Overview
Auditory brainstem response (ABR) audiometry is a neurologic test of auditory brainstem function in response to auditory (click) stimuli. First described by Jewett and Williston in 1971, ABR audiometry is the most common application of auditory evoked responses. Test administration and interpretation is typically performed by an audiologist. This article provides an overview of the test and its most common applications. For purposes of clarity and brevity, specialized ABR techniques and more technical issues have been omitted. [1]
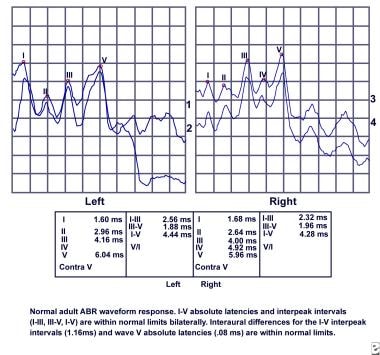
ABR audiometry refers to an evoked potential generated by a brief click or tone pip transmitted from an acoustic transducer in the form of an insert earphone or headphone. The elicited waveform response is measured by surface electrodes typically placed at the vertex of the scalp and ear lobes. The amplitude (microvoltage) of the signal is averaged and charted against the time (millisecond), much like an EEG. The waveform peaks are labeled I-VII. These waveforms normally occur within a 10-millisecond time period after a click stimulus presented at high intensities (70-90 dB normal hearing level [nHL]). (See image below.)
Although the ABR provides information regarding auditory function and hearing sensitivity, it is not a substitute for a formal hearing evaluation, and results should be used in conjunction with behavioral audiometry whenever possible.
Physiology
Auditory brainstem response (ABR) audiometry typically uses a click stimulus that generates a response from the basilar region of the cochlea. The signal travels along the auditory pathway from the cochlear nuclear complex proximally to the inferior colliculus. ABR waves I and II correspond to true action potentials. Later waves may reflect postsynaptic activity in major brainstem auditory centers that concomitantly contribute to waveform peaks and troughs. The positive peaks of the waveforms reflect combined afferent (and likely efferent) activity from axonal pathways in the auditory brain stem.
In the United States, the waveforms are typically plotted with the vertex site electrode in the positive voltage input of the amplifier, resulting in I, III, and V wave peaks. In other countries, the waves are plotted with a negative voltage.
Waveform components
Wave I
The ABR wave I response is the far-field representation of the compound auditory nerve action potential in the distal portion of cranial nerve (CN) VIII. The response is believed to originate from afferent activity of the CN VIII fibers (first-order neurons) as they leave the cochlea and enter the internal auditory canal.
A study by Lin et al indicated that in the assessment of ABR in patients with idiopathic sudden sensorineural hearing loss (ISSNHL), wave I latency is significantly associated with hearing outcomes, with a trend toward prolongation found between patients with complete hearing recovery and those experiencing only slight recovery. [2]
A study by Bramhall et al indicated that in persons with normal pure-tone auditory thresholds, those with a history of greater noise exposure tend to have smaller ABR wave I amplitudes at suprathreshold levels. The study included military veterans exposed to high levels of military noise and non-veterans with a history of firearm use, as well as veterans and non-veterans with less noise exposure. Suprathreshold ABR measurements were made at 1, 3, 4, and 6 kHz, using alternating polarity tone bursts, with the ABR wave I amplitudes at suprathreshold levels being smaller at all four frequencies in the high-noise-level groups. The amplitude differences between the groups could not be attributed to either sex or outer hair cell function variability. The investigators could not confirm whether the differences were due to synaptopathy without postmortem temporal bone examination. [3]
However, a literature review by Barbee et al suggested that ABR wave I amplitude, as well as the summating potential-to-action potential ratio and speech recognition in noise with and without temporal distortion, offers an effective nonbehavioral measure of cochlear synaptopathy. [4]
A study by Silva et al indicated that heart rate variability interacts with the ABR, specifically with regard to wave I and particularly in the right ear, suggesting that autonomic control of the heart rate is associated with brainstem auditory processing and that vagal tone/cochlear nerve interaction occurs. [5]
Wave II
The ABR wave II is generated by the proximal VIII nerve as it enters the brain stem.
Wave III
The ABR wave III arises from second-order neuron activity (beyond CN VIII) in or near the cochlear nucleus. Literature suggests wave III is generated in the caudal portion of the auditory pons. The cochlear nucleus contains approximately 100,000 neurons, most of which are innervated by eighth nerve fibers.
Wave IV
The ABR wave IV, which often shares the same peak with wave V, is thought to arise from pontine third-order neurons mostly located in the superior olivary complex, but additional contributions may come from the cochlear nucleus and nucleus of lateral lemniscus.
Wave V
Generation of wave V likely reflects activity of multiple anatomic auditory structures. The ABR wave V is the component analyzed most often in clinical applications of the ABR. Although some debate exists regarding the precise generation of wave V, it is believed to originate from the vicinity of the inferior colliculus. The second-order neuron activity may additionally contribute in some way to wave V. The inferior colliculus is a complex structure, with more than 99% of the axons from lower auditory brainstem regions going through the lateral lemniscus to the inferior colliculus.
A study by Spitzer et al of 71 preschoolers aged 3.12-4.99 years found a systematic decrease in wave V latency in these subjects, indicating that the ABR is not fully mature by age 2 years, as has been thought, but instead continues to develop through a child’s preschool years. [6]
Waves VI and VII
Thalamic (medial geniculate body) origin is suggested for generation of waves VI and VII, but the actual site of generation is uncertain.
In a study of children and adolescents with attention deficit hyperactivity disorder (ADHD), Claesdotter-Knutsson et al found that the right-side ABR wave VI demonstrated lower activity prior to the start of methylphenidate treatment than it did when treatment had reached a steady state. With wave VI possibly being of thalamic origin, the investigators proposed that the results support “the growing body of research suggesting that specific ABR peaks correlate to certain psychiatric symptoms.” [7]
Applications
Identification of retrocochlear pathology
Auditory brainstem response (ABR) audiometry is considered an effective screening tool in the evaluation of suspected retrocochlear pathology such as an acoustic neuroma or vestibular schwannoma. However, an abnormal ABR finding suggestive of retrocochlear pathology indicates the need for MRI of the cerebellopontine angle.
Symptoms of eighth nerve pathology
Clinical symptoms may include but are not limited to the following:
-
Asymmetrical or unilateral sensorineural hearing loss
-
Asymmetrical high-frequency hearing loss
-
Unilateral tinnitus
-
Unilaterally or bilaterally poor word recognition scores as compared with degree of sensorineural hearing loss
-
Perceived distortion of sounds when peripheral hearing is essentially normal
Auditory brainstem response evaluation
In addition to retrocochlear pathologies, many factors may influence ABR results, including the degree of sensorineural hearing loss, asymmetry of hearing loss, test parameters, and other patient factors. These influences must be factored in when performing and analyzing an ABR result.
Findings suggestive of retrocochlear pathology may include any 1 or more of the following:
-
Absolute latency interaural difference wave V (IT5) - Prolonged
-
I-V interpeak interval interaural difference - Prolonged
-
Absolute latency of wave V - Prolonged as compared with normative data
-
Absolute latencies and interpeak intervals latencies I-III, I-V, III-V - Prolonged as compared with normative data
-
Absent auditory brainstem response in the involved ear
In general, ABR exhibits a sensitivity of over 90% and a specificity of approximately 70-90%.
Sensitivity for small tumors is not as high. For this reason, a symptomatic patient with a normal ABR result should receive a follow-up audiogram in 6 months to monitor for any changes in hearing sensitivity or tinnitus. The ABR may be repeated if indicated. Alternatively, MRI with gadolinium enhancement, which has become the new criterion standard, can be used to identify very small (3-mm) vestibular schwannomas.
The ABR sensitivity in the diagnosis of CN VIII tumors by size according to several studies is as follows:
-
In a 1994 study by Dornhoffer, Helms, and Hoehmann, the sensitivity was 93% for tumors smaller than 1 cm. [8]
-
In 1997, Zappia, O'Connor, Wiet, and Dinces reported a sensitivity of 89% for small tumors smaller than 1 cm, 98% for medium tumors 1.1-2 cm, and 100% for tumors larger than 2 cm. The overall sensitivity was 95%. [9]
-
In a 1995 study, Chandrasekhar, Brackmann, and Devgan reported a sensitivity of 83.1% for tumors smaller than 1 cm and a sensitivity of 100% for tumors larger than 3 cm. Overall sensitivity was 92%. [10]
-
In 1995, Gordon and Cohen reported the following sensitivities: 69% for tumors smaller than 9 mm, 89% for tumors 1-1.5 cm, 86% for tumors 1.6-2 cm, and 100% for tumors larger than 2 cm. [11]
-
In a 2001 report by Schmidt, Sataloff, Newman, Spiegel, and Myers, the sensitivity was 58% for tumors smaller than 1 cm, 94% for tumors 1.1-1.5 cm, and 100% for tumors larger than 1.5 cm. The overall sensitivity was 90%. [12]
-
In a large prospective study that compared ABR with contrast-enhanced MRI (the criterion standard) in 312 patients with asymmetric sensorineural hearing loss, Cueva found that APR yielded a sensitivity and specificity of 71% and 74%, respectively, in revealing the cause of lesions for asymmetric sense and oral hearing loss (including, but not limited to, vestibular schwannoma). The ABR-positive predictive value was only 23%, whereas its negative predictive value was 96%. Seven of 31 positive cases had other lesions that ABR could not identify as a cause of the hearing loss. [13]
Although traditional ABR measures decrease in sensitivity as a factor of tumor size, recent studies have shown that by using a new stacked derived-band ABR that measures amplitude, very small tumors may be detected more accurately. This new technique, combined with traditional ABR audiometry, may soon make possible the detection of very small tumors with accuracy approaching 100% using ABR audiometry.
Other applications of auditory brainstem response
Other applications of ABR continue to evolve. Recent research suggests that although the overall ABR wave latencies are within normal limits in patients with tinnitus, those patients have longer latencies than control patients without tinnitus. [14] This suggests that ABR may be useful in monitoring and understanding tinnitus. ABR has also been used for prognostication in patients with coma. Researchers have found that patients with a Glasgow coma scale of 3 and who also have a significantly abnormal ABR had a greater probability of dying than those with a normal ABR [15] (see the Glasgow Coma Scale calculator).
A study by Sköld et al indicated that ABR wave patterns are significantly different between patients with bipolar disorder type I (BPI) and those with schizophrenia, suggesting that ABR may be useful as a BPI biomarker. The study, which involved 23 patients with BPI and 20 patients with schizophrenia, as well as 20 controls, found that wave III and VII amplitudes were significantly higher in the patients with BPI than in those with schizophrenia. The report also found that in BPI patients, as well as (somewhat less strongly) those with schizophrenia, the portion of the ABR curve containing waves VI and VII did not correlate well will that belonging to the controls. According to the investigators, the study’s results indicate that BPI may be associated with thalamocortical circuitry abnormalities. [16]
Newborn Hearing Screening
Auditory brainstem response (ABR) technology is used in testing newborns. Approximately 1 of every 1000 children is born deaf; many more are born with less severe degrees of hearing impairment, while others may acquire hearing loss during early childhood.
Historically, only infants who met one or more criteria on the high-risk register were tested. Universal hearing screening has been recommended because about 50% of the infants later identified with hearing loss are not tested when neonatal hearing screening is restricted to high-risk groups. Recently, hospitals across the United States have been implementing universal newborn hearing screening programs. These programs are possible because of the combination of technological advances in ABR and otoacoustic emissions (OAE) testing methods and equipment availability, which enables accurate and cost-effective evaluation of hearing in newborns.
Several clinical trials have shown automated auditory brainstem response (AABR) testing (eg, Algo-1 Plus) as an effective screening tool in the evaluation of hearing in newborns, with a sensitivity of 100% and specificity of 96-98%.
When used as a threshold measure to screen for normal hearing, each ear may be evaluated independently, with a stimulus presented at an intensity level of 35-40 dB nHL. Click-evoked ABR is highly correlated with hearing sensitivity in the frequency range from 1000-4000 Hz. AABRs test for the presence or absence of wave V at soft stimulus levels. No operator interpretation is required. AABR can be used on the ward and during oxygen therapy without disturbance from ambient noise.
In its 2019 position statement, the Joint Committee on Infant Hearing recommended that infants who have passed the newborn hearing screen undergo follow-up testing in the presence of the following risk factors. [17]
Perinatal risk factors include the following [17] :
-
Early, progressive, or delayed-onset permanent childhood hearing loss in the infant’s family history
-
More than 5 days of neonatal intensive care
-
Hyperbilirubinemia with exchange transfusion, regardless of length of stay
-
More than 5 days of aminoglycoside administration
-
Asphyxia or hypoxic ischemic encephalopathy
-
Extracorporeal membrane oxygenation
-
In utero infection such as herpes, rubella, syphilis, toxoplasmosis, or cytomegalovirus
-
Mother positive for Zika, while infant exhibits no laboratory evidence of the infection and no clinical findings
-
Mother positive for Zika, with infant exhibiting laboratory evidence of the infection and clinical findings
-
Mother positive for Zika, with infant exhibiting laboratory evidence of the infection but no clinical findings
-
Birth conditions or findings such as microtia/atresia, ear dysplasia, oral facial clefting, white forelock, microphthalmia, congenital microcephaly, congenital or acquired hydrocephalus, and temporal bone abnormalities
-
The presence of one of over 400 syndromes characterized by atypical hearing thresholds
Perinatal or postnatal risk factors include the following [17] :
-
Positive cultures for infections linked to sensorineural hearing loss, including confirmed bacterial and viral (especially herpes viruses and varicella) meningitis or encephalitis
-
Events that can result in hearing loss, including significant head trauma (particularly basal skull/temporal bone fractures) and chemotherapy
-
Concerns by caregivers in terms of hearing, speech, language, developmental delay, and/or developmental regression
ABRs may be used to detect auditory neuropathy or neural conduction disorders in newborns. Because ABRs are reflective of auditory nerve and brainstem function, these infants can have an abnormal ABR screening result even when peripheral hearing is normal.
Infants that do not pass the newborn hearing screenings do not necessarily have hearing problems. When hearing loss is suspected because of an abnormal ABR screening result, a follow-up diagnostic threshold ABR test is scheduled to determine frequency-specific hearing status. Estimation of hearing at specific frequencies may be obtained through use of brief tone stimulation, such as a tone burst.
Auditory Brainstem Response in Surgery
Intraoperative monitoring
Auditory brainstem response (ABR), often used intraoperatively with electrocochleography, provides early identification of changes in the neurophysiologic status of the peripheral and central nervous systems. This information is useful in the prevention of neurotologic dysfunction and the preservation of postoperative hearing loss. For many patients with tumors of CN VIII or the cerebellopontine angle, hearing may be diminished or completely lost postoperatively, even when the auditory nerve has been preserved anatomically.
Auditory brainstem response evaluation
Wave I, which is generated by the cochlear end of CN VIII, provides valuable real-time information regarding blood flow to the cochlea. Because ischemia is a primary cause of surgery-related hearing loss, wave I is monitored closely for any shift in latency or decrease of amplitude.
Wave I-II and I-III interpeak intervals can provide distal and proximal information during CN VIII surgeries.
Wave V and the I-V interpeak interval latencies are monitored for shifts or alterations in latency and amplitude. The I-V latency provides information regarding the integrity of CN VIII to the auditory brain stem.
Limitations
Wave V alterations occurring intraoperatively do not necessarily reflect changes in hearing status. Changes in latency may instead be caused by desynchronization of neurons or other outside factors. Also, a potential time delay exists between the actual occurrence of insult and when the shift in wave V appears. Patients with preexisting sensorineural hearing loss may have poor waveform morphology and no wave I response.
Typical uses of intraoperative auditory brainstem response
Monitoring cochlear function directed at hearing preservation
-
Cerebellopontine angle tumor resection (acoustic neuroma surgery)
-
Vascular decompression of trigeminal neuralgia
-
Vestibular nerve section for the relief of vertigo
-
Exploration of the facial nerve for facial nerve decompression
-
Endolymphatic sac decompression for Mèniére disease
Monitoring brainstem integrity
-
Brainstem tumor resection
-
Brainstem aneurysm clipping or arteriovenous malformation resection
Conclusion
Auditory brainstem response (ABR) audiometry has a wide range of clinical applications, including screening for retrocochlear pathology, universal newborn hearing screening, and intraoperative monitoring. Additional applications include ICU monitoring, frequency-specific estimation of auditory sensitivity, and diagnostic information regarding suspected demyelinating disorders (eg, multiple sclerosis). As technology continues to evolve, ABR will likely provide more qualitative and quantitative information regarding the function of the auditory nerve and brainstem pathways involved in hearing.
-
Normal adult auditory brainstem response (ABR) audiometry waveform response.