Initial Thyroid Embryology
The thyroid gland is the first of the body's endocrine glands to develop, on approximately the 24th day of gestation. This occurs under the influence of fibroblast growth factor signaling pathways. [1] The thyroid originates from two main structures: the primitive pharynx and the neural crest. The rudimentary lateral thyroid develops from neural crest cells, while the median thyroid, which forms the bulk of the gland, arises from the primitive pharynx.
The thyroid gland forms as a proliferation of endodermal epithelial cells on the median surface of the developing pharyngeal floor. The site of this development lies between 2 key structures, the tuberculum impar and the copula, and is known as the foramen cecum. The thyroid initially arises caudal to the tuberculum impar, which is also known as the median tongue bud. This embryonic swelling develops from the first pharyngeal arch and occurs midline on the floor of the developing pharynx, eventually helping form the tongue as the two lateral lingual swellings overgrow it.
The foramen cecum begins rostral to the copula, also known as the hypobranchial eminence. This median embryologic swelling consists of mesoderm that arises from the second pharyngeal pouch (although the third and fourth pouches are also involved). The thyroid gland, therefore, originates from between the first and second pouches.
The initial thyroid precursor, the thyroid primordium, starts as a simple midline thickening and develops to form the thyroid diverticulum. This structure is initially hollow, although it later solidifies and becomes bilobed. The stem usually has a lumen, the thyroglossal duct, that does not descend into the lateral lobes. The 2 lobes are located on either side of the midline and are connected via an isthmus.
Descent of the Thyroid Gland
The initial descent of the thyroid gland follows the primitive heart and occurs anterior to the pharyngeal gut. At this point, the thyroid is still connected to the tongue via the thyroglossal duct. The tubular duct later solidifies into a cord of cells that will form the follicular elements. The proximal segment retracts and subsequently obliterates entirely, leaving only the foramen cecum at the posterior aspect of the tongue. Nonetheless, in some individuals, remnants of this duct may still persist.
The foramen cecum represents the opening of the thyroglossal duct into the tongue; its remains may be observed as a small blind pit in the midline between the anterior two thirds and the posterior third of the tongue at the apex of the sulcus terminalis.
A pyramidal lobe of the thyroid may be observed in as many as 50% of patients. This lobe represents a persistence of the inferior end of the thyroglossal duct that has failed to obliterate. [2] As such, the pyramidal lobe itself may be attached to the hyoid bone, similar to a thyroglossal duct cyst, or may be incorporated into a thyroglossal duct cyst. However, the pyramidal lobe can usually be identified along the superior edge of the isthmus, more commonly on the left side.
The caudal segment of the thyroglossal duct develops as a bilobed, encapsulated gland while reaching its final, orthotopic position.
Descent of the thyroid gland carries it anterior (or ventral) to the hyoid bone and, subsequently, anterior (or ventral) to the laryngeal cartilages. As the thyroid gland descends, it forms its mature shape, with a median isthmus connecting the two lateral lobes. The thyroid completes its descent in the seventh gestational week, coming to rest in its final location immediately anterior to the trachea. (Defective embryogenesis of the thyroid gland as it descends to its target location can result in ectopic thyroid tissue, found in approximately 1 in 100,000-300,000 persons and in 1 in 4000-8000 patients with thyroid disease. [3] )
The caudal segment of the thyroglossal duct undergoes histologic differentiation into follicular elements at gestational weeks 10 and 11. The division of these primary follicles results in follicle formation during weeks 11 and 12. Intracellular canaliculi form connections with adjacent cells, and these spaces subsequently fuse, forming a confluent lumen. Desmosomes, molecular complexes of adhesion proteins, connect the cells, keeping follicular contents confined within the lumen. Colloid then accumulates within the follicles during gestational week 13. The connective tissue capsule and the interlobar septa develop from the fourth pharyngeal pouch's neural crest mesenchyme.
A study by López-Márquez et al, utilizing mice, indicated that the Sox9 transcription factor is “likely at the center of an important regulatory network of signals that govern thyroid differentiation both in embryos and in adults.” [4]
Another study in mice, by Johansson et al, indicated “that embryonic thyroid differentiation is an asynchronous process,” one that spans “several developmental stages of thyroid organogenesis.” [5]
For further reading, please see the Medscape Reference article Thyroid Anatomy.
Parafollicular Cells
The ultimobranchial body develops from the pharyngeal endoderm and in turn gives rise to the parafollicular cells. Also known as C cells, these are a special subset of cells within the thyroid gland that secrete calcitonin, a hormone necessary for the regulation of calcium. This cell differentiation is regulated by the basic helix-loop-helix (bHlH) transcription factor MASH1. [1]
The ultimobranchial (ultimopharyngeal) body represents the last structure derived from the branchial pouches (also called pharyngeal pouches); hence its name. It arises from the ventral portion of the fourth pharyngeal pouch (see the image below). The existence of a fifth pharyngeal pouch is debated. The ventral portion of the fourth pharyngeal pouch attaches to the posterior aspect of the thyroid at the fifth week of gestation and increases the weight of the thyroid by 30%.
The ultimobranchial body is incorporated into the thyroid gland, disseminating its cells into the gland as the lateral and medial thyroid primordia fuse. The cause of the fusion is unknown, but the site at which these rudimentary structures fuse has been identified as the tubercle of Zuckerkandl. [6] Neural crest parafollicular mesenchyme supplies neurovascular and lymphatic structures. [7]
Although it was previously thought that neural crest cells invaded the ultimobranchial body and consequently gave rise to C cells, subsequent research has indicated that the C cells are actually endodermal in origin. It is now believed that once pharyngeal pouch endoderm gives rise to ultimobranchial body epithelial cells, the epithelial cells disseminate, following fusion, into the parenchyma of the embryonic thyroid and form C cells.
The integrative process explains why C cells are not found throughout the entire thyroid but are instead restricted to an area within the middle to upper third of the lateral lobes, while the upper and lower poles and the isthmic areas are largely devoid of C cells. [8]
Thyroid Embryology Clinical Correlations
The thyroglossal duct is an epithelialized, ductlike structure that connects the developing thyroid gland and the foramen cecum. During the fifth week of development, this structure loses its lumen and breaks into fragments. The mesoderm condenses, forming the hyoid bone, which subsequently undergoes chondrification. This development divides the thyroglossal duct intro suprahyoid and infrahyoid divisions. The attenuated duct then atrophies by the end of gestational week 8.
If the thyroglossal duct does not atrophy, then the remnant can manifest clinically as a thyroglossal duct cyst (TDC). These cysts are the most common congenital cervical anomalies, being approximately three times as common as branchial cleft cysts. While half of these generally midline cystic masses are located at or just below the level of the hyoid bone, they may be located and can track anywhere from the thyroid cartilage up to the base of the tongue, along the embryonic course of descent. [9, 10]
A TDC presents as a painless, asymptomatic midline swelling. [11] TDCs are classically perceived to be rising in the neck upon protrusion of the tongue or swallowing, due to their physical attachment to the hyoid bone and muscles of the tongue. Cysts are smooth, round, well-defined, and slightly mobile on physical exam. Although most are observed during childhood, they can present at any age. If the cyst ruptures, it may go on to form a thyroglossal duct sinus or a thyroglossal duct fistula that exits through the overlying skin. Because the hyoid bone develops in an anterior direction and may surround the thyroglossal duct, the surgeon should resect the central portion on the hyoid bone along with the cyst (the Sistrunk procedure), unless the thyroglossal duct tract can clearly be observed coursing away from the bone. [12, 13]
Thyroid imaging and thyroid function tests should be done in all patients preoperatively to define the anatomy of the normal thyroid gland. Thyroid scintigraphy or high-resolution ultrasonography can be implemented for excluding ectopic thyroid tissue and identifying the normal thyroid gland. An aberrant or ectopic thyroid gland may occur anywhere along the path of initial descent of the thyroid, although it is most common at the base of the tongue, just posterior to the foramen cecum. In this location, an aberrant or ectopic thyroid gland is known as a lingual thyroid and represents a failure of the thyroid to descend. This failure contrasts with the incomplete descent of the thyroid, in which case the resulting final resting point of the gland may be high in the neck or just below the hyoid bone. [14, 15]
Most cases of ectopic thyroid are detected in early childhood and may be associated with hypothyroidism. The tissue may enlarge due to an elevation of thyroid-stimulating hormone (TSH), resulting in localized symptoms. If present in the anterior neck, the enlarged tissue may be mistaken for a TDC. It is important to differentiate between these lesions, as THS-related enlarged tissue is frequently the only thyroid tissue present.
Accessory thyroid tissue can also occur, arising from remnants of the thyroglossal duct. While the accessory thyroid tissue may be functional, it is generally insufficient for normal function if the main thyroid gland is entirely removed. This accessory tissue may appear anywhere along the path of the thyroglossal duct tract. [16]
Inferior Parathyroid Embryology
The inferior parathyroid glands are also known as parathyroid IIIs, because they arise from the dorsal wing of the third pharyngeal pouch. The third pharyngeal pouch differentiates at gestational weeks 5-6, with the ventral wing becoming the thymus. (These structures develop under the influence of fibroblast growth factor receptor substrate 2 [FRS2]. [1] ) This common origin is why the parathyroid IIIs are often referred to as the thymic parathyroids and the two structures are described as the parathymus. [17]
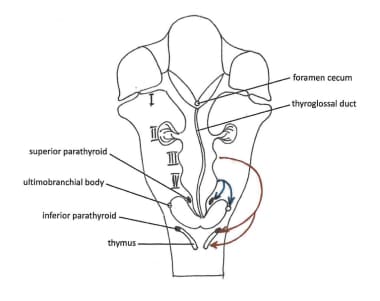
The thymus and parathyroids both lose their connections to the pharynx at gestational week 7. The thymus then migrates caudally and medially, pulling the parathyroids with it; therefore, parathyroid IIIs are in a more inferior position than are parathyroid IVs. The parathyroids, in turn lose their connection with the thymus. The inferior parathyroid glands usually stop at the dorsal surface of the thyroid gland, outside of the fibrous capsule of the gland itself. An image of the inferior parathyroids can be seen below.
Superior Parathyroid Embryology
The superior parathyroid glands are also known as parathyroid IVs, because they arise from the dorsal wing of the fourth pharyngeal pouch, differentiating at gestational weeks 5-6. The fourth pharyngeal pouch forms the caudal pharyngeal complex, giving rise to the ultimobranchial body. Due to this close origin with the lateral thyroid, the superior parathyroids are sometimes called the thyroid parathyroids.
At gestational week 7, the glands lose connections with the pharynx and attach themselves to the thyroid gland, which is migrating caudally. However, it migrates far less than the thymus (with parathyroid IIIs, as described above). Because of the shorter migration length, the superior parathyroid glands (IV) are in a more constant location than the inferior parathyroids (III). The superior parathyroids are generally located more posterior and medial than the inferior parathyroids, and their final resting point is usually on the dorsal surface of the thyroid gland, outside the fibrous capsule of the thyroid gland. Histologically, differentiation of the chief cells occurs during the embryonic period, while that of the oxyphil cells takes place 5-7 years after birth. [1] An image of the superior parathyroids can be seen below.
Parathyroid Embryology Clinical Correlations
Accessory or supernumerary parathyroid glands are found in approximately 13% of individuals at autopsy. These glands most likely result from tissue fragmentation that occurs during the migration of the glands rather than from an initial division of the primordia of the glands themselves. [16] Separation of cells from the main mass during the migratory process produces microscopic rests, or fragments, of ectopic tissue. Rudimentary rests of parathyroid tissue can be differentiated from true supernumerary glands based on size. The former typically weigh less than 5 mg, while true supernumerary glands have an average weight of 24 mg. [18] Primary and secondary hyperplasia may result in stimulation of embryologic fragments, resulting in the development of supernumerary glands proper.
Absence of parathyroids (ie, < 4 glands) is noted in approximately 3% of individuals at autopsy. This absence may result from a failure of the primordia to differentiate into parathyroid glands or may be the result of parathyroid gland atrophy early in development.
Ectopic parathyroid glands occur in 15-20% of patients. The glands may be located anywhere near or even within the thyroid or thymus. For example, if parathyroid IVs do not descend entirely, they may be located as high as the bifurcation of the common carotid artery. Conversely, if parathyroid IVs do not release from the thymus, they may be located intrathoracically, as low as the aortopulmonary window. Other common ectopic locations include the anterior mediastinum, posterior mediastinum, and retroesophageal and prevertebral regions. However, even when the parathyroid glands are in an ectopic location, they still often are symmetrical from side to side, making localization somewhat easier.
Ectopic location may also be acquired due to enlargement of a gland, with gravitational forces causing migration, or as a result of regional dynamics, such as laryngeal movement during swallowing or the influence of negative intrathoracic pressure. [19] Congenital ectopias are generally caused by anomalies in the migration of parathyroid IIIs, whereas acquired ectopias usually affect parathyroid IVs.
In some cases, after adhering to the thyroid capsule, the parathyroid gland may actually enter the thyroid gland and embed there, rather than remain outside the gland. This situation results in an intrathyroidal parathyroid gland. The underlying origin of this phenomenon has not yet been elucidated. However, it has been concluded that parathyroid IIIs and IVs and supernumerary glands can all be the source of intrathyroidal parathyroid glands.
DiGeorge syndrome manifests as congenital thymic aplasia and absent parathyroid glands. This syndrome results from failure of the third and fourth pharyngeal pouches to differentiate. DiGeorge syndrome is also associated with facial abnormalities from abnormal development within first arch structures. No genetic cause is known, but teratogens are the assumed mechanism. Symptoms include neonatal tetany and impaired cellular immunity with normal humoral immunity.
-
Superior parathyroids.
-
Inferior parathyroids.
-
Descent of the thyroid and parathyroids.