Practice Essentials
The parotid glands are small exocrine glands that rarely call attention to themselves. Perfect function throughout life is normal. Dry mouth, drooling, swelling, and pain are essentially the only symptoms caused by dysfunction of the salivary glands.
The major salivary glands and their ducts are strategically situated on either side of the dental occlusal planes to irrigate and saturate a food bolus with saliva during chewing. The parotid gland contacts the mandibular ramus and muscles of mastication, which massage the gland during chewing. The mechanical squeezing and the parasympathetic nervous system, which analyzes a number of sensory inputs, cause the glands to inject an appropriate quantity and quality of saliva into the oral cavity. Minor salivary glands are scattered throughout the oral cavity and pharynx to assist the major glands in moistening, lubricating, and protecting the teeth and mucosa. The normal flow of saliva though the duct prevents oral bacteria from ascending the duct to cause infection.
Signs and symptoms of parotitis
The signs and symptoms of infectious parotitis include the following:
-
Acute bacterial parotitis - The patient reports progressive, painful swelling of the gland and fever; chewing aggravates the pain
-
Acute viral parotitis (mumps) - Pain and swelling of the gland last 5-9 days; moderate malaise, anorexia, and fever occur; bilateral involvement is present in most instances
-
Human immunodeficiency virus (HIV) parotitis - Nonpainful swelling of the gland occurs; otherwise, patient is asymptomatic
-
Parotitis in tuberculosis - Chronic, nontender swelling of one parotid gland occurs, or a lump is noted within the gland; symptoms of tuberculosis are found in some cases.
The signs and symptoms of chronic punctate parotitis (chronic autoimmune parotitis) include the following [1] :
-
Sjögren syndrome - Recurrent or chronic swelling of one or both parotid glands with no apparent cause is noted; it is frequently associated with autoimmune disease; discomfort is modest in most cases and is related to dry mouth and eyes.
Diseases of uncertain etiology have the following signs and symptoms:
-
Recurrent parotitis of childhood - Repetitious episodes of unilateral or bilateral mumps-like episodes in a young child are indicative
-
Sarcoidosis - Chronic, nontender swelling of parotid gland occurs
-
Chronic, nonspecific parotitis - Most commonly, patients experience episodes of painful parotid inflammation that last for hours to weeks with relative asymptomatic periods between; pain varies from mild to incapacitating
Workup in parotitis
Imaging studies include the following:
-
Computed tomography (CT) scanning and magnetic resonance imaging (MRI) with gadolinium enhancement - These studies may be used to determine the size, shape, and some qualities of neoplasms or swelling within the gland
-
Sialography - Injury to the ducts or acini is demonstrated with this study
-
Scintigraphy
-
Ultrasonography - This demonstrates solid masses or fluid collections within the gland; it also can detect hypoechoic areas that correspond to punctate sialectasis by sialography
Procedures include the following:
-
Interventional sialendoscopy - This procedure is useful for the assessment and treatment of several inflammatory disorders of the gland
-
Incisional biopsy
Management of parotitis
Sialogogues, local heat, gentle massage of the gland from posterior to anterior, and hydration provide variable symptomatic relief. When pus is expressed from the Stensen duct, culture and sensitivity studies guide antibiotic selection.
When surgery is required for chronic parotitis, the standard treatment is superficial parotidectomy, but if CT scanning or surgery reveals significant involvement of the deep lobe, that portion of the gland is dissected from beneath the nerve.
In tympanic neurectomy, the parasympathetic supply to the parotid gland is sectioned within the middle ear so as to cause gland atrophy.
In ligation of the parotid duct, the duct can be ligated so as to cause atrophy of the gland and prevent ascending bacterial infections from entering the mouth.
Background
Inflammatory swelling of the glands may present a serious diagnostic challenge. Parotitis presents in many forms, and the symptoms vary from modest to prostrating. Reading the numerous journal articles on parotitis reveals frequent contradictions in the classification, etiology, and treatment of the disorders. A pure viral or bacterial infection, an autoimmune inflammation, or a combination of these can be the etiology. In this article, evolution of the knowledge of parotitis, as well as the diagnosis and treatment, is discussed.
Infectious parotitis
This group of diseases is caused by known infectious agents.
Acute bacterial parotitis
Acute bacterial parotitis is now infrequent, but its historical importance and occasional occurrence today necessitate in-depth knowledge of this entity by the otolaryngologist. Mumps and bacterial parotitis were differentiated by 1800, but neither was effectively treated. The mortality rate for bacterial parotitis was 80%. Before antibiotics and intravenous administration of fluids were available, bacterial parotitis occurred in postoperative patients or other severely ill patients who became dehydrated and contributed to their demise as an incurable sepsis.
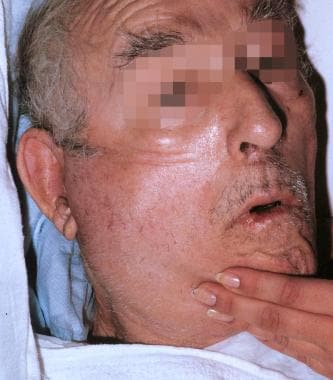
Early in the 20th century, surgeons were hesitant to incise and drain parotid abscesses and frequently used ineffective conservative measures until the process was irreversible. They feared the consequences of the unsightly scar and facial paralysis. Parotid abscess is depicted in the image below.
In 1917, Lilienthal described a surgical treatment that was very similar to what is used today. [2] He called parotid abscesses celiac parotitis because they were believed to be metastatic from abdominal infections. Other authors used names such as acute surgical parotitis, acute necrotic parotitis, acute gangrenous parotitis, and other historical designations according to Hemenway and English in 1971. [3]
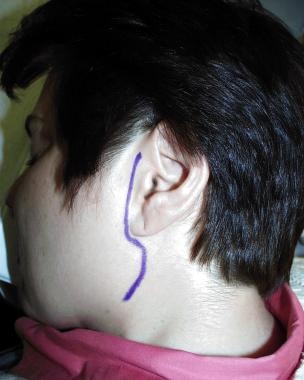
Lilienthal designed a vertical incision just anterior to the auricle that coursed posteriorly and inferiorly below the ear to join and follow an upper cervical skin crease that paralleled the lower mandibular border as seen in the image below. [2] He elevated the outlined skin flap forward to expose the parotid gland and made multiple incisions into the gland parallel to the facial nerve branches. He then opened the fascia behind the angle of the mandible to drain deeper spaces. The wound was packed and healed by secondary intention, resulting in a surprisingly good cosmetic result. The number of patients treated by this drainage is not known, but this treatment was probably almost anecdotal to Lilienthal’s contemporaries.
In 1919, Zachary Cope, a British Army surgeon, described 7 patients with parotitis that he had treated in Baghdad during the exceptionally hot summer of 1917. [4] He recorded that these soldiers had heatstroke or were severely affected by the extreme heat. The patients developed parotid swelling accompanied by fever and general malaise. Cope made wide T-shaped incisions in the gland to allow drainage. Four of the 7 survived after sloughing gangrenous parotid tissue. Cope stated that although the disease was a bacterial infection, the excessive heat and debilitating illness predisposed to its development.
In 1923, Blair and Padgett of St. Louis published an article stating that early surgical drainage of the infected gland was safe and frequently was life saving. [5] They stated that acute suppurative parotitis was an ascending duct infection related to decreased salivary flow, fever, and general debilitation. They cultured the pus and found that Staphylococcus aureus was the most common organism. The treatment proposed by Blair and Padgett did not become the standard practice for several more years. However, the high mortality rate decreased early in the 20th century and was 30-50% by 1930, probably because of more prompt and effective drainage of the abscesses.
From the 1930s to the 1960s, irradiation treatment of numerous diseases became popular, and several authors advocated 4-6 Gy delivered over 4-5 days for bacterial parotitis. Most patients with severe infections required surgical drainage despite radiation treatment. By 1960, most published papers stressed large doses of antibiotics, improved oral hygiene, and increased fluid intake as treatment, with incision and drainage for failures. They found that the parotid capsule and septations required wide exposure and extensive deep incisions parallel to the facial nerve branches to exteriorize the diseased gland. Physicians recognized the importance of hydration and oral hygiene for debilitated patients, and the incidence of bacterial parotitis plummeted.
Parotitis is now more common in elderly patients because many take medications with an atropine effect that retards salivary flow and predisposes to ascending infection. Many psychotropic drugs are relatives of antihistamines.
Acute parotitis in neonates
This rare form of parotitis is lethal without treatment. In January 2004, Spiegel et al reviewed the literature and stated that only 32 cases had been reported in journals during the previous 3 decades. [6] The characteristic clinical picture was of a sick premature infant with unilateral parotid swelling and inflammation. Seventy-five percent of the cases were in male infants. Pus expressed from the duct cultured S aureus in more than half of the cases. Most all of the cultured bacteria were from organisms present in the oral cavity, which suggests an ascending infection from the mouth.
Treatment is prompt administration of gentamicin and antistaphylococcal antibiotics plus adequate hydration, with a cure in approximately 80% of cases. Failure to improve after 24-48 hours of treatment necessitates surgical drainage. Recurrence is uncommon. Acute bacterial parotitis in children between one year of age and adolescence is extremely rare and only a few have been reported. The etiology and treatment is the same as for adults.
Chronic bacterial parotitis
Chronic bacterial parotitis may exist in the presence of calculi or stenosis of the ducts secondary to injury. A number of articles and book chapters describe that chronic infection is a sequela of acute bacterial infection, but the evidence is scant. Most authors have suggested that decreased salivary flow was the common denominator, but reduced flow may be due to the inflammation. In most instances, the chronic disease is either autoimmune or of unknown etiology with superimposed bacterial infections and should not be designated as a chronic bacterial infection.
Acute viral parotitis (mumps)
Mumps, one of the classic childhood infections, is spread by droplets or by direct spread from oropharyngeal secretions that contain the paramyxovirus. Universal immunization, which began in 1977, has made the clinical disease unusual in developed countries. The child should receive the first measles, mumps and rubella (MMR) vaccine at age one year and a second at age 4-6 years. [7, 8, 9]
Occasional outbreaks of mumps are seen, mostly in teenagers or patients in their early twenties who did not receive the second shot. Before the vaccines were available, exposure was almost universal, and clinical disease resulted in 60-70% of those who were exposed. The disease was characterized by grossly enlarged and modestly tender parotid glands. Parotid stimulation caused pain in the gland and ear. Mumps was a benign disease in the vast majority of cases but was occasionally complicated by meningoencephalitis, pancreatitis, orchitis, or deafness especially in young adults. Treatment was and is symptomatic and supportive.
HIV parotitis
Generalized lymphadenopathy has long been associated with HIV, but the localized enlargement of the parotid gland is less well known. The course of the disease is different enough between children and adults to warrant a separate description.
HIV parotitis in children
Salivary gland involvement in children with HIV is well recognized and is much more common than involvement in adults. Characteristically, the gland is firm, nontender, and chronically enlarged (unilateral or bilateral) and usually causes few symptoms. Lymphoepithelial cysts are less common than in adults. Xerostomia with decreased salivary flow rates occurs in adults but is infrequent in children. Infiltration of CD8-positive lymphocytes, possibly as a result of HIV, Epstein-Barr virus (EBV), or an interaction between the 2, enlarges the gland. The diagnosis of HIV parotitis is usually clinical with the typical findings. Other forms of chronic parotitis are rare in children. [10]
The picture is not typical for acute bacterial infection. No specific treatment exists for this parotitis, and none is usually required. Some evidence indicates that parotid involvement is a good prognostic sign.
HIV parotitis in adults
The name lymphoepithelial lesion is frequently applied to HIV parotitis and adds confusion to the many names of parotid inflammatory diseases. A group at the State University of New York (SUNY) presented a series of 50 patients with HIV and masses in the tail of the parotid. Most of the patients were prison inmates and intravenous (IV) drug users. Parotidectomy was performed in 35 patients, with patients earlier in the series undergoing lateral lobectomy and later patients undergoing local excision of involved tissue.
Microscopic examination of the excised specimen revealed 3 types of involvement: (1) follicular hyperplasia of the parotid lymph nodes; (2) diffuse infiltration of the gland by lymphocytes (CD8 T cells) with appearance much like Sjögren disease; and (3) benign lymphoepithelial cysts that are the type of involvement most frequently described in the literature. The cyst walls are lined by stratified epithelium that may be squamous, columnar, cuboidal, or pseudostratified. Aggregates of lymphoid tissue are present within the cyst walls. The salivary parenchyma has a normal appearance. MRI shows the multiple cysts within the enlarged glands and is almost diagnostic. If the enlarged gland causes cosmetic deformity or pain, antiviral therapy, low-dose radiation therapy, or surgical excision may decrease the size of the gland. [11] Most authors suggest enucleation of large cysts. In some individuals, the parotid gland symptoms are very similar to and may be confused with Sjögren syndrome.
Parotitis in tuberculosis
The incidence of pulmonary tuberculosis steadily decreased in the United States until 1985, but the trend reversed and has been slowly increasing since that time with a concurrent rise in extrapulmonary tuberculosis. [12] Tuberculosis is an uncommon cause of parotitis and is not particularly important, except that approximately 25% of patients have pulmonary tuberculosis and may infect their associates. Approximately 3% of patients with AIDS have tuberculosis, and almost 70% of these patients have pulmonary tuberculosis.
In the past, extrapulmonary tuberculosis was apt to be due to atypical tuberculosis, such as the bovine variety, but today, most cases are due to Mycobacterium tuberculosis. Patients have enlarged, nontender, but moderately painful glands. Involvement is most frequently confined to the parotid lymph nodes, but the gland may become diffusely involved with the disease.
The diagnosis is made by typical chest radiograph findings, cultures, or histologic diagnosis after the gland has been removed. The long delay for cultures prolongs the public’s exposure to the disease. A positive skin test is not particularly helpful because of the high incidence of positive skin tests in the general population. Fine-needle aspiration biopsy occasionally yields Langerhans giant cells, which suggest tuberculosis. When diagnosed and treated with antitubercular medications, the gland may return to normal in 1-3 months. Untreated cases progress to draining fistulas and fibrosis.
Influenza-associated parotitis
A reported 256 cases of influenza-associated parotitis were found in 27 states during the 2014-2015 US influenza season, according to a study by Rolfes et al. Of 50 cases evaluated, parotitis occurred primarily in patients under age 20 years (73%) and tended to be painful (86%) and unilateral (68%), with the majority of cases arising in association with influenza A(H3N2) virus infection. A median period of 4 days passed between systemic or respiratory symptom onset and parotitis development. [13]
A study by Labuda et al comparing the epidemiology of influenza-related parotitis with that of mumps parotitis, as observed during a mumps outbreak in Arkansas between August 2016 and July 2017, found influenza-related parotitis to be more prevalent in males, while mumps parotitis was distributed equally between the sexes. The investigators also reported that while influenza-related parotitis was more common in the white population, mumps parotitis arose most often in the Pacific Islander population from the Republic of the Marshall Islands, living in Washington County, Arkansas. All cases of influenza-related parotitis emerged from influenza A. [14]
Chronic punctate parotitis (chronic autoimmune parotitis)
Although acute bacterial parotitis is fairly well understood, chronic enlargement of the salivary glands with recurring infection has caused confusion for more than a century. Numerous terms found in the literature, such as Mikulicz disease, Sjögren syndrome, benign lymphoepithelial lesion of Godwin, chronic punctate sialectasis, and recurrent parotitis of childhood (as seen in the image below), are confusing to the physician. Sialography was commonly used in the workup of parotid disease in the past, and this group demonstrates punctate sialectasis, which implies point like dilatations within the gland.
Mikulicz disease
Johann Mikulicz-Radecki (1850-1905), a professor of surgery in Breslau, Poland, trained under Theodore Billroth of Vienna. In 1888, he encountered a 42-year-old farmer who had experienced lachrymal gland swellings followed by enlargement of the submaxillary and parotid glands. The enlarged lacrimal glands interfered with his vision and were excised by Mikulicz. The submaxillary glands were also excised, but the parotid glands were not removed. Mikulicz recorded in his publication that this was a benign disease and was not related to tuberculosis, leukemia, or malignant lymphoma. He believed the causative factor to be infection. No other patient with an identical disease has been described.
Unfortunately, the name Mikulicz has been associated with numerous conditions that involve enlarged parotid glands. Early in the 20th century, numerous reports described conditions associated with enlargement or inflammation of the salivary glands. In 1927, Schaffer and Jacobson presented a simplified grouping with only 2 major divisions: [15] (1) cases in which tuberculosis, leukemia, or some other disease had enlarged the gland and (2) Mikulicz disease, in which these other diseases were ruled out. The term "Mikulicz disease" probably should be discarded because of ambiguous definition. However, the term is so popular that it will not soon go away.
Sjögren syndrome
The next major milestone in the understanding of chronic parotitis was the publication of a paper in 1933 by Henrik Sjögren, a Swedish ophthalmologist. [16] In 1930, Sjögren observed his first cases of keratitis sicca and began a systematic study of the disease that lasted more than 20 years. In his landmark publication, he named the disorder keratoconjunctivitis sicca. Sjögren published so extensively that by 1936, the entity was referred to as Sjögren syndrome, a name that today is widely employed throughout the world.
Sjögren reviewed the available literature on the subject and published histopathologic studies of the cornea, conjunctiva, and lachrymal glands. He reported that this disease affected menopausal women in whom the keratoconjunctivitis sicca was a local phenomenon. He stressed that arthritis was a significant feature of the disease and occurred in most patients. Patients had an elevated erythrocyte sedimentation rate. Hypochromic anemia and fever were occasionally present. Sjögren revived the Schirmer test for measuring tear secretion and popularized the use of the Rose Bengal staining technique for the diagnosis.
Sjögren syndrome, similar to other connective tissue diseases, is a multisystem disorder with diverse features. The entity is classified by some as a primary condition (primary Sjögren syndrome) or is associated with autoimmune diseases such as lupus erythematosus or rheumatoid arthritis (secondary Sjögren syndrome). In the fully developed syndrome, most organs seem to be involved.
Most authors classify the disease as definite Sjögren syndrome, which includes (1) objective evidence of keratoconjunctivitis sicca or (2) characteristic pathologic features of the salivary glands. The probable Sjögren syndrome requires 2 out of 3 of the following: (1) recurrent chronic idiopathic salivary gland swelling, (2) unexplained xerostomia, and (3) connective tissue disease.
The disease most commonly appears in people aged 40-60 years, but it may affect small children. In Sjögren syndrome, the prevalence of parotitis in women versus men is approximately 9:1. The involved parotid gland is enlarged and tender at times. Massage of the gland produces clear saliva with flocculated clumps of coagulated proteins.
The next major development in the evolution of knowledge of chronic parotitis was a collective pathologic study of "Mikulicz Disease" by Morgan and Castleman in 1953. [17] They reported 18 cases and stated that the pathologic involvement was uniform in all of these cases. The basic features are massive lymphoid infiltration with atrophy of the acini, proliferation of the cells of the small ducts that leads to narrowing of the lumen, and, finally, obliteration.
The functioning parts of the gland (acini) are destroyed, leaving the appearance of large lymph follicles. However, among this distorted architecture, islands of ductal epithelial cells and myoepithelial cells that appear fairly healthy can be observed. These groupings are called epimyoepithelial islands. The large ducts appear to be normal. In the Morgan and Castleman series, some of the surgical procedures were radical parotidectomies, including severing the facial nerve, because the surgeon believed that the condition was a malignancy.
For further reading on Sjögren syndrome, please see the Medscape Reference articles on the subject in our Pediatrics, Ophthalmology, Emergency Medicine, Rheumatology, and Dermatology sections.
Lymphoepithelial lesion of Godwin
John T. Godwin, a New York pathologist, published a series of 11 parotidectomy cases in 1952 that he diagnosed as benign lymphoepithelial lesion of the parotid gland. Two of the patients had undergone radical parotidectomy followed by irradiation because of the mistaken belief that the lesions were malignant. Three other patients were treated with irradiation. Some had bilateral enlargement of the parotid glands and dry eyes. As a pathologist, he had not interviewed the patients, and the recorded information was incomplete. [18]
Godwin discussed the work of Mikulicz but did not mention Sjögren, although the history of the cases reveals that some undoubtedly were Sjögren syndrome. Several of the patients underwent sialography that revealed punctate sialectasis. He suggested that sialography might be helpful in the diagnosis but that a needle-aspiration biopsy was not adequate for diagnosis. He suggested incisional biopsy for diagnosis and determined that extensive surgery was not necessary. Godwin noted that several of the glands were diffusely involved, while some had well-circumscribed areas of tumor.
The Godwin name is most frequently associated with a circumscribed tumor with the histologic features of Sjögren syndrome. Why an autoimmune process directed against the salivary gland tissue would be so localized within the gland is difficult to understand.
Pathogenesis of autoimmune parotitis
The autoimmune diseases listed above appear to be the same disease process with different manifestations. Initial insult to the gland may be a viral infection. Peptides derived from viral antigens and autoantigens become associated with class II histocompatibility molecules in the cytoplasm of the epithelial cell, and the human leukocyte antigen (HLA) complex is subsequently expressed on the cell surface. CD4+ T cells recognize these antigens and release a series of cytokines, which promote further T-cell activation.
B cells enter the gland and produce autoantibodies, including anti–Sjögren syndrome antibodies (ie, anti–SS-A, anti–SS-B) and rheumatoid factor (RF). B cells with cell surface RF can concentrate immune complexes and present antigens to CD4+ T cells. The acini are destroyed by this autoimmune mechanism. Continued cell division of specific B cells leads to oligoclonal expansion and increased chance of karyotypic error associated with neoplastic transformation.
Diseases of uncertain etiology
Salivary stone (sialolithiasis)
Formation of stones within the duct system is one of the more frequent disorders of the salivary glands. The exact cause is not known, but most agree that the stone begins as a small nidus and grows by concentric deposition of inorganic crystals in an organic matrix. Calculi are much less common in the parotid gland than in the submandibular gland, possibly because the secretions are more serous than the mucoid saliva of the submandibular gland.
Calculi do not cause symptoms until they become large enough to impede the flow of saliva. Partial obstruction causes the gland to inflate itself with marked stimulation to secrete saliva, as occurs in eating. The gland swells while eating and soon becomes painful. The swelling and pain subside in 30-60 minutes only to recur at the next meal. Total obstruction causes pain, swelling, and infection. Complications of the calculi include infection within the gland (sialadenitis), scarring with stenosis, fistula formation, and rarely the stone migrates outside the duct to appear as an inflammatory mass in the neck
The diagnosis is confirmed by imaging studies including plain radiographs with or without injection of contrast media into the duct. CT scans also show single or multiple stones. (A retrospective study by Jáurequi et al suggested that it is uncommon to find multiple parotid calcifications in chronic parotitis, with the investigators reporting more than one calcification in the parotid gland region in just 13 out of 133 patients (10%) following parotid sialendoscopy for chronic sialadenitis. [19] )
Treatment is removal of the stone. Massage of the gland from posterior to anterior may occasionally remove stones, but most require surgical removal. Lidocaine injected around the duct orifice and into the duct allows serial dilatation with graduated dilators. The duct is filleted with sharp scissors and then massage may deliver the stone. This is the simplest method of removal in most instances because the required instruments are available in the ENT or dental office. Extracorporeal shock wave lithotripsy is an alternate treatment to fragment the stone.
Interventional sialendoscopy is growing in popularity and availability and seems to be the best method of treatment. The duct is anesthetized and dilated to insert a telescope for inspection of the large ducts. A working channel in the telescope permits irrigation, suction, and insertion of forceps, wire loop, or even laser energy via a glass fiber to remove the calculi. The clinician has much more information as to the condition of the duct system. This instrument is useful for the assessment and treatment of several inflammatory disorders of the gland.
Chronic recurrent parotitis (chronic nonspecific parotitis)
This general term is used for patients in whom no definite etiology is found. Hundreds of papers on chronic parotitis have discussed the nature and treatment of the disease or diseases. The theories of etiology are diverse. Many authors are convinced that sialoliths or scarring of the ducts cause stasis of salivary flow and predispose the gland to infection is the etiology, but this is probably true for only a minority of cases.
All authors agree that the spectrum of symptoms varies from mild to incapacitating. Episodes may last for several days, paralleling the time course of a bacterial or viral illness. Others may experience episodes that last only a few hours from onset to resolution. Some episodes may last for several weeks. Quiescent periods between episodes last for hours, days, or even years. This range suggests that more than one disease may be the cause. The cases in which the painful episodes last for hours are probably caused by mediator release rather than infection.
Sialography generally shows marked dilatation (ectasia) of the major ducts, with narrowed areas that give the appearance of a string of sausage. Most authors believe that the narrowed areas represent strictures of the ducts. The minor ducts are frequently not patent and punctuate sialectasis is not seen. This suggests that the disease arises in the ductal system. A number of articles from Great Britain and Japan report a condition called "sialodochitis," which would be included in the chronic recurrent parotitis group. This word is not in most American medical dictionaries but implies inflammation of the ducts. The acini may be histologically normal, at least in the early stages of the disease.
Most authors suggest that the treatment of all forms of chronic parotitis should be proportional to the symptoms, which are subjective rather than objective. Treatment escalates with the symptoms, from massage, sialogogues to antibiotics, and analgesics Pain seems to be the driving symptom. Periodic irrigation of the Stensen duct with saline, antibiotics, and/or steroids has been advocated with good rationale in those patients with sialectasia of the duct. This procedure removes debris from the duct and deposits the drugs to the needed location. Frequent irrigation is probably important, but the patient cannot perform the treatment. If periodic irrigation is successful, it should be performed rather than removal of the gland. Because the pathophysiology is poorly understood, the rationale of several surgical treatments is rather weak. Parotidectomy removes the diseased gland but begs the question of specific treatment.
Recurrent parotitis of childhood
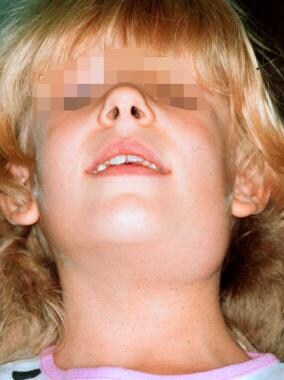
Another uncommon syndrome that has been recognized for the past 50 years is recurrent parotitis of childhood, in which recurring episodes clinically resemble mumps. Generally, episodes begin by age 5 years, and virtually all patients become asymptomatic by age 10-15 years. The duration of attacks averages 3-7 days but may last 2-3 weeks in some individuals. The spectrum varies from mild and infrequent attacks to episodes so frequent that they prevent regular school attendance. The child, although not ill, is regularly sent home with the diagnosis of mumps until school officials are informed of the nature of the disease.
During the attacks, the parotid gland is enlarged, moderately red, and tender. Massaging the gland from back to front produces clear saliva with lots of "snowflakes" or little white curds from the Stensen duct. The disease is unilateral most commonly and, if bilateral, is most apt to be asymmetric. The child generally is not very sick during the episodes.
Bacterial cultures from saliva generally produce Streptococcus viridans or another low-virulence bacterium that is considered normal oral flora. Even between attacks, bacteria are present in the saliva. Ultrasonography and sialography reveal punctate sialectasis as in Sjögren syndrome. Even when symptoms are unilateral, sialectasis is demonstrated by sialography in the opposite gland in most instances. Sialographic changes persist even after all other symptoms have ceased. Findings may eventually disappear, but the natural history of gland findings is not clear.
The histopathology is essentially the same as Sjögren and Mikulicz disease. Some children with recurrent parotitis may actually have Sjögren syndrome and may develop the full-blown clinical picture. The cause of the disease is unknown. Sialectases may precede infections and may be a site of lowered resistance. A number of etiologies have been suggested. The disease is unrelated to mumps, and when viral studies have been performed, elevated serum titers to numerous other viruses have been found. One theory is that infection of the glands at a young age affects the immune system, and the disease may represent immaturity of the immunologic response. Searches for autoantibodies have not been successful. The benign self-limiting nature of this entity makes autoimmunity doubtful. No evidence suggests that allergy is a cause. Resolution of symptoms with age may be due to regeneration of glandular elements and return to normal function.
A retrospective study by Hildago-Santos et al found that out of 36 patients with juvenile recurrent chronic parotitis, 16 (44%) had serologic abnormalities of an immunologic nature, including nonspecific abnormalities (such as positivity for antinuclear antibodies, high immunoglobulin G (IgG), or low complement factor), or those “associated with a specific final diagnosis,” including selective IgA deficiency, Sjögren syndrome that was or was not associated with systemic lupus erythematosus, celiac disease that was or was not associated with diabetes mellitus, Hashimoto thyroiditis, and acquired immunodeficiency syndrome (AIDS). [20]
A study by Xie et al indicated that amylase activity is important in the assessment of juvenile recurrent parotitis. The study, of 44 patients with the disease, found that the degree of parotid gland function was significantly related to serum amylase activity, with the severity of the parotitis associated with the extent of glandular function degradation. [21]
Applying local heat applied to the gland, massaging the gland from back to front, and taking penicillin usually cure individual episodes. Treatment of individual infections may prevent injury to the gland parenchyma. Severe disease may be treated by parotidectomy. Parotidectomy is rarely indicated.
Sialadenosis (sialosis)
In this disorder, both parotid glands may be diffusely enlarged with only modest symptoms. Patients are aged 20-60 years at onset, and the sexes are equally involved. The glands are soft and nontender. Biopsy shows the acinar cells to be enlarged to almost twice the normal diameter and the cytoplasm packed with enzyme granules. The cause is unknown, but inappropriate autonomic nervous system stimuli are frequently suggested. Approximately half of the patients have endocrine disorders such as diabetes, nutritional disorders such as pellagra or kwashiorkor, or have taken drugs such as guanethidine, thioridazine, or isoprenaline. If the symptoms are mild, treatment is not required. If the glands are disfiguring, partial parotidectomy should improve the appearance.
Sarcoidosis
Sarcoidosis is a chronic multisystem disorder of unknown cause that is characterized by accumulations of T lymphocytes and mononuclear phagocytes, noncaseating epithelioid granulomas, and the derangement of normal tissue architecture. Skin anergy and depressed cellular immune processes in the blood are common. In the United States, the incidence is much higher in African Americans and generally begins in people aged 20-40 years.
Diagnosis requires the typical clinical picture and biopsy reveals noncaseating granuloma, plus exclusion of other diseases associated with such granulomas. In most instances, the process does not attack involved organs, but the bulk of the accumulated cells may distort the normal architecture enough to impair function. The lungs, skin, and lymph nodes are most often affected, but the salivary glands are involved in approximately 10% of cases. Bilateral firm, smooth, and nontender parotid enlargement is classic. Xerostomia occasionally occurs. The Heerfordt-Waldenstrom syndrome consists of sarcoidosis with parotid enlargement, fever, anterior uveitis, and facial nerve palsy.
Sarcoidosis is benign in most instances, and treatment is generally not advisable unless organ dysfunction occurs. The only effective treatment is corticosteroids administered for several weeks. Treatment of the parotid glands is not necessary, but most would treat facial paralysis because of the fear of permanent function loss.
Pneumoparotitis
Pneumoparotitis is air within the ducts of the parotid gland with or without inflammation. The duct orifice normally functions as a valve to prevent air from entering the gland from a pressurized oral cavity. Rarely, an incompetent valve allows insufflation of air into the duct system. Pneumoparotitis most commonly occurs in wind instrument players, glass blowers, and scuba divers. Occasionally, a person learns how to perform parotid insufflation voluntarily. The condition is harmless unless bacteria from the mouth cause parotitis. Rarely, rupture of the ductal system causes extensive subcutaneous emphysema.
Miscellaneous causes of inflammation and enlargement of the parotid
Several lymph nodes reside within the parotid gland as a superficial and deep group of nodes. These nodes may be involved with any process that affects lymph nodes, including bacterial, fungal, viral, and neoplastic processes. Rarely, drugs such as iodides, phenylbutazone, thiouracil, isoproterenol, heavy metals, sulfisoxazole, and phenothiazines cause parotid swelling. The parotid glands may be incidentally involved in numerous systemic conditions such as uremia and kwashiorkor. Diseases such as Wegener granuloma or Kimura disease involve the parotid glands as rare causes of parotitis.
Pathophysiology
The pathophysiology varies with the type of parotitis and is discussed under Background.
Epidemiology
Mortality/Morbidity
Death from parotitis is extremely unusual. Parotitis most frequently is a complication of an underlying process. Morbidity is generally proportional to the original disease.
Race
Parotitis occurs with equal frequency in people of all races. However, a retrospective study by Benaim et al determined that in a single tertiary care pediatric teaching hospital, juvenile recurrent parotitis was found most often in Black males aged 2-8 years. [22]
Sex
Chronicparotitis occurs with equal frequency in both sexes. The parotitis of Sjögren syndrome has a male-to-female ratio of 1:9. Recurrent parotitis of childhood is more common in males.
Age
Viral parotitis (mumps) occurs most frequently in children. Parotitis that accompanies systemic diseases (eg, rheumatoid arthritis or HIV) mirrors the occurrence of those diseases.
-
Elderly man with parotid abscess.
-
Six-year-old girl with recurrent parotitis of childhood.
-
Sialogram of patient with sialectasis. Notice the appearance of a tree with leaves.
-
Incision outlined for incision and drainage of parotid abscess.
-
Parotid gland anatomy.