Overview
Multiple radiolucent or mixed radiolucent/radiopaque lesions of the mandible may present as incidental findings on radiographs or as the chief symptom of a patient. This article is not intended to be an all-inclusive discussion of such lesions; instead, it confines itself to an overview of the major odontogenic cysts and tumors with a brief discussion of other mandibular lesions that are often called cysts but are not true cystic lesions.
Although often similar in radiographic presentation, malignant tumors (both primary and metastatic), benign salivary tumors, and vascular lesions are not addressed herein. However, such lesions should be included in the differential diagnoses of a patient presenting with mandibular radiolucency or swelling. As a corollary, before the biopsy of any such lesions, the area should be aspirated to exclude a vascular lesion.
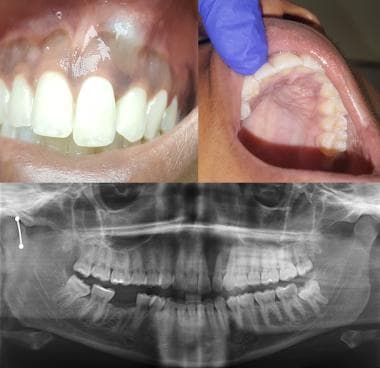
 Initial radiographic appearance. The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. The second radiograph depicts the appearance of the lesion at the second presentation.
Initial radiographic appearance. The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. The second radiograph depicts the appearance of the lesion at the second presentation.
Odontogenic Mandibular Cysts
Odontogenic cysts are defined as epithelial-lined structures derived from odontogenic epithelium. Most odontogenic cysts are defined more by their location than by any histologic characteristics. Accordingly, the surgeon must provide the pathologist with appropriate history and radiographs when submitting such specimens for examination.
Periapical cyst
A periapical (radicular) cyst is the most common odontogenic cyst. The usual etiology is a tooth that becomes infected, leading to necrosis of the pulp. Toxins exit the apex of the tooth, leading to periapical inflammation. This inflammation stimulates the Malassez epithelial rests, which are found in the periodontal ligament, resulting in the formation of a periapical granuloma that may be infected or sterile. Eventually, this epithelium undergoes necrosis caused by a lack of blood supply, and the granuloma becomes a cyst. The lesions are not usually clinically detectable when small but most often are discovered as incidental findings on radiographic survey.
Radiographically, distinguishing between a granuloma and a cyst is impossible, although some say that if the lesion is quite large it is more likely to be a cyst. [1] They both present as radiolucent lesions in association with the apex of a nonvital tooth. Occasionally, these lesions can become quite large because they grow in response to pressure. However, granulomas and cysts are not neoplastic.
Microscopically, the epithelium is a nondescript stratified squamous epithelium without keratin formation. Inflammatory changes may be observed in the cyst wall, and these changes, in turn, may lead to epithelial changes (eg, ulceration, atrophy, hyperplasia). In particularly inflamed lesions, cholesterol slits and/or foamy macrophages may be apparent.
Several treatment options exist for such cysts. Many cysts resolve with endodontic therapy of the involved tooth. Those lesions should be monitored radiographically to ensure such resolution. Lesions that fail to resolve with such therapy should be surgically removed and histopathologically examined. Although these cysts arise from a mature resting epithelium and thus have a relatively low growth potential, a squamous cell carcinoma occasionally may arise de novo in a radicular cyst, thus the recommendation for histopathologic examination of all tissues removed.
Dentigerous cyst
The second most common odontogenic cyst is the dentigerous cyst, which develops within the normal dental follicle that surrounds an unerupted tooth. The dentigerous cyst is not thought to be neoplastic. It most frequently is found in areas where unerupted teeth are found: mandibular third molars, maxillary third molars, and maxillary canines, in decreasing order of frequency.
Dentigerous cysts can grow very large and can move teeth, but, more commonly, they are relatively small. Most dentigerous cysts are asymptomatic, and their discovery is usually an incidental finding on radiography.
The usual radiographic appearance is that of a well-demarcated radiolucent lesion attached at an acute angle to the cervical area of an unerupted tooth. The border of the lesion may be radiopaque. The radiographic differentiation between a dentigerous cyst and a normal dental follicle is based merely on size.
However, histologically, a distinction other than size is found. The dental follicle is normally lined by the reduced enamel epithelium (see Odontogenesis), while the dentigerous cyst is lined with a stratified squamous nonkeratinizing epithelium. Dystrophic calcification and clusters of mucous cells may be found within the cysts.
Dentigerous cysts develop from follicular epithelium, and follicular epithelium has greater potential for growth, differentiation, and degeneration than the epithelium from which radicular cysts arise. Occasionally, other more ominous lesions arise within the walls of the dentigerous cyst, including mucoepidermoid carcinoma arising from mucous cells within the cyst walls, ameloblastoma (see Odontogenic tumors; 17% of ameloblastomas arise within a dentigerous cyst), and squamous cell carcinoma. As previously mentioned, dentigerous cysts also can become quite large and can place the patient at risk for pathologic jaw fracture.
These findings comprise most of the medical rationale for removal of impacted third molars with pericoronal radiolucencies; however, impacted teeth with small pericoronal radiolucencies (suggesting the presence of normal dental follicle rather than dentigerous cyst) may also be monitored with serial radiographic examination. Any increase in the size of the lesion should prompt removal and histopathologic examination. Any lesion that appears larger than a normal dental follicle indicates removal and histopathologic examination.
Primordial cyst
By definition, the primordial cyst develops instead of a tooth. Presumably, the dental follicle forms and subsequently undergoes cystic degeneration without ever completing odontogenesis. This is the rarest odontogenic cyst, and lesions designated as primordial cysts may represent residual cysts. The histology of these lesions is a nondescript stratified squamous epithelium. A complete dental history is important to establish a diagnosis of primordial (versus residual) cyst, although such a diagnosis often has little clinical significance in terms of treatment planning and decision making.
Residual cyst
Residual cyst is a term of convenience because no teeth are left by which to identify the lesion. Most commonly, these are actually retained periapical cysts from teeth that have been removed. The histology is a nondescript stratified squamous epithelium.
Lateral periodontal cyst
The name lateral periodontal cyst is a misnomer. These cysts are not inflammatory, they do not arise from periodontitis, and they are not a phenomenon associated with lateral canals within the tooth structure. These cysts are always well demarcated, relatively small, and radiolucent (sometimes with a radiopaque roof). They are most commonly associated with the mandibular premolar area and are occasionally found in the maxillary anterior. They are usually not clinically apparent but, rather, are detected through radiographic examination. These cysts have a distinctive histology consisting of a thick fibrous noninflamed cyst wall, and the lining epithelium is made of thin cuboidal cells. This lining is incomplete and easily sloughs away with mural thickenings of clear cells at periodic intervals. These cysts develop from the postfunctional dental lamina, and no good explanation is known for the localization that is shown.
Gingival cyst of the newborn
Gingival cysts of newborns generally occur in multiples but occasionally occur as solitary nodules. They are located on the alveolar ridges of newborns or young infants. These structures originate from remnants of the dental lamina and are located in the corium below the surface epithelium. Occasionally, they may become large enough to be clinically noticeable as discrete white swellings on the ridges. They are generally asymptomatic and do not produce any discomfort for the infant.
Bohn nodules and Epstein pearls are 2 similar lesions with which gingival cysts sometimes may be confused; however, the location and etiology of these lesions are somewhat different. Epstein pearls are cystic keratin-filled nodules found along the midpalatine raphe and are thought to be derived from entrapped epithelial remnants along the line of fusion. Bohn nodules are keratin-filled cysts scattered all over the palate, but they are most apparent at the junction of the hard and soft palate. These are thought to be derived from palatal salivary gland structures.
Histologically, the gingival cyst of the newborn is a true cyst with a thin epithelial lining. The lumen is usually filled with keratin but may contain some inflammatory cells, dystrophic calcifications, and hyaline bodies, such as those often found in dentigerous cysts.
No treatment is required for these lesions, which usually disappear either by opening onto the surface mucosa or through disruption by erupting teeth. These cysts are most likely what older literature describes as predeciduous dentition.
Gingival cyst of the adult
Gingival cysts of the adult are found only in soft tissue in the lower premolar areas. These cysts present as tense, fluctuant, vesicular, or bullous lesions. Histologically, they look like lateral periodontal cysts, and they probably represent the same lesion when found in soft tissue.
Odontogenic keratocyst
This lesion is relatively common. Originally described in 1956, by a Danish surgeon, it was first called an odontogenic keratocyst. In 2006, the parakeratinized (and more common) variant of the lesion was renamed the keratocystic odontogenic tumor (KCOT), to reflect the fact that this lesion represents a benign odontogenic tumor rather than a simple cyst. Of note, the much less common orthokeratinized version, which has a far lower incidence of recurrence, is still considered a cyst. In 2017, with the publication of the fourth edition of the WHO Classification of Head and Neck Tumours, from the World Health Organization (WHO), the official name of this entity reverted to odontogenic keratocyst (OKC). [2]
Clinically and radiographically, the OKC may have any appearance; it is a great mimic, and the diagnosis is a histologic one. These lesions can be both aggressive and difficult to remove, growing quite rapidly, and recurrences are frequent. They are the third most common odontogenic cyst/tumor and belong in the differential diagnoses of any radiolucency of the jaws. Although 40% of OKCs appear in a dentigerous relationship, 9% of dentigerous cysts are OKCs when the histology is examined. These lesions are additionally found as part of the basal cell nevus syndrome, also known as Gorlin syndrome (see the next section, Basal cell nevus syndrome).
Histologically, OKCs are formed with a stratified squamous epithelium that produces orthokeratin (10%), parakeratin (83%), or both types of keratin (7%). The epithelial lining appears corrugated when viewed under a microscope. A well-polarized, hyperchromatic basal layer is observed, and the cells remain basaloid almost to the surface. No rete ridges are present; therefore, the epithelium often sloughs from the connective tissue (94% of the time). The epithelium is thin, and mitotic activity is frequent; therefore, OKCs grow in a neoplastic fashion and not in response to internal pressure. The lumen frequently is filled with a foul-smelling, cheeselike material that is not pus but rather collected degenerating keratin.
The lesions grow in a multilocular, bosselated fashion, with daughter cysts that extend into the surrounding bone. Because of this relationship, the tendency for recurrence is high, particularly if the original surgical treatment does not result in complete removal of the lesion. Enucleations with peripheral ostectomy and/or cryosurgery are the most common forms of treatment. [3] Preliminary marsupialization and decompression prior to definitive treatment is particularly helpful when treating lesions that are large or in close proximity to important anatomic structures such as the inferior alveolar nerve or maxillary sinus. Long-term (lifetime) clinical and radiographic follow-up is imperative. If these lesions are left untreated, they can become quite large and locally destructive.
As mentioned, the variant that produces only orthokeratin acts somewhat differently than the lesions that produce parakeratin. The orthokeratin variation is almost always found in a dentigerous association, usually around the mandibular third molar, and is much less aggressive. It does not exhibit a hyperchromatic basal layer; in fact, the basal layer is flattened. This variant is not associated with basal cell nevus syndrome.
Basal cell nevus syndrome
This symptom complex includes hypertelorism, midface hypoplasia, relative frontal bossing and prognathism, intellectual disability, schizophrenia, multiple basal cell carcinomas, calcification of the falx cerebri, bifid ribs, palmar pitting (the pits later develop into basal cell carcinoma), and multiple OKCs. Multiple OKCs are diagnostic for basal cell nevus syndrome until proven otherwise. This is a hereditary disease with autosomal dominant inheritance and high penetration. Of patients with OKC, 5% have basal cell nevus syndrome. Early identification of these patients and their lesions is key to improving long-term survival and quality of life.
Glandular odontogenic cyst (GOC)
The glandular odontogenic cyst (GOC) is a very rare and relatively new entity. First reported in 1987, by Padaychee and Van Wyk, it was initially called a sialodontogenic cyst. By 1992, however, it had become obvious that this cyst is not salivary but odontogenic in origin and as such the name was changed to glandular odontogenic cyst. This lesion represents approximately 0.2% of all odontogenic cysts, making reliable statistics difficult to obtain. One of the largest literature reviews of the cyst was performed by Kaplan et al and included 111 published case reports. In this cohort, the age ranged from 14-75 years, with a mean age of 45.7; there was a slight male predilection (1.3:1) and a significant mandibular over maxillary predilection (70:30), with no significant difference in the number of cases found between anterior and posterior tooth-bearing structures. [4]
GOCs range in size from very small to rather large and may present as a unilocular or multilocular radiolucency. They can be very aggressive, not only expanding but also causing thinning (24.4%) and perforation (61%) of cortical bone. Recurrence rates after treatment vary and appear to be proportional to a number of factors, including the size of the initial lesion at diagnosis and the extent of the treatment offered. Published recurrence rates thus range from as low as 1.44% in very small lesions to 85.6% in large lesions. Kaplan et al defined a large lesion as one that involves more than two teeth and/or extends beyond alveolar bone or into adjacent structures.
GOCs, though quite rare, are significant because they are easily confused with multiple types of lesions, most of which are benign (eg, mucous metaplasia in an odontogenic cyst, surgical ciliated cysts, botryoid cysts). Importantly, they are also easily confused with low-grade mucoepidermoid carcinoma, a slow-growing but relentless malignancy with excellent short-term survival rates but a very poor long-term prognosis. Thus, it is essential to identify and appropriately treat GOCs not only to address local destruction but also to ensure that a proper diagnosis is made.
Histologically, GOCs are characterized by a nonkeratinized, stratified squamous epithelium of varying thickness with focal areas of proliferation. There is a flat interface with the underlying connective tissues, and there is no palisading of the basal layer. Hobnail (cuboidal eosinophilic) cells are common in the lining epithelium, and periodic acid-Schiff–positive material and/or clefts lined by mucous cells within the lining, along with intraepithelial ducts, are common. Less commonly, papillary conformations, cilia, vacuolated cells, and daughter cysts have been described. An excellent flow chart for the histologic differentiation of these lesions is provided in the following study: Kaplan I, Anavi Y, Manor R, Sulkes J, Calderon S. The use of molecular markers as an aid in the diagnosis of glandular odontogenic cyst. Oral Oncol. 2005 Oct. 41 (9):895-902. [5]
Treatment options vary depending on the size and location of the GOC at discovery. Minor treatment for a very small lesion confined to the apex of a single tooth may consist of endodontic therapy, apicoectomy, curettage, and submission for histopathologic diagnosis with peripheral osteotomy. Less aggressive surgical treatment of larger lesions may include enucleation/curettage with subsequent treatment with Carnoy’s solution (now difficult to obtain in United States, as the chloroform in this preparation has been deemed a carcinogen), cryosurgery, or peripheral osteotomy. For lesions that are very large and/or in close proximity to a significant anatomic structure, marsupialization and decompression prior to definitive treatment may improve success, decrease morbidity, and increase patient acceptance of treatment. Finally, for very large lesions, marginal or segmental resection may be indicated.
Nonodontogenic Mandibular Cysts
Stafne bone cyst
A Stafne bone cyst is an unusual form of slightly aberrant salivary gland tissue wherein a developmental inclusion of glandular tissue is found within or, more commonly, adjacent to the lingual surface of the body of the mandible within a deep and well-circumscribed depression. The oldest described occurrence of this phenomenon is in a skull dated to the sixth to fourth centuries BC. The phenomenon was first recognized by Stafne in 1942, hence the eponym.
However, this cyst has been referred to by many other names, including static bone cavity, defect of the mandible, lingual mandibular bone cavity, static bone cyst, latent bone cyst, and Stafne bone defect. The incidence of occurrence has been reported as 0.1-1.3% in various studies. The general consensus is that this is a congenital defect, although it rarely has been observed in children. These lesions may generally be regarded as developmental rather than pathologic defects. A predilection for males over females seems to exist.
Radiographically, the lesion usually appears as an ovoid radiolucency located between the inferior alveolar canal and the inferior border of the mandible in the region of the second or third molars. It can be differentiated from the traumatic or hemorrhagic bone cyst, which by location almost invariably lies superior to the inferior alveolar canal.
Although the classic Stafne cyst is described in the posterior mandible, an anterior variant presenting as a round or ovoid radiolucency in the area between the central incisors and first premolars exists; however, it is far less common.
These lesions generally represent benign developmental anomalies that normally do not require any treatment. A complication occasionally reported in the literature is the development of a true salivary gland neoplasm in the tissue associated with one of the cortical defects. Therefore, recording the finding of these lesions and periodically observing them radiographically seem prudent. Clinical or radiographic changes may indicate the need for further investigation.
Traumatic bone cyst
The traumatic bone cyst also is known as solitary bone cyst, hemorrhagic cyst, extravasation cyst, unicameral bone cyst, simple bone cyst, and idiopathic bone cavity.
The traumatic bone cyst is a relatively frequent lesion both in the jaws and elsewhere in the skeleton. The specific etiology of the lesion is unknown, although several mechanisms have been proposed. The most widely accepted is that these lesions originate from intramedullary hemorrhage caused by trauma. In these cases, failure of organization of the blood clot occurs followed by subsequent degeneration of the clot, eventually leading to an empty bone cavity. Restricted venous drainage leads to increasing edema, which in turn causes continued resorption of trabeculae and expansion of the lesion. Expansion of the lesion tends to stop when cortical bone is reached, thus these lesions are not characterized by any cortical expansion. Instead, they are usually incidental findings on radiographs taken for other purposes. However, it is not unusual for a patient to be unable to recall any trauma to the involved jaw.
The lesion is most commonly found in young persons (median age, 18 y); the male-to-female incidence ratio is 3:2. The lesions occasionally have been reported in the maxilla but are far more common in the mandible. When the cavities are opened surgically, they are generally either empty or filled with a small amount of straw-colored fluid. Shreds of necrotic clot and fragments of fibrous connective tissue have been reported less commonly. Histologically, these cysts may have a thin connective tissue membrane lining or no lining at all.
Radiographically, these lesions tend to appear as smoothly outlined radiolucencies that scallop around the roots of the teeth. They do not displace teeth or resorb roots, and the lamina dura is left intact. They may range from very small (< 1 cm) to very large (involving most of the mandible). They tend to occur above the inferior alveolar canal.
These lesions are usually surgically explored to establish a diagnosis, which is made upon finding an empty cavity. No further treatment is generally necessary because surgical manipulation causes the cavity to fill with blood. Soft tissues are closed, and the lesion tends to heal without further intervention. The extreme rarity of such lesions in older patients suggests that the lesions may be self-limiting and/or subject to resolution over time.
Focal osteoporotic bone marrow defect
Bone marrow may be stimulated in response to unusual demands for increased blood cell production. This hyperplastic marrow may present as focal radiolucencies in the jaws. Of jaw lesions, 75% are reported to occur in female patients, and 85% of jaw lesions are found in the mandible. The lesions are almost always asymptomatic and are discovered as incidental findings on radiographs taken for other indications.
Radiographically, these lesions present as ill-defined radiolucencies of variable size, more commonly in edentulous areas. This suggests that in some cases, the lesions represent failure of normal bone regeneration postextraction. Histologically, the tissue in these areas is composed primarily of red marrow, yellow marrow, or a combination of both with long thin irregular trabeculae that are missing the osteoblastic layer.
The radiographic appearance of these lesions is not pathognomonic; therefore, these lesions are usually diagnosed surgically. Once diagnosed, they require no further specific treatment; however, if the etiology for the increased hematopoietic demands is unknown, consider an investigation.
Aneurysmal bone cyst
In 1942, Jaffe and Lichtenstein first classified the aneurysmal bone cyst as a distinct lesion; it is neither a cyst nor an aneurysm. It was not reported in the jaws until 1958, and although theories abound, the etiology and pathogenesis of this lesion are still unknown. Proposed mechanisms for the formation of the aneurysmal bone cyst include alterations in local hemodynamics leading to venous engorgement, resorption, and replacement with connective tissue and osteoid; futile attempts at repair of a hematoma (eg, what occurs in the giant cell granuloma); and microcyst formation secondary to cellular edema associated with other lesions. Often, but not always, these lesions appear in association with other lesions of bone, such as the unicameral cyst, dentigerous cyst, osteoclastoma, central giant cell tumor, fibrous dysplasia, and osteosarcoma.
Aneurysmal bone cysts have been observed in every part of the skeleton, although more than 50% of lesions occur in the long bones and vertebral column. They occur in jaws of people of all ages, but a predilection for younger patients and female patients exists. Aneurysmal bone cysts are more likely to occur in the mandible than in the maxilla. They may displace but usually do not resorb the dentition, and sensory disturbances generally are not present. The radiographic appearance often is described as cystic, honeycomb, or soap bubble with eccentric expansion. Cortical bone may be thinned or destroyed, and a periosteal reaction may be present.
Histologically, the aneurysmal bone cyst reveals a fibrous connective tissue stroma with many cavernous or sinusoidal blood-filled spaces. Young fibroblasts are present throughout the stroma, and multinucleated giant cells are scattered throughout the lesion. Without the cavernous spaces, this lesion would appear almost identical to the central giant cell granuloma.
Treatment of the aneurysmal bone cyst requires complete removal, and complete removal of the lesion with aggressive curettage is the most common treatment modality. Excellent exposure to facilitate this treatment is necessary because these lesions may bleed copiously, and timely but thorough removal of the lesion helps to decrease blood loss. Failure to completely remove all traces of the lesion carries a significant risk (21-59%) of recurrence. Recommendations for bone grafting of the resultant defect vary according to the remaining clinical situation after removal of the lesion. Some authors recommend excision with cryosurgery for recurrent lesions, while other authors advocate block excision or resection with reconstruction. In the past, radiation was proposed for the treatment of these lesions, but radiation may fail to arrest the lesion and, more importantly, can result in sarcomatous changes.
Odontogenesis
Odontogenic tumors represent the results of interruptions in or reactivation of tissues involved in the normal sequence of odontogenesis. The nature of the neoplasm is determined by the stage of development at which the arrest occurs. A brief review of odontogenesis is helpful in understanding the pathogenesis and behaviors of odontogenic tumors.
At the sixth week of gestation, odontogenesis begins with proliferation of certain areas of oral ectoderm to form the dental lamina. At each of the locations where a tooth will be formed, a downgrowth from the dental lamina forms the beginning of the enamel organ. Together, the enamel organ, dental papilla, and dental sac are the formative structures for the entire tooth and supporting structures. The dental lamina, which originally connected the enamel organ to the oral epithelium, breaks up, thus separating the developing tooth bud from the epithelium of the oral cavity.
Each stage in the development of the tooth is associated with certain events that are summarized below. Interruptions in this sequence may lead to the formation of odontogenic tumors.
-
Bud stage: Initiation and formation of the enamel organ occurs.
-
Cap stage: Proliferation occurs. Unequal growth leads to the characteristic shape. The peripheral cells are cuboidal and are termed the outer enamel epithelium (OEE), and the cells in the concavity are tall columnar cells termed the inner dental epithelium. At the same time, polygonal cells between outer and inner enamel epithelia begin to separate and form the delicate cellular network known as the stellate reticulum (SR), the spaces of which are filled with a mucoid fluid. Histologically, this material resembles Wharton jelly. The proliferation of the epithelial components just discussed leads to condensation of the enclosed ectomesenchyme and formation of the dental papilla. The cells of the dental papilla eventually form tooth pulp and dentin. Similarly, condensation of the ectomesenchyme surrounding the enamel organ leads to creation of the dental sac. The cells of the dental sac eventually form cementum and the periodontal ligament.
-
Bell stage: Histodifferentiation (early) and morphodifferentiation (late) occur. The enamel organ now assumes an elongated bell shape and has 4 distinct types of epithelial cells: inner enamel epithelium (IEE), stratum intermedium, SR, and OEE.
The IEE organizes and induces the adjacent cells of the dental papilla to differentiate into odontoblasts, which form dentin. The dentin, in turn, induces the IEE to differentiate into ameloblasts, which lay down enamel matrix opposite the dentin. This reciprocal induction is essential for the formation of the tooth.
The stratum intermedium consists of a few layers of squamous cells between the IEE and the SR. This layer is apparently essential to enamel formation because it is absent in the part of the tooth germ that outlines areas of the tooth without enamel.
The SR expands by increasing the amount of intracellular fluid. It collapses before the formation of enamel, leaving the ameloblasts closer to the nutrient capillaries adjacent to the OEE.
The OEE, which was formerly smooth, becomes folded. In the folds, the adjacent mesenchyme of the dental sac forms papillae with capillary loops to provide a supply of nutrients for the metabolic activity of the avascular enamel organ. The enamel organ also forms Hertwig epithelial root sheath, which determines the shape of the roots and initiates the formation of dentin in the roots.
-
Apposition: The deposition of the matrix of the hard dental structures occurs next. These structures then begin calcification, eruption, and, with time, attrition.
Odontogenic Tumors
Ameloblastoma
Ameloblastoma (see the images below) is an entirely epithelial tumor arising from the dental lamina, Hertwig sheath, the enamel organ, or the lining of dental follicles/dentigerous cysts. Ameloblastoma is the most common epithelial odontogenic tumor. Ameloblastomas usually occur in individuals aged 20-40 years; however, the unicystic variant (see Surgical considerations, below) most often occurs in adolescents. This lesion occurs in both the maxilla and mandible, but the posterior mandible is the most common location; only 20% of lesions are found in the maxilla. The lesion is distributed equally between males and females.
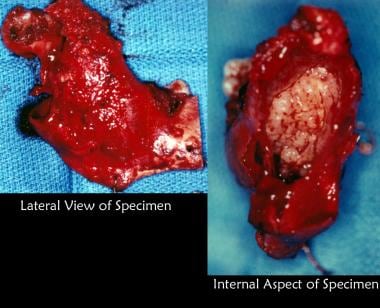 The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. Incisional biopsy revealed the lesion to be an ameloblastoma. Treatment consisted of segmental resection of the entire mandible from the condyle to the area of the second premolar. The lateral and internal aspects of the resected specimen are depicted.
The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. Incisional biopsy revealed the lesion to be an ameloblastoma. Treatment consisted of segmental resection of the entire mandible from the condyle to the area of the second premolar. The lateral and internal aspects of the resected specimen are depicted.
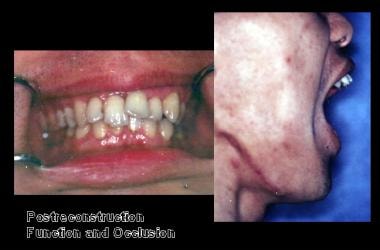 The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow-up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. Incisional biopsy revealed the lesion to be an ameloblastoma. Treatment consisted of segmental resection of the entire mandible from the condyle to the area of the second premolar. The defect was reconstructed using autogenous rib, iliac crest, and tibial bone. Note the stable occlusion and excellent function obtained.
The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow-up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. Incisional biopsy revealed the lesion to be an ameloblastoma. Treatment consisted of segmental resection of the entire mandible from the condyle to the area of the second premolar. The defect was reconstructed using autogenous rib, iliac crest, and tibial bone. Note the stable occlusion and excellent function obtained.
Although ameloblastoma generally is not classified as a malignant lesion (a rare malignant variant exists), it is extremely aggressive and infiltrative. Many have suggested that this lesion should be considered a low-grade or indolent malignancy, similar to basal cell carcinoma. Many histologic and behavioral similarities are found between the 2 lesions. It generally does not metastasize but is slow growing, persistent, and hard to eradicate. If ameloblastoma is not noticed as an incidental finding on radiographs taken for other purposes, the first symptom is usually painless bony expansion.
Based on a literature review and meta-analysis, Chae et al estimated that ameloblastomas have a mean growth rate of 87.84% per year, with solid, multicystic ameloblastomas having the highest growth rate, and the peripheral form of the tumor having the lowest such rate. [6]
Radiographic findings
Ameloblastomas typically appear as an expansile multilocular radiolucency in the area of the lower third molar, but they may be found anywhere in the jaws (see the image below). These lesions may be unilocular when small, and they often resorb the teeth they contact. These lesions are never radiopaque.
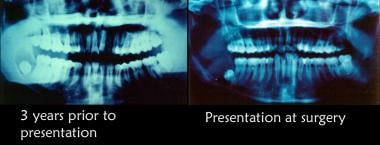
 Initial radiographic appearance. The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. The second radiograph depicts the appearance of the lesion at the second presentation.
Initial radiographic appearance. The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. The second radiograph depicts the appearance of the lesion at the second presentation.
Histologic characteristics
Ameloblastoma does not have a capsule. The neoplastic component is purely epithelial and resembles the cap stage of odontogenesis (ie, polarized tall columnar cells on the outer aspect of the lesion with SR on the inner aspect, which may form a cyst). The lesion may have a reactive connective tissue component that is not neoplastic. This is a nonfunctional tumor, ie, it does not induce the surrounding connective tissue, which in turn is unable to induce enamel formation. In effect, these tumors represent arrested odontogenesis. Multiple histologic varieties exist, eg, the acanthomatous type in which the SR is replaced by squamous cells and pearls, the granular cell type in which the SR is replaced by granular cells, and the plexiform type in which the SR is reduced or absent.
Treatment
The treatment of ameloblastoma is surgical excision with wide free margins (see Surgical considerations, below). Appropriate reconstruction may be performed at the same time. All patients with ameloblastoma, regardless of surgical treatment method or histologic type, must be monitored radiographically throughout their lifetime. If excision is inadequate, recurrence is common.
Surgical considerations
See the list below:
-
The maxillary ameloblastoma is not confined by the strong cortical plate found in the mandible. In addition, the posterior maxilla lies in close relationship to many vital structures. These factors make strong arguments for aggressive and definitive surgical treatment of the maxillary ameloblastoma.
-
In the mandible, 1-cm clear margins are considered the standard. This may be accomplished with block or segmental resection, depending on the relationship of the lesion to the inferior cortical border.
-
The single exception to this may be the unicystic ameloblastoma. This variant most commonly appears in late adolescence and, as the name suggests, is characterized by a unicystic radiolucency that most commonly is found in the area of the mandibular third molars. Unlike other types of ameloblastomas, it is believed that this lesion is encapsulated and can be removed with enucleation/curettage procedures alone. These lesions may recur, and recurrences may require more aggressive treatment. Most authors believe that if left untreated, this lesion becomes an ameloblastoma of one of the classic varieties, leading to the corollary conclusion that these lesions simply represent an early stage in the development of ameloblastoma.
-
For peripheral ameloblastoma, a more conservative excision with close clinical follow-up is the standard of care.
Relationship to other lesions
See the list below:
-
Basal cell carcinoma: Basal cell carcinoma is another infiltrative, essentially nonmetastasizing adnexal neoplasm. Basal cell carcinomas and ameloblastomas are slow growing but persistent, and they may cause death via local extension into vital structures. If one considers that the tooth is an oral adnexal structure, then it is easy to understand why ameloblastoma may be seen as an analogue to basal cell carcinoma.
-
Tibial adamantinoma: This lesion is histologically similar to the plexiform variety of ameloblastoma. It is considered a low-grade malignancy and, as the name suggests, is found in the tibia.
-
Craniopharyngioma: This pituitary tumor arises from Rathke pouch, part of the oral stomadeum that histologically appears somewhat like ameloblastoma. However, it is actually more like the Gorlin cyst.
-
Peripheral ameloblastoma: This lesion is histologically identical to the central ameloblastoma, but it does not involve bone and is confined entirely to the gingiva. It has a lower potential for growth and invasion than the central ameloblastoma, and, quite possibly, it is responsible for reported cases of basal cell carcinoma in the gingiva.
-
Malignant ameloblastoma: Approximately 2% of ameloblastomas metastasize, usually to the lungs. These lesions may actually be the result of aspiration of material from fungating lesions in the oral cavity and, therefore, do not represent true metastases.
-
Ameloblastic carcinoma: These are cytologically malignant lesions with hyperchromatism, pleomorphism, and high mitotic activity. Real metastases occur with ameloblastic carcinoma.
Adenomatoid odontogenic tumor
The adenomatoid odontogenic tumor (AOT) is a fairly uncommon tumor, but it usually can be easily identified from its clinical and radiographic appearance. It often is remembered as the "two-thirds tumor." It most commonly occurs in the second and third decades of life (12-20 y). Two thirds of the cases occur in the anterior maxilla, one third occur in the anterior mandible, and it is never found posterior to the premolars. Two thirds of the cases occur in females, and two thirds of the cases are associated with an impacted tooth (usually the cuspid).
This tumor originates from the reduced enamel epithelium of the dental follicle and histologically reproduces the IEE. It is generally asymptomatic but may present with mild swelling or in association with a clinically missing tooth.
Radiographic findings
This lesion generally appears as a well-demarcated radiolucency. In 75% of cases, it is associated with an unerupted tooth, usually the canine. It may contain radiopaque flecks, which represent calcified material. If associated with a tooth, it generally attaches to the tooth further apical on the root than the typical dentigerous cyst.
Histologic characteristics
Technically, this is a hamartoma rather than a true neoplasm because it has a limited growth potential. It has a thick fibrous capsule filled with a proliferation of epithelial elements that form nodules and ductlike structures (eg, organoid nodules of cuboidal or low columnar cells separated by spindly epithelium). In the absence of connective tissue to induce the formation of enamel, the product of these cells, a pre-enamel matrix, is thought to degenerate and ultimately leave areas of dystrophic calcification and amyloid.
Treatment
The recommended treatment of these lesions is simple removal. If left alone, these structures probably involute. However, they can become quite large. Most are removed at biopsy. If AOT is incompletely removed at biopsy, the literature suggests that the remainder of the lesion degenerates. They are not known to recur.
Calcifying epithelial odontogenic tumor
The calcifying epithelial odontogenic tumor (CEOT), or Pindborg tumor, is a benign infiltrative odontogenic tumor that is one of the rarest. It is named after Jens Pindborg, the Danish oral pathologist. It is most often found in the mandibular molar/premolar region, but 33% of cases are found in the maxilla. It is associated with an unerupted or impacted tooth in 50% of cases. CEOT is an infiltrative neoplasm and causes destruction with local expansion. It is derived from the stratum intermedium and has a lower growth potential than ameloblastoma. Not surprisingly, it is less aggressive than ameloblastoma.
In a review of 20 cases of CEOT, by Ruddocks et al, the condition was found equally in males and females, with more of the tumors arising in the mandible than in the maxilla (60% and 40%, respectively). With regard to CEOT variants, two patients had incipient CEOT, and two had peripheral CEOT. [7]
Radiographic findings
These lesions can be radiolucent, but they more characteristically are mixed lucent and opaque masses, exhibiting a snow-driven appearance.
Histologic characteristics
The histologic view of this lesion is worrisome because it appears as invasive infiltrative islands in bone. These islands look like pure squamous cells with a high degree of nuclear pleomorphism; however, Liesegang rings (ovoid dystrophic calcifications), a normal mature cytoplasm (large polyhedral cells with good intercellular bridges and filled with mature keratin granules), and the lack of mitotic figures help to distinguish this lesion from squamous cell carcinoma. The pleomorphism noted is secondary to degeneration of the nuclei and necrobiosis, and dystrophic calcification and amyloid conversion are characteristic in dying epithelial cells.
Treatment
The treatment of this lesion is complete surgical excision. The recurrence rate for CEOT is 4%. The lesion is slow growing and requires long-term follow-up monitoring for recurrence (at least 5-10 y). No cases of malignant transformation are reported.
Keratinizing and calcifying odontogenic cyst/calcifying odontogenic cyst
The keratinizing and calcifying odontogenic cyst (KCOC), or Gorlin cyst, is not actually a cyst but rather a neoplasm with cystic tendencies. Some KCOC lesions are actually solid. This is a very rare lesion with no age, sex, or location predilections. KCOC may be found anywhere in the jaws, and one fourth of lesions are found in peripheral soft tissue (eg, gingiva). If KCOC is not discovered as an incidental finding on radiographic examination, the earliest clinical presentation usually is a localized swelling.
These lesions arise from a more mature enamel epithelium than ameloblastoma, and, accordingly, they have less growth potential.
Radiographic findings
These are nondescript radiolucencies that may contain flecks of opacity. They may become quite large if not discovered serendipitously.
Histologic characteristics
These lesions are lined by an epithelium that is similar in appearance to ameloblastoma (eg, polarized basal layer and SR are present). KCOC appears somewhat similar to the unicystic ameloblastoma, with masses of keratinized squamous epithelial cells within the SR. However, these cells have no nuclei and are called ghost cells. This ghost epithelium eventually herniates into the connective tissue, causing a connective tissue foreign body response that results in dentinoid dystrophic calcification and the formation of granulation tissue. More simply, this lesion represents enamel epithelium that has a tendency to mature but is unable to form enamel. The result is the formation of ghost keratin that may, in turn, induce dentinoid. The similarities between this lesion and the craniopharyngioma have previously been mentioned.
Treatment
These lesions are surgically removed and rarely recur after excision. A case report by Emam et al found that in KCOC surgery, use of a two-stage approach, in which decompression was followed by enucleation and curettage, led to a significant reduction in deformity. However, long-term follow-up was necessitated by the possibility of recurrence. [8]
A note on terminology: Often there is discussion, particularly amongst pathologists, as to the correct name for a specific entity and, from time to time, terminology changes. A recent discussion on the Bulletin Board of Oral Pathology noted that at one institution the term calcifying odontogenic cyst (Gorlin cyst) is used for the cystic version of this lesion, while dentinogenic ghost cell tumor is used for the “solid, non-cystic lesions” composed of dentinoid and ghost cells. A similar terminology discussion has simultaneously been entertained on the subject of the odontogenic keratocyst. The important take-home point is that good communication with the pathologist examining the lesion is a necessity to make sure that the treating surgeon and the pathologist are indeed talking about the same lesion and to ensure that the treatment options to be offered are appropriate to the disease entity sampled.
Odontogenic myxoma
This is a benign infiltrative lesion that is clinically indistinguishable from ameloblastoma. It is found in tooth-bearing areas, and a slight predilection for the mandible exists. It generally appears in the early third to fourth decades of life as a slow-growing expansile lesion. If odontogenic myxoma is left untreated, it is invasive and destructive. It is derived from dental mesenchyme (papilla) or follicle.
Radiographic findings
The radiographic appearance of this lesion is not distinctive. It appears quite similar to ameloblastoma (eg, multilocular radiolucency), though some authors believe that the individual loculations are somewhat smaller in odontogenic myxoma (myxofibroma).
Histologic characteristics
A few stellate fibroblasts with copious amounts of hyaluronic acid, scant collagen fibrils, and no capsule describe the histologic appearance of this lesion. The hyaluronic acid component stains with Alcian blue, reminding one of the appearance of Wharton jelly. This lesion looks like developing pulp and may be confused with a developing third molar.
Treatment
As with ameloblastoma, this lesion is treated with block excision. Recurrences occur although with somewhat less frequency than with ameloblastoma.
Ameloblastic fibroma
Ameloblastic fibroma is a true mixed tumor arising from a combination of 2 embryonic tissues. The epithelial component is able to induce mesenchyme but not to the extent of developing dental hard tissues. It is a relatively uncommon tumor of young people (aged 5-20 y); 75% of ameloblastic fibromas are found in the posterior mandible in the area of a developing tooth. It is benign and expansile, growing as a pushing front rather than invading surrounding tissues.
Radiographic findings
This lesion appears as a uniocular or bilocular radiolucency, most often in the posterior mandible. The radiographic appearance is identical to that of unicystic ameloblastoma, and both lesions should be differential diagnoses because they affect similar age groups and have similar clinical and radiographic appearances. Histologic examination differentiates the two.
Histologic characteristics
The epithelial component of this lesion is almost identical to that of ameloblastoma; however, the connective tissue component looks like dental pulp. It is a young, cellular, homogenous connective tissue without much dense collagen. The epithelial and connective tissue components grow together inside a capsule.
Treatment
The treatment of this lesion is block excision with a border of normal bone. With simple enucleation, recurrence rates of 20-40% have been reported. Sarcomatous change (ameloblastic fibrosarcoma) has also been reported with recurrence or inadequate excision.
Ameloblastic fibro-odontoma
Ameloblastic fibro-odontoma is an extremely rare odontogenic lesion that develops dental hard tissues (eg, dentin, enamel, cementum). It appears most often before age 20 years and has a slight predilection for the premolar area in either jaw. Although these lesions can become quite large, they generally do not have much growth potential.
Radiographic findings
Ameloblastic fibro-odontoma appears as a well-demarcated radiolucency with a large central opacity. These lesions almost always are found in association with a tooth. Some similarity exists in radiographic appearance to that of the Pindborg tumor and Gorlin cyst because this is a mixed radiolucent/radiopaque lesion. If located in the anterior mandible, AOT also may be included in the radiographic differential diagnoses.
Histologic characteristics
This lesion has the histologic characteristics of ameloblastic fibroma. However, epithelial induction of connective tissue occurs to the point of histodifferentiation, leading to presence of dentin, enamel, and/or cementum in the microscopic presentation.
Treatment
Surgical excision of ameloblastic fibro-odontoma is the usual proposed treatment. These lesions do not often recur. The literature contains some support for sarcomatous change with recurrence.
Complex odontoma
Complex odontoma lesions represent further histologic differentiation of the odontogenic pluripotential epithelium. Findings from this lesion resemble those of ameloblastic fibro-odontoma but extended one step further. The epithelium in this lesion has involuted, leaving disorganized dental hard tissues in place. These are common lesions, and they persist throughout life. They are usually detected in adolescence and have a predilection for the mandibular molar regions; however, they can be found in other areas of the jaws.
Radiographic findings
These lesions are generally described as sunburst radiopacities surrounded by a thin, uniform, radiolucent rim. Although this description may have some superficial resemblance to the radiographic description of osteosarcoma, the association with a tooth, the clear demarcation of the lesions' borders, and the lack of pain and/or swelling serve to delineate this very benign lesion from osteosarcoma.
Histologic characteristics
The histodifferentiation of this lesion is extended one step further than that of ameloblastic fibro-odontoma. In complex odontoma, a physiologic reduction of the ameloblastic epithelium is found. A mixed honeycomb presentation of enamel, cementum, dentin, and pulpal tissue is present. Histodifferentiation but no morphodifferentiation is observed in this lesion.
Treatment
Simple removal or radiographic observation is the method of treatment. These lesions do not recur.
Compound odontoma
This is the most common odontogenic tumor. It represents the product of both histodifferentiation and morphodifferentiation of odontogenic tissues, resulting in what appears as a cluster of multiple abortive teeth. It is most commonly found in the maxillary anterior alveolar bone but may be located anywhere within the tooth-bearing segments of the jaws. It is often responsible for preventing normal tooth eruption, thus it is usually discovered during adolescence.
Radiographic findings
Multiple tiny toothlike structures are contained within a fine radiolucent rim.
Histologic characteristics
Histology of compound odontomas approaches normal tooth structure. Gross clinical examination is usually sufficient for diagnosis.
Treatment
Simple removal is the method of treatment. These lesions do not recur.
Cementoblastoma
Cementoblastoma, as distinguished from cementoma, is a true neoplasm of cementum. This benign neoplasm is rare and is usually observed in patients younger than 25 years. It is most often found in association with the apex of the mandibular first molars (50% of lesions), and it is never found in association with the anterior dentition. The lesion is usually asymptomatic, although occasionally the associated tooth may be slightly sensitive to percussion.
Radiographic findings
A round opaque sunburst mass attached to the apex of a tooth that is well-demarcated and surrounded by a thin radiolucent rim is observed. The lesion obscures the lamina dura. Students sometimes confuse it with condensing osteitis, a common lesion resulting from low-grade periapical irritation that stimulates bone growth. Although the most usual location for the 2 lesions is the same, condensing osteitis does not obscure the periodontal ligament (PDL) space and tends to be more irregular in outline. The mature cementoma, also known as periapical cemental dysplasia, is another common lesion that students may confuse with cementoblastoma. However, cementoma is usually located in the mandibular anterior region and does not obscure the PDL space. Cementomas actually have 3 developmental stages: osteolytic (at which point the lesion appears as a radiolucency), cementoblastic (mixed radiolucent/radiopaque), and mature (radiopaque).
Histologic characteristics
Plump cementoblasts separated by cemental partitions form the histology of this encapsulated lesion.
Treatment
Removal of attached tooth and tumor is the traditional method of treatment. No recurrences are reported. A case report by Borges et al described a conservative, tooth sparing methodology involving endodontic treatment of the affected tooth, followed 30 days later by excision of the lesion and the apical third of the tooth’s root. In the first 7 years of follow-up, no recurrence was observed, and the tooth remained functional. [9]
-
Initial radiographic appearance. The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. The second radiograph depicts the appearance of the lesion at the second presentation.
-
The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. Incisional biopsy revealed the lesion to be an ameloblastoma. Treatment consisted of segmental resection of the entire mandible from the condyle to the area of the second premolar. The lateral and internal aspects of the resected specimen are depicted.
-
The patient was advised that biopsy of cystic lesion was indicated; however, he did not schedule an appointment because he was planning to leave the country. He did not follow-up for 3 years. He returned to the United States and sought care from a general dentist when he noted some mild facial swelling and discomfort. He was referred to the oral and maxillofacial surgery (OMFS) clinic. Incisional biopsy revealed the lesion to be an ameloblastoma. Treatment consisted of segmental resection of the entire mandible from the condyle to the area of the second premolar. The defect was reconstructed using autogenous rib, iliac crest, and tibial bone. Note the stable occlusion and excellent function obtained.
-
Glandular odontogenic cyst.