased extraluminal pressure due to obesity, and increased neck circumference, he or she is at a very high risk for developing OSA.
Bernoulli principle and Venturi effect
Two basic principles of fluid flow, the Bernoulli principle and the Venturi effect, can be applied to give additional insight to the effects of airway narrowing.
The Bernoulli principle describes fluid flow in a column. A partial vacuum exists at the outer edges of a column of moving fluid. As airflow speed increases, the partial vacuum pressure increases. The smaller the column is, the faster the flow. This principle is illustrated by a drinking straw: If too much negative pressure is generated within the straw, it collapses; as the negative pressure decreases, the straw becomes more rigid and does not collapse.
The Venturi effect describes the acceleration of airflow that occurs as a current of air enters a narrow passageway. The wind blowing between buildings or the water spraying out of a hose that is partially occluded by the thumb are examples of this effect. In the human anatomy, the pharynx is the passageway, and the distensibility and movable walls of the pharynx govern the width of the named passageway; thus, inhaled air accelerates through the pharynx.
Airway pressures, resistance, and collapse
Because of the different anatomy and pliability of the pharyngeal walls, patients with OSA and individuals without sleep apnea differ in their closing pressures and airway resistance. In a nonsnoring adult, the negative pressure required to close the upper airway is less than (more negative than) –25 cm water. Snoring adults have a much more pliable airway, with closure during sleep occurring at pressures that range from –2 to –10 cm water.
Physiologically, the pharyngeal muscles work to keep the airway open. During sleep, the cortical input to these muscles decreases and therefore increases the chance of collapse. In a normal person, negative intrapharyngeal pressures during sleep reflexively stimulate pharyngeal dilator tone to keep the airway patent. In a patient with OSA, whose airway is likely narrower than that of the normal patient, the reflexive increase in tone does not counteract the increased pressure of the narrowed airway of sleep and is not enough to prop the airway open. This results in pharyngeal collapse and causes either a hypopneic or an apneic event, which self-terminates in an arousal as the body strives to open the airway and restore ventilation.
There is significant person-to-person variability in chemoreceptor and mechanoreceptor sensitivity, and this impacts the duration of apneic events and the likelihood of significant oxygen desaturation, hypoxemia, and hypercapnia. For example, if a patient has a low sensitivity to high levels of carbon dioxide (CO2), the apnea associated with a pharyngeal collapse may last for several seconds before arousal is stimulated.
Sleep-disordered breathing and cardiovascular and pulmonary effects
Acutely, a single apneic event has many cardiovascular consequences. During an apneic episode, there is often associated bradycardia, an initial drop then increase in blood pressure, increased pulmonary artery pressure, decreased cardiac output, decreased left ventricular end-diastolic pressure (LVEDP), and increased afterload. Bradycardia is due to increased vagal tone from hypoxemia.
Following an apneic episode, sympathetic nerve activity increases, resulting in tachycardia, increased cardiac output, and a rise in blood pressure.
The cardiovascular sequelae and other sequelae of OSA include the following: pulmonary hypertension, diabetes mellitus, hypertension, heart disease, and stroke. OSA has been shown in studies to cause mild pulmonary hypertension (mean pulmonary arterial pressure [MPAP] of 20-30 mm Hg). [20, 21] Concurrent chronic obstructive pulmonary disease (COPD) also plays an important role in the development of pulmonary hypertension. OSA has also been associated with glucose intolerance, which is thought to be secondary to sleep fragmentation, repetitive hypoxemia, and higher levels of proinflammatory cytokines associated with insulin resistance.
A study by Mokhlesi et al indicated that an independent association exists between REM OSA and nondipping of nocturnal blood pressure, the latter being a phenomenon linked to target organ damage and cardiovascular disease. The study found that the risk of nondipping in either systolic or diastolic blood pressure, as assessed in 199 and 215 subjects, respectively, increased in relation to the severity of the OSA in REM sleep. [22]
American Heart Association scientific statement
A scientific statement from the American Heart Association (AHA) asserts that the “interplay of SDB, sleep disorders, and cardiac arrhythmia is complex and linked through multifactorial mechanisms.” SDB-induced autonomic responses, according to the statement, are implicated in arrhythmogenesis by strong preclinical data. However, the AHA contends that arrhythmogenic risk is also enhanced by adverse cardiac remodeling, in which repetitive episodes of hypoxia, intrathoracic pressure alterations, and increased systemic inflammation and free radicals, all sequelae of SDB, play a part. [23]
Also according to the AHA statement, many observational studies have indicated that there is an association between treatment of SDB and improvement of outcomes in atrial fibrillation, but the AHA reports that “these findings have not been confirmed in randomized controlled clinical trials.” The AHA acknowledges, however, that “trials to date have been limited by modest sample size, inadequate power, short duration of follow-up, or patient selection.” [23]
Consensus statements in the AHA statement include, but are not limited to, the following [23] :
-
Because SDB is highly prevalent in atrial fibrillation, patients with atrial fibrillation who are at increased risk for OSA should especially be considered for OSA screening, including persons with obesity and hypertension and in whom atrial fibrillation episodes are predominant at night
-
Sleepiness is suggested to have limited clinical utility in atrial fibrillation screening, with the predictive ability of self-reported sleepiness for SDB being variable and the role of sleepiness in SDB with atrial fibrillation being unestablished
-
Patients with profound or severe nocturnal bradyarrhythmia (ie, bradycardia, atrioventricular conduction delay, or prolonged sinus pauses) should be considered for SDB screening, with follow-up diagnostic testing (as indicated), to evaluate these individuals for bradyarrhythmic improvement; this strategy should be considered, as sleep medicine resources and patient medical stability permit, before a pacemaker is placed
-
It is reasonable that patients under consideration for permanent pacemaker placement for bradyarrhythmia be evaluated for SDB as a potential targetable risk factor, especially in cases where nocturnal (or sleep-time) bradyarrhythmia episodes are predominant
-
SDB treatment can be considered in cases of profound or severe nocturnal bradyarrhythmia and SDB
Hypertension
Whether OSA is a cause of or a common comorbidity of hypertension is undetermined. This distinction is difficult to make, because the 2 syndromes occur in similar demographics. Studies show that 30-50% of patients with hypertension also have OSA. The incidence of hypertension in patients with OSA linearly increases with respect to increases in the apnea-hypopnea index (AHI). [20]
Other studies have shown an extremely high prevalence of OSA in refractory hypertension (defined as hypertension, excluding secondary hypertension, that is not controlled by >3 antihypertensive medications despite compliance). The associated increase in sympathetic tone following the repetitive apneas and hypopneas (as documented by the AHI) is believed to be a possible causal factor for hypertension in some patient populations. In fact, some studies have shown that patients with OSA have an increase in serum and urine catecholamines. [20]
A study by Martynowicz et al indicated that in individuals with hypertension, the AHI is higher, as evaluated with polysomnography, in those with lower blood telomerase activity, as well as in hypertensive patients with impaired vascular endothelial function. [24]
Definitive data that suggest a causal relationship between OSA and hypertension do not exist, and numerous studies conflict with each other and themselves. Bassetti et al demonstrated an increase in long-term mortality with an increased AHI, but the investigators found no associated increased recurrence of vascular events or stroke. [25] In current clinical practice, ruling out OSA in a hypertensive patient is accepted, especially if the patient has additional risk factors.
Heart disease
Some authors suggest that OSA should be a separate risk factor for heart disease (along with hyperlipidemia, hypertension, diabetes, and smoking). The Sleep Heart Health Study showed that the incidence of stroke was higher in patients with OSA than in those with any other cardiovascular disease. [26] Additionally, some studies suggest that sleep-disordered breathing (SDB) is associated with an increased long-term mortality after cerebrovascular accidents. OSA was 5 times more frequent in stroke patients than in healthy patients of the same age and sex. [25]
A further suggested link between heart disease and OSA is the inflammatory effects of apneic events. Hypoxemia is a trigger for the activation of neutrophils, which adhere to endothelial cells and are responsible for the release of oxygen free radicals. Repeated oxidative stress can lead to endothelial dysfunction, increased vasoconstriction, decreased vasodilation, and, ultimately, increased risk of atherosclerosis. Inflammatory markers, such as C-reactive protein, are often elevated in patients with OSA. [27]
Studies by Suzuki et al have shown an increase in interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in patients with OSA. [28] After initiation of continuous positive airway pressure (CPAP) therapy, IL-6 levels were noted to decrease. This may suggest that OSA contributes to the oxidative stress and systemic inflammation present in atherosclerosis. Additional studies have shown a reduction in objective sleepiness when immune modulators such as TNF-α were neutralized. Thus, these cytokines may be possible targets of symptomatic treatment of OSA.
Still, many of the studies regarding cardiovascular disease and OSA and/or SDB are contradictory or inconclusive because of the overlap of disease in many patients. OSA may be regarded as an immunologic disease with multisystem consequences, because immune modulators have been associated with OSA. However, the cardiovascular system seems to be the most affected.
The Epworth Sleepiness Scale
Patients often underestimate their level of sleepiness, probably because sleep apnea is such a chronic, insidious problem, and they consider their present state to be normal. The Epworth sleepiness scale (see Table 2, below) is a commonly used and statistically validated questionnaire for daytime sleepiness. Several situations are listed, and the patient is asked to evaluate their sleepiness. The following scale is then used to choose the most appropriate number for each situation:
-
0 = Would never doze
-
1 = Slight chance of dozing
-
2 = Moderate chance of dozing
-
3 = High chance of dozing
Table 2. Example of Scoring on Epworth Sleepiness Scale (Open Table in a new window)
Situation |
Chance of Dozing |
Sitting and reading |
3 |
Watching television |
2 |
Sitting inactive in a public place (eg, theater) |
0 |
Being a car passenger for 1 hour without a break |
2 |
Lying down to rest in the afternoon |
3 |
Sitting and talking to someone |
0 |
Quietly sitting quietly after lunch without alcohol |
2 |
In a car, while stopping for a few minutes in traffic |
0 |
Total |
12 |
The generally accepted interpretations of the Epworth sleepiness scale are as follows:
-
A score of 0-5 should be interpreted as supernormal.
-
A score of 5-10 should be interpreted as normal.
-
A score of 10-15 should be interpreted as sleepy.
-
A score of 15-20 should be interpreted as very sleepy.
-
A score of more than 20 should be interpreted as dangerously sleepy. (Arrange transportation for patient.)
Mallampati Airway Classification
A patient being evaluated for obstructive sleep apnea (OSA) should be assessed for surgically correctable causes of OSA. Patients without the typical risk factors, such as obesity, should raise a clinical suspicion of abnormal anatomy as a cause of OSA. For example, if a young, athletic male is experiencing excessive daytime somnolence and is receiving complaints from his bed partner regarding loud snoring, it is important to assess the patient’s airway because of the potential implications of treatment.
The Mallampati score of the oropharynx is an airway assessment classification system that was initially designed to predict ease of intubation. Currently, this system has been used to predict the severity of OSA or to select patients for specific OSA procedures by describing the tongue size in relation to the oropharynx.
To measure tongue size, the patient holds the head in a neutral position, opens the mouth as wide as possible, and sticks out the tongue. The patient should relax the tongue and remain quiet so that the tongue and palate relationship can be determined.
The Mallampati score is defined as follows:
-
Class I: The soft palate, fauces, uvula, anterior and posterior pillars are visualized.
-
Class II: The soft palate, fauces, and uvula are visualized.
-
Class III: The soft palate and base of the uvula are visualized.
-
Class IV: The soft plate is not visualized.
Friedman Classification
The Friedman classification was developed to predict the successful outcome of surgery, specifically uvulopalatopharyngoplasty (UPPP), for patients with obstructive sleep apnea (OSA). The system includes an evaluation of palate position, tonsil size, and body mass index (BMI) to predict the success of UPPP. A modified version of the Mallampati classification is used to evaluate palate position relative to oropharyngeal size. In the Friedman classification, the patient keeps his tongue in a neutral position, and 4 stages are used to describe the airway. [29]
The palate position is graded from I-IV, as follows:
-
I: The uvula, soft palate, and tonsils/pillars are clearly visible.
-
II: The uvula and soft palate are visible, but the tonsils are not.
-
III: Only part of the soft palate is visible.
-
IV: Only the hard palate is visible.
The tonsil size is graded from 0-4, as follows:
-
0+: A previous tonsillectomy has been performed.
-
1+: The tonsils are hidden within the tonsillar pillars.
-
2+: The tonsils extend to the tonsillar pillars.
-
3+: The tonsils extend beyond the pillars but not to the midline.
-
4+: The tonsils extend to the midline.
BMI is loosely included in the Friedman classification. The stages of disease are defined as follows:
-
Stage I disease includes patients with palate position I or II, tonsil size 3 or 4, and a BMI of less than 40 kg/m2.
-
Stage II disease includes patients with palate position I or II and tonsil size 0, 1, or 2—or palate position III and IV and tonsil size 3 or 4—and BMI less than 40 kg/m2.
-
Stage III disease includes patients with palate position III or IV and tonsil size 0, 1, or 2.
All patients with a BMI of greater than 40 kg/m2 are considered stage III. Patients with stage I classification are predicted to have successful results with UPPP. Stage II and III patients should have adjunctive treatment if they undergo UPPP, because the procedure does not have a high likelihood of cure. The strength of this classification system is still under review, but it has consistently shown to be a reliable indicator of prognosis after surgery.
Diagnostic Considerations
Obstructive sleep apnea syndrome (OSAS) is associated with a number of problems that may be evaluated by history, physical examination, and blood tests. These evaluations must be performed in all patients; especially note any cardiac or pulmonary problems. Other problem areas include the following:
-
Cardiopulmonary (myocardial infarction [MI], arrhythmia, cor pulmonale)
-
Memory (short-term memory loss)
-
Dyslipidemia (elevated cholesterol, triglycerides)
-
Red blood cells (polycythemia)
-
Blood pressure (independently associated with snoring)
-
Gastroesophageal reflux
-
Ulcer
-
Headache (Morning headaches may result from carbon dioxide buildup that occurs during apneic episodes.)
Pulse Oximetry
Nocturnal pulse oximetry is often used as a screening tool to identify the patients who have a high probability of a positive polysomnography (PSG) result. Oximetry alone is sensitive but not specific enough to make a definitive diagnosis of obstructive sleep apnea (OSA).
When combined with the Epworth sleepiness scale, pulse oximetry is a very good screening tool for deciding which patients should undergo PSG (see The Epworth Sleepiness Scale). A number of algorithms have been used with pulse oximetry in order to decide who is likely to have a positive PSG result.
Gyulay et al reported that the percentage of time a patient is saturating at 92% (CT92) or 90% (CT90) is a critical landmark with regard to predicting the presence of OSA and the likelihood of a positive PSG. [30] In normal sleep, the CT92 should be less than 2% and the CT90 less than 1% of the total sleep time. For example, if the patient sleeps for 100 minutes and has an oxygen saturation of less than 92% for 2 minutes, then sleep apnea is likely. In this case, the 2 minutes need not be (and are usually not) contiguous. For those patients with a respiratory disturbance index (RDI) of greater than 15 and symptoms severe enough to warrant use of continuous positive airway pressure (CPAP), the CT90 has 100% sensitivity in predicting OSAS, according to Gyulay et al. [30]
Counting the number of desaturation events (rather than the total time of desaturation) is no more predictive than the clinical assessment. Respiratory events that cause sleep fragmentation but not arterial desaturation probably lessen the predictive value of this count. No connection exists between desaturations and sleep fragmentation; therefore, oximetry readings are markers for sleep-disordered breathing (SDB) and not measurements of SDB.
Polysomnography
Full-night polysomnography (PSG) is the criterion standard for diagnosing obstructive sleep apnea (OSA). [8] Split-night PSG is performed when the first one half of the total sleep episode is dedicated to diagnosis and the latter half is dedicated to a continuous positive airway pressure (CPAP) trial. The patient must be asleep for at least 2 hours before the initiation of CPAP.
Multiple Sleep Latency and Maintenance of Wakefulness Tests
The Multiple Sleep Latency Test (MSLT) measures the length of time required for a person to fall asleep. This test is a good measure of narcolepsy and rapid eye movement (REM) sleep deprivation, which may be present in severe obstructive sleep apnea (OSA). The patient is given the opportunity to fall asleep 5 times throughout the day, separated by 2 hours between each test. The patient is allowed up to 20 minutes to fall asleep.
The interpretation of the MSLT is as follows:
-
0-5 minutes: Pathologic sleepiness
-
6-10 minutes: Indeterminate sleepiness
-
Longer than 10 minutes: Normal sleepiness
The Maintenance of Wakefulness Test is similar to MSLT and can be used to predict occupational safety in patients with OSA.
Management Overview
The clinician must fit the appropriate treatment to the needs of the patient. This requires a detailed patient history and physical examination, as well as an exhaustive explanation of the problem, treatment options, and possible complications. [31] Understanding the pathophysiology and the statistical probabilities associated with each treatment is essential for adequate treatment of patients with sleep-disordered breathing (SDB). The resolution of symptoms with a surgical procedure does not necessarily equate to the resolution of apnea. Resolution of daytime somnolence does not necessarily mean obliteration of SDB. Because of this potential problem and the progressive nature of the disease, patients should be followed in clinic with periodic polysomnography (PSG) to ensure efficacy of treatment.
Nonsurgical Behavioral Therapy
As a solitary treatment, oxygen is minimally effective, and drugs are largely ineffective for obstructive apnea. Other nonsurgical behavioral therapies include the following:
-
Weight loss (very effective in patients who are very obese): A single-center, prospective observational follow-up study evaluated 63 men aged 30-65 years with body mass index (BMI) of 30-40 and moderate-to-severe obstructive sleep apnea. After a 1-year weight loss program followed by a weight loss maintenance program, the results found that treatment with a very low energy diet may improve obstructive sleep apnea, especially in those who lost the most weight and had the most severe sleep apnea prior to treatment. [32]
-
Improved sleep hygiene (Avoid alcohol or sedative-hypnotic medication before sleep. Alcohol and sedative-hypnotic medications aid in more rapid sleep onset, but the sleep is not as deep or "refreshing.")
-
Positional therapy (avoidance of the supine position, to decrease snoring and, potentially, obstruction)
-
Continuous positive airway pressure (CPAP) (discussed further below)
-
Oral appliances (discussed further below)
Continuous positive airway pressure
CPAP is the criterion standard of therapy for obstructive sleep apnea (OSA) and is the most commonly used initial treatment. This therapy acts as a pneumatic splint that props open the pharyngeal walls of the upper airway to prevent collapse. Patients may feel improvement after just one night; however, the major cause of failure of treatment is noncompliance. (Dry mouth, nasal congestion, and rhinorrhea [CPAP rhinitis] are common side effects of CPAP therapy that may lead to noncompliance.)
The specific pressure needed to maintain patency of the patient’s airway is determined during a sleep study. CPAP is titrated upward until all sleep-disordered breathing (SDB) events cease, and oxygen saturation is maintained above 90%.
CPAP self-adjusts against various aspects of the patient’s airflow patterns based on an algorithm within the unit (autotitration). Overall, this reduces the pressure at which a patient is maintained, compared with the standard CPAP. However, if the machine does not titrate properly, treatment may be subtherapeutic. Patients may tolerate autotitration better than standard CPAP, although this has not been directly measured.
Bilevel positive airway pressure (BiPAP) is the delivery of different pressures during inspiration and expiration. It may be used in patients with concomitant hypoventilatory syndromes that require more ventilation at night or in patients who require very high CPAP pressures (>16 cm water) but who cannot tolerate the high pressure. There is no evidence that BPAP has greater compliance rates than CPAP.
CPAP may not simply act mechanically to reduce the deleterious effects of OSA. Increased sympathetic tone associated with OSA has been shown to resolve after CPAP treatment. However, studies of whether CPAP has been shown to resolve hypertension have yielded conflicting results. [33] Nonetheless, a literature review by Liu et al suggested that CPAP can reduce blood pressure in OSA patients with resistant hypertension. Assessing data from five randomized, controlled studies, the investigators reported that, following CPAP treatment in these patients, pooled changes of -4.78 mm Hg and -2.95 mm Hg were seen for 24-hour ambulatory systolic and diastolic blood pressure, respectively. [34]
Oral appliances
Dental devices are simple, nonsurgical alternatives that are generally effective for simple snoring and mild sleep apnea. These devices fall under the categories of mandibular advancement devices (MAD) and tongue-retaining devices. [9] Dental devices reposition the upper airway anatomy to prevent obstruction by increasing the air space, either by stabilizing the mandible anteriorly, advancing the soft palate and tongue, or both (see Snoring and Obstructive Sleep Apnea, Oral Appliances for more details). [35]
Interest and usage of dental devices has increased since the 1990s; with advancements in appliances and design, patients tolerate these devices relatively well. Compared with CPAP, the primary advantages of oral appliances are portability, increased tolerance, ease of care, and quietness. The disadvantages are that they are not as effective in patients with severe apnea, they may be uncomfortable, and they may cause temporary temporomandibular joint (TMJ) pain.
Ferguson et al assessed the efficacy of mandibular advancing splints and found they improved apnea slightly. [36] Subjective reports, such as reduction of daytime somnolence, were greater than objective measures. Coruzzi focused on the effectiveness of oral appliances and suggested that personalized oral jaw-positioning devices improve SDB. [37] In turn, this may reduce cardiac variability, a marker for cardiovascular risk.
Minimal risks to the teeth are present when making the impression at fitting. Occasionally, the jaw-thrust device and CPAP must be used in concert to control sleep apnea.
Contraindications in the use of oral appliance are TMJ arthritis, poor dentition, nasal obstruction, poor motivation, and pharyngeal stenosis.
OSA Surgical Therapy
Surgical intervention for treating obstructive sleep apnea (OSA) is typically reserved for patients in whom continuous positive airway pressure (CPAP) treatment fails, whether due to noncompliance, intolerance, or ineffectiveness. Broadly speaking, the effectiveness of surgical treatment is quite controversial and studies have yielded conflicting results. Uvulopalatopharyngoplasty (UPPP), the most common surgical procedure for the correction of OSA, has only a 40% success rate, at best, and has even been shown to worsen OSA in several cases. [10]
Surgical interventions for OSA can be divided into those that bypass the upper airway and those that reduce upper airway obstruction. Procedures that bypass the upper airway are much more effective, but they are typically too extreme and not the treatment of choice in most situations.
Tracheostomy
Tracheostomy is the creation of a direct opening of the trachea (via tracheotomy) into which a tube is placed to bypass a potential upper airway obstruction. During waking hours, the patient can cap the tracheotomy tube; upon sleeping, the patient may uncap the tracheotomy tube to allow unobstructed ventilation. Although this is a very effective treatment for OSA, tracheostomy is reserved for special cases, such as a life-threatening disease secondary to cor pulmonale, arrhythmias, or hypoxemia that cannot otherwise be treated by CPAP.
LAUP, RFUP, and UPPP
More commonly, surgical correction seeks to relieve upper airway obstruction. Soft-palate surgery is the most common type of attempted surgical correction of OSA. Laser-assisted uvuloplasty (LAUP), radiofrequency uvuloplasty (RFUP), and uvulopalatopharyngoplasty (UPPP) have been used as the first step in surgical intervention.
UPPP consists of removal of the palatine tonsil, uvula, a portion of the soft palate, and the lateral pharyngeal wall. Outcomes of soft-palate surgery are less than ideal. With respect to long-term results, Boot et al suggested that resolution of OSA declined years after surgery. [38]
Using LAUP, RFUP, or both may be effective in mild sleep apnea but none has been proven in moderate or severe sleep apnea. The RFUP has a 41% recurrence rate of simple snoring after 14 months; repeat treatment in 8 of 9 patients resulted in a significant decrease in snoring. LAUP has also been shown to improve exercise tolerance in OSA patients during cardiovascular stress tests. [39]
Advancement procedures
Advancement procedures are often attempted in patients whose OSA does not respond to soft-palate procedures. These procedures have a 40-70% cure rate. Genioglossus, hyoid, and suture tongue advancement are common advancement procedures. Bimaxillary advancement is used when all other attempts have failed, but this procedure can be attempted at any time, particularly in patients with craniofacial abnormalities.
Nasal and tongue surgery
Other attempted surgical procedures include nasal and tongue surgery. Correction of nasal obstruction alone does not usually correct sleep apnea, particularly because patients with apnea often breathe through their mouths. Nasal procedures are often used as an adjunct to make CPAP available in the postoperative period. Tongue resection and submucosal radiofrequency surgery have been shown to be efficacious in patients whose OSA does not respond to soft-palate procedures. [10]
Radiofrequency surgery
Radiofrequency surgery is a minimally invasive technique used to reduce the bulk of the tongue at its base. The beneficial effects of radiofrequency surgery are likely a result of changes in upper airway collapsibility and not alteration of the upper airway anatomy. Tongue-base suspension procedures, in combination with UPPP, have been shown to be more efficacious than UPPP alone. [40]
Multistep procedures
Surgical treatment for OSA is often managed with a multistep approach. According to Jacobowitz, combined palatal and hypopharyngeal surgery effectively reduced symptoms (eg, daytime somnolence) and the apnea-hypopnea index (AHI) in patients with apnea who were CPAP intolerant and had diffuse airway narrowing and severely elevated AHI. [10] Multilevel surgery in patients with Friedman stages 2 and 3 yielded a decrease in symptoms and AHI.
A study by Friedman et al found a higher incidence of complications in patients with OSA who underwent multilevel surgery that included UPPP than in those who were treated with UPPP alone (4.63% vs 1.6%, respectively), although values for fatal complications were too small to compare. [41]
Using a staged surgical approach, Riley et al demonstrated a long-term surgical success rate of 90% (average follow-up duration, 50.7 mo). [42] Phase I consisted of UPPP, genioglossus advancement, and hyoid advancement. Phase II was reserved for phase I failures and consisted of bimaxillary advancement. The amount of skeletal advancement in the procedures was the most significant predictor of outcome. [42] The investigators used the respiratory disturbance index (RDI) and oxyhemoglobin desaturation nadir (LSAT) to compare preoperative CPAP control of OSA with postoperative outcomes. The differences were not statistically significant in 90% of cases. [42] In other words, OSA was managed equivocally by both advancement procedures and CPAP.
A study by Verse et al that focused on creating a protocol for multilevel surgery for OSA found better short-term results in their patients who underwent multistep procedures than those who received fewer surgical procedures. [43] All patients underwent uvula flap, tonsillectomy, and radiofrequency treatment of the tongue base, and one group additionally underwent hyoid suspension, whereas the other did not. In both groups, nasal surgery was performed if necessary. The group that received the additional hyoid suspension yielded better short-term results. [43]
Generally, surgical treatment of OSA has increasing efficacy with increasing number of procedures and a multistep approach. Note that most surgical candidates have a relatively low body mass index (BMI) compared with the classic OSA patient.
Potential Outpatient Procedures
Two potential outpatient procedures in the treatment of obstructive sleep apnea (OSA) are palatal implantation and cautery-assisted palatal stiffening operation (CAPSO).
Palatal implants
Palatal implantation has attracted attention as a potential treatment for mild to moderate OSA. [44] This is a minimally invasive approach in which 3 implants are placed inside the soft palate centered around the uvula. The morbidity is minimal, because this approach can be performed in a clinic and no mucosa is cut. If successful, the patient avoids both the operating table and the chronic use of the CPAP machine.
The palatal implant method has the potential to significantly improve the apnea-hypopnea index (AHI) in patients with mild to moderate OSA, minimal tonsillar hypertrophy, uvula elongation, and a body mass index (BMI) of less than 30 kg/m2. Short-term results are comparable with those reported for uvulopalatopharyngoplasty (UPPP) without the associated morbidity. In a study by Friedman et al, palatal implants were found to be a valuable mode of treatment for patients whose symptoms failed to improve after surgery, namely UPPP. [45] The subjective improvement of symptoms was significant.
Cautery-assisted palatal stiffening operation
CAPSO is another outpatient procedure that has been studied for the treatment of OSA. Unlike other oropharyngeal procedures (UPPP and laser-assisted uvuloplasty [LAUP]), CAPSO is a short, minimally invasive procedure with low morbidity. It has, however, been linked to increased pain compared with UPPP and LAUP. [46]
-
Snoring and obstructive sleep apnea syndrome (OSAS) treatment algorithm. ESS - Epworth sleepiness scale; LAUP – laser-assisted uvuloplasty; RFUP - radio frequency uvuloplasty; CPAP - continuous positive airway pressure; UPPP - uvulopalatopharyngoplasty.
-
Airway collapse.
Tables
What would you like to print?
- Practice Essentials
- Terminology in Sleep-Disordered Breathing
- Sleep Studies and Stages
- Sleep Physiology
- Relevant Anatomic Features in OSA
- Risk Factors for Sleep Apnea
- Pathophysiology of Airway Obstruction
- The Epworth Sleepiness Scale
- Mallampati Airway Classification
- Friedman Classification
- Diagnostic Considerations
- Pulse Oximetry
- Polysomnography
- Multiple Sleep Latency and Maintenance of Wakefulness Tests
- Management Overview
- Nonsurgical Behavioral Therapy
- OSA Surgical Therapy
- Potential Outpatient Procedures
- Show All
- Media Gallery
- Tables
- References