Overview
Skin transplanted from one location to another on the same individual is termed an autograft. These grafts consist of the entire epidermis and a dermal component of variable thickness. [1, 2] If the entire thickness of the dermis is included, the appropriate term is full-thickness skin graft (FTSG). If less than the entire thickness of the dermis is included, this graft is referred to as a split-thickness skin graft (STSG). STSGs are categorized further as thin (0.005-0.012 in), intermediate (0.012-0.018 in), or thick (0.018-0.030 in), based on the thickness of the harvested graft. Based on reconstructive needs, STSGs meeting the above criteria can be readily harvested using commercial dermatomes.
Anatomy
Skin covers the entire external surface of the human body, representing the largest single organ. The integument acts as a protective barrier from environmental insults including trauma, radiation, harsh environmental conditions and infection. Other functions include thermoregulation (through sweating, vasoconstriction or vasodilatation) and control of insensible fluid loss. Restoration of an intact skin barrier is of utmost importance following wounding to prevent infection, minimize wound contraction to maintain function, and minimize cosmetic disfigurement and to avoid volume depletion. Skin grafting was first performed in India 2000 years ago but widespread interest did not develop until the 19th century. Skin grafting currently represents the most rapid, effective method of reconstructing large skin defects.
A thorough understanding of pertinent skin anatomy is required to perform a skin graft. The skin consists of 2 layers, the epidermis and dermis. The epidermis is stratified squamous epithelium, consisting primarily of keratinocytes in progressive stages of differentiation from deeper to more superficial layers. The epidermis is subdivided into 4-5 layers, depending upon the anatomic location. These include the stratum basale (basal layer), stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum. The stratum corneum represents a significant protective barrier from the external environment and prevents desiccation of the skin. Melanocytes are found in the stratum basale and are responsible for an individual’s skin color. Of note, the epidermis has no blood vessels, so it must receive nutrients by diffusion from the underlying dermis.
The dermis is a more complex structure and is composed of 2 layers, a superficial papillary dermis and deeper reticular dermis. The papillary dermis is thinner, consisting of loose connective tissue containing capillaries, elastic fibers, reticular fibers, and some collagen. The epidermis articulates with the papillary dermis by way of a series of invaginations, Rete pegs, that strengthen the cohesion between these layers.
These pegs become blunted with age, increasing the risk of shear injury to the skin. [3] The reticular dermis consists of a thicker layer of dense connective tissue containing larger blood vessels, closely interlaced elastic fibers, and coarse branching collagen fibers arranged in layers parallel to the surface. The reticular layer contains fibroblasts, nerve endings, lymphatics, and epidermal appendages. Surrounding the components of the dermis is the gel-like ground substance, composed of mucopolysaccharides (primarily hyaluronic acid), chondroitin sulfates, and glycoproteins.
Epidermal appendages play a critical role in re-epithelialization after epidermal or superficial dermal injury, including healing of split-thickness skin graft (STSG) donor sites. Injuries that ablate these structures or underlying conditions that interfere with their function (Accutane treatment) increase the risk of cosmetically and functionally poor wound healing. These intradermal epithelial structures (sebaceous glands, sweat glands, hair follicles) are lined with epithelial cells that have the potential for division and differentiation. They are often found deep within the dermis. In the face, they may lie in the subcutaneous fat beneath the dermis, which accounts for the remarkable ability of the face to reepithelialize even the deepest cutaneous wounds.
Sebaceous glands (holocrine glands) secrete sebum that serves to lubricate the skin and make it more impervious to moisture. They are found over the entire surface of the body, except the palms and the soles and dorsum of the feet. They are largest and most concentrated in the face and scalp, where they are an anatomic focus for the development of acne.
Sweat glands (eccrine glands) are found over the entire surface of the body, except the lips, external ear canal, and labia minora. They are most highly concentrated in the palms and soles of the feet. The normal function of these glands is to produce sweat, which cools the body by evaporation. Apocrine glands are similar in structure to eccrine glands. They are concentrated in the axilla and anogenital regions. They probably serve a vestigial sexual function because they produce odor and do not function prior to puberty. The hair follicle is another important source of epithelial cells, and many of the other dermal appendages actually open into the hair follicle, rather than directly onto the skin.
The skin varies in thickness based on its anatomic location and the sex and age of the individual. Skin is thickest on the palms and soles of the feet, while the thinnest skin is found on the eyelids and in the post-auricular region. Male skin characteristically is thicker than female skin in all anatomic locations. Children have relatively thin skin that progressively thickens until the fourth or fifth decade of life, when it begins to atrophy. This thinning is primarily a dermal change, with loss of elastic fibers, epithelial appendages, and ground substance.
Graft Selection
The choice between full- and split-thickness grafting depends on wound condition, location, thickness, size, and aesthetic concerns. [1] Split-thickness skin grafts (STSGs) require less ideal conditions for survival and have a much broader range of application than FTSGs. STSGs are used to resurface large wounds, line cavities, resurface mucosal deficits, close flap donor sites, and resurface muscle flaps.
However, STSGs do have significant disadvantages that must be considered. STSG are more fragile, especially when placed over areas with little underlying soft tissue support, and usually do not withstand subsequent radiation therapy. They can contract significantly during healing. They tend to be hypo- or hyperpigmented, particularly in darker-skinned individuals. Their thinness, abnormal pigmentation, and frequent lack of smooth texture and hair growth make STSGs more functional than cosmetic. When used to resurface large burns of the face, STSG may yield an undesirable masklike appearance. Although both FTSG and STSG donor sites leave a second wound, the STSG donor site must reepithelialize and often causes significant discomfort and has an ongoing wound care requirement until healed. However, these sites may be reharvested once healing is complete.
More characteristics of the normal donor skin are maintained following grafting when thick STSG or FTSG are harvested, because more collagen content, dermal vascular plexuses, and epithelial appendages are contained within thicker grafts. However, thicker grafts have higher metabolic needs. Thus, they require optimal conditions for survival and have higher incidence of graft failure than STSGs. FTSGs, on the other hand, have a better color match to the recipient site due to their thicker nature and inclusion of additional dermal structures. They tend to contract to a much lesser degree than STSGs, providing optimized cosmetic and functional results. Further, FTSGs are often a better thickness match for full thickness skin/dermis defects. The donor site is typically closed primarily and requires a much less intensive wound care regimen.
Donor Site Selection
Split-thickness skin grafts (STSGs) may be harvested from any surface of the body, but the sites chosen should be concealed easily in recreational clothing and minimize the discomfort during reepithelialization.
Common sites include the upper anterior and lateral thighs. [4] The buttocks may be used as a donor site, but patients may have significant postoperative pain and will require assistance in caring for the wound. The scalp may be used for resurfacing areas of the face that are too large for full-thickness skin grafts (FTSGs) and in severe burns in which donor-site availability is limited. [5] Because of its thickness, scalp skin may be harvested repeatedly with minor risk of alopecia or subsequent hair growth at the recipient site. For hand wounds, the upper inner arm is a cosmetically superior donor site to the more accessible forearm. As an alternative, in cases of free tissue transfer, especially when the flap is being placed intraorally or subcutaneously, the STSG should be harvested from the cutaneous portion of the free flap. [6]
A retrospective study by Rotatori et al indicated that in pediatric burn patients who undergo treatment with STSGs, the thigh, if used as a donor site, is particularly likely to develop hypertrophic scarring, while, among all possible sites, the scalp, arm, foot, and lower legs are less likely. [7]
Facial wounds, for the above-noted reasons, are often repaired with FTSGs. Preauricular or postauricular skin, as well as supraclavicular skin, is often a good match in these locations. A study by Hexsel et al indicated that the postauricular region is an excellent site for harvesting small to moderate-sized STSGs (< 10 cm) for the head, neck, and upper chest. In the study, patients who underwent Mohs micrographic surgery with STSGs (total of 41 surgical defects) healed well at the postauricular donor site, scoring low on the Vancouver Scar Scale at 6-11 weeks (18 donor sites), 3-6 months (7 donor sites), and 6 months or more (16 donor sites), postsurgery. [8]
In cases requiring full-thickness skin resurfacing as well as cartilaginous tissue for reconstruction, composite grafts consisting of skin and cartilage can be harvested from the helical root or conchal bowl.
Wound Preparation
The wound bed must be optimally prepared to ensure skin graft survival. Improper wound preparation is the source of most skin graft failures. [9, 10, 11] Skin grafts will not survive on tissue with limited blood supply (cartilage, tendon, nerve). Skin grafts will survive on periosteum, perichondrium, peritenon, perineurium, dermis, fascia, muscle, and granulation tissue. Wounds that develop secondary to radiation are also less likely to support split-thickness skin grafts (STSGs) and often require adjunctive measures to optimize survival. [12]
Underlying conditions that compromise wound healing, venous stasis, and arterial insufficiency should be optimized prior to grafting to increase the likelihood of graft survival. The wound must be free of necrotic tissue and relatively uncontaminated by bacteria. [9, 11] Bacterial counts greater than 100,000 per square centimeter are associated with a high likelihood of graft failure. Debridement, dressing changes, and topical or systemic antibiotics may be indicated prior to grafting to achieve an adequate wound bed.
An additional option that is relatively new to the facial plastic surgeon’s armamentarium is the wound vacuum-assisted closure (VAC) device. [13, 14, 15] This device has been shown to stimulate granulation tissue and decrease the bacterial counts of contaminated wounds. Further, following application of a nonadherent dressing, this device can be applied on top of STSG, creating a sandwich dressing consisting of the wound bed, skin graft, and VAC device. A randomized, prospective trial comparing VAC devices to a non-negative pressure control was performed in 2006 and showed significantly fewer skin graft losses, shorter hospital stays, and fewer needs for second procedures in the VAC group. [16] There is a significant difference in cost, however, with VAC devices costing about 50 times more than the standard cotton bolster dressing.
A prospective, randomized, controlled trial by Nguyen et al indicated that the use of gauze dressings and wall suction is as effective as VAC in aiding wound closure through negative pressure therapy. The study found that by postoperative day 4 or 5, STSGs had fully taken in 60 out of 77 wounds treated with VAC and in 64 out of 80 wounds treated with gauze sealed with an occlusive dressing and wall suction. [17]
A 2010 study by Demirtas et al found that, among 5 tested dressing materials, Comfeel Plus Transparent was the least painful and one of the most economical materials, although none of the tested materials was found to be ideal based on the authors' criteria. [18]
Operative Technique
Careful operative technique is necessary to maximize graft survival. After initiation of appropriate anesthesia, which can include either topical/local [19, 20] with or without sedation or general anesthesia, the wound is prepared for grafting. In the case of posttraumatic or postoncologic ablation reconstruction, these patients are often under general anesthesia due to the significant anesthesia requirement of their trauma repair or oncologic procedure. Wound preparation involves cleansing with saline, judicious debridement, and meticulous hemostasis. Minimal use of electrocautery is recommended because it creates devitalized tissue. Use of epinephrine at the donor or recipient site does not compromise graft survival.
Harvesting
Split-thickness skin grafts (STSGs) may be harvested in a variety of ways. [21] The most commonly used technique involves a dermatome, which provides rapid, consistent harvest of large uniform-thickness grafts. Dermatomes are typically air-powered or electric, although manually operated devices exist. Commonly used dermatomes include the Padgett and Zimmer, among others. All of these dermatomes harvest with a rapidly oscillating blade. The thickness is easily adjusted on the instrument. The width is typically adjusted in 1- to 2-inch increments by applying blade guards of different widths. Lastly, the length is dictated by the surgical technique.
Regardless of technique, adequate anesthesia must be established because harvesting of skin grafts is a painful procedure. Lidocaine with epinephrine injected at the donor site may reduce blood loss and provide greater tissue turgor that assists in harvesting.
Surgeons must be familiar with the installation of the blade and depth settings and must check these before operating the device. The blade has a correct and an incorrect orientation, and inexperienced operating room personnel may easily confuse the two. Insertion of a Bard-Parker No. 15 scalpel blade (beveled edge) simulates a thickness of 0.015 inches (intermediate thickness) and can be used to quickly verify that depth settings are correct. Wash off Betadine or other agent to prepare the donor site to allow the device to easily slide over the skin. Avoid use of DuraPrep to prepare the donor site because its removal is difficult and it interferes with smooth advancement of the dermatome over donor skin. To this end, lubricating the skin and dermatome with mineral oil allows easy gliding of the dermatome. These substances may be gently washed from the graft with saline following harvesting and do not compromise graft survival. [22]
The dermatome is held in the dominant hand of the operator at a 30-45° angle from the donor skin surface. Greater angulation of the dermatome leads to gouging or trenching of the donor site skin. With the nonoperating hand providing traction behind the dermatome, the assistant provides traction in front of the dermatome to help stretch and flatten the skin. The dermatome is running when it engages the skin surface and is then advanced in a smooth continuous motion over the skin with gentle downward pressure. After an appropriate length has been harvested, the dermatome is tilted away from the skin and lifted off of the skin to cut the distal edge of the graft and complete the harvesting.
The graft may then be gently washed of lubricant and used for grafting with or without meshing. Typically, the donor site exhibits numerous small punctate bleeding spots with thin to intermediate-thickness grafts. Harvesting thicker grafts yields fewer bleeding points that bleed more copiously. Any exposure of fat indicates that excision of the graft was performed too deeply, probably because of technical error in assembly of the dermatome.
Drum dermatomes (Reese, Padgett-Hood) deserve special mention but now are used less frequently. The oscillating blade is manually powered as the drum is rolled over the skin surface. These dermatomes are useful when the donor site is irregular or has a convexity, concavity, or bony prominence (neck, flank, buttock) because the skin to be harvested is first made adherent to the drum with a special glue or adhesive tape. These dermatomes also allow harvesting of precise irregular patterns by varying the pattern of adhesive applied to the skin and drum. Disadvantages include injury to operating personnel by the swinging blade, flammable defatting agents necessary to prepare the donor site skin (acetone, ether), and greater technical expertise required to safely and effectively operate these devices. These devices are rarely necessary in head and neck reconstruction given that the more commonly used donor sites (thigh) are available and provide generous donor tissue. [4]
Another method for harvesting STSGs is freehand with a knife. Although this may be performed with a scalpel, other devices (Humby knife, Weck blade, Blair knife) are also available. Disadvantages include grafts with irregular edges and varying thicknesses. As with drum dermatomes, greater technical expertise is necessary, and graft quality tends to be more operator-dependent when compared with air- or electric-powered dermatomes.
Placement
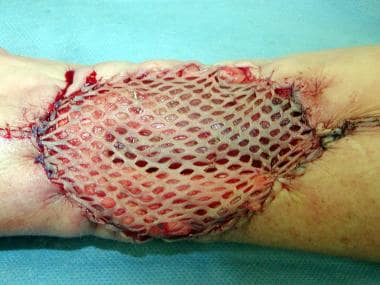
Once harvested, a STSG may be meshed by placing the graft on a carrier and passing it through a meshing device.
The carrier has a grooved side that must be directed superiorly and upon which the graft should be laid out. This technique allows expansion of the graft surface area up to 9 times the donor site surface area. This technique is indicated when insufficient donor skin is available for large wounds, as in major burns or when the recipient site is irregularly contoured and adherence is a concern. [23]
Expansion slits allow wound fluid to escape through the graft rather than accumulating beneath the graft and preventing adherence. Alternatively, this can be performed in a more limited fashion by “pie crusting” the graft with a No. 15 blade. However, neither technique will prevent graft loss due to underlying hematoma. Expansion slits must heal by re-epithelialization and may contract significantly. Also, the healed wound characteristically has a crocodile skin or checkerboard appearance. Because of secondary contraction and poor cosmesis, avoid meshing STSGs placed on the face, on the hands, over joints, and in other highly visible areas. In these regions, STSGs may be pie-crusted to allow drainage of wound fluid from beneath the graft.
Several adjunctive maneuvers can be used to reduce or eliminate the surgical defect that must be resurface. In many cases, a purse-string closure can be used to significantly reduce the surgical defect size. [24] In the authors’ experience of reconstructing forearm wounds, we have found that this technique can reduce the defect size by almost 50%. Further, V-to-Y advancement closure can further reduce the area that requires skin graft coverage. [25] As an aside, this technique can sometimes obviate the need for a skin graft on the face with a cosmetically acceptable result. [26]
Once the recipient site has been prepared, the graft may be placed over the wound bed. Placing the dermal, typically white or lighter color, side down is important. Take care to prevent wrinkling or excessive stretching of the graft. The graft must then be secured in place to provide stability during initial adherence and healing. This is most often accomplished by suturing the graft to the surrounding wound bed. Avoid using staples because they are painful to remove and may disrupt graft adherence to the wound when removed at approximately 7 days postoperatively. Absorbable sutures, such as 5-0 fast absorbing gut, are preferable because they do not require removal.
Usually, 4-corner sutures are placed to hold the graft in the proper orientation. Then a running suture is placed around the periphery. Passing the needle first through the graft and then through the surrounding wound margin to prevent lifting of the graft from the wound bed is helpful. Perfect epidermal-to-epidermal, graft to native skin, approximation ensures optimal cosmetic results. Tacking sutures may also be used focally to ensure adherence of the graft over a concave portion of the wound and to prevent serous fluid accumulation.
Dressings and wound care
A dressing is then chosen to provide uniform pressure over the entire grafted area through a nonadherent, semiocclusive, absorbent dressing material. These dressings are meant to immobilize the graft, prevent shearing, and prevent seroma or hematoma formation beneath the graft. “Tie-over” bolster dressings are useful over joints or other areas where motion is difficult to avoid, in wounds with irregular contours, and in wound locations where securing a dressing is difficult (oral and nasal cavities, nasal tip).
These bolsters may be constructed from foam rubber, N-terface, Adaptic, or Xeroform folded over moistened cotton balls. When sewn into place, these provide a constant light pressure that is molded to the contour of the wound. These dressings are then secured by placing nonabsorbable sutures radially around the wound and tying them to each other over the bolster dressing. Alternatively, sutures used to hold the graft in place may be nonabsorbable and left long to tie subsequently over the bolster.
Another dressing choice for irregularly contoured wounds or wounds with high levels of exudate is the vacuum-assisted closure (VAC) sponge. [15, 14] These vacuum-molded sponge dressings conform to the wound surface by suction and promote skin graft adherence while removing exudate and edema from surrounding tissues. A nonadherent surface (Adaptic) must be placed as an interface between the skin graft and the sponge to prevent peeling off the graft when removing the sponge. Burn netting may also be useful for securing dressings in difficult locations (pelvic and shoulder regions).
Graft adherence is maximal during the first 8 hours postgrafting, but the initial dressing should be left in place for 3-7 days unless pain, odor, discharge, or other signs of complications exist. When removing dressings, moisten them with saline to reduce adherence to the graft. The dressing may then be carefully removed to prevent lifting the graft off of the underlying wound bed. Treat hematomas or seromas encountered at dressing change by making a small incision over the collection and expressing the underlying contents. Rolling the fluid out from under the edge of the graft is not recommended because it disrupts adherence of the entire graft, not just the area of hematoma or seroma formation.
The donor site must also be dressed appropriately at the conclusion of the operation. [27] A variety of dressing options exists for STSGs donor sites. After hemostasis has been achieved, apply a dressing with application of moist gauze containing epinephrine solution. (A literature review by Brown and Holloway indicated that with regard to dressings at STSG donor sites, healing rates and pain reduction are greater with moist products than with nonmoist ones. [28] )
The ideal donor site dressing should be one that promotes rapid reepithelialization, causes little pain, requires little care, is inexpensive, and has a low rate of infection. [29] Options include occlusive dressings (DuoDerm), semiocclusive dressings (Op-Site, Tegaderm), [29, 30] semiopen dressings (Vaseline gauze, Xeroform, scarlet red), and no dressing. [31]
Multiple synthetic dressings have been studied in comparison to the standard Vaseline gauze dressing for promotion of reepithelialization. Examples include Veloderm (hemicellulose dressing) and Biobrane (semipermeable silicone film with imbedded porcine collagen thread). A randomized controlled trial in 2012 demonstrated significantly improved healing times, pain, and exudate scores in the Veloderm group. There was also a significantly lower need for dressing changes when compared with the Vaseline gauze group. [32]
A prospective, comparative, randomized study by Moellhoff et al found polylactic acid membranes to be superior to polyurethane film as dressing for STSG donor sites. Patients who received the polylactic acid membrane had a lower median Vancouver Scar Scale score (2 [interquartile range, 1-3]) than did those in the polyurethane film group (3 [interquartile range, 2-4]). Pain during dressing change and mobilization were also lower in association with polylactic acid membranes, with the membranes additionally requiring fewer changes per day during the patients’ hospital stay than did polyurethane film (0.28 vs 0.44, respectively). [33]
In general, most studies show that semiocclusive dressings are superior to occlusive or open dressings. These products have been shown to have the fastest healing rates [30] (average of 9 days to re-epithelialization), [29] lowest subjective pain scores, lowest infection rates (3%) and are among the lowest in cost. They have the advantage of being transparent, which allows ongoing inspection of the site while maintaining sterility. Fluid collects under these materials, which promotes moist wound healing and probably accounts for the more rapid healing rates and decreased subjective pain scores. Remove the dressing and use another technique if the fluid becomes cloudy or otherwise suggestive of infection. If the fluid accumulation is significant enough that the covering appears tight and likely to rupture, the fluid may be withdrawn with a sterile needle and a patch of similar material may be used to close the needle-stick site.
Ottomann et al found that a single extracorporeal shock wave treatment (ESWT) can significantly accelerate epithelialization of the donor site after skin graft harvesting. In a randomized trial, a single defocused shock wave treatment (100 impulses/cm2 at 0.1 mJ/mm2) applied immediately after skin harvest reduced the time to complete epithelialization from 16.7 days to 13.9 days. [34] Animal models have shown that the mechanism of accelerated wound healing occurs because of proangiogenic and anti-inflammatory effects of the ESWT. [35]
The rate of healing is proportional to the number of epithelial appendages remaining and inversely proportional to the thickness of graft harvested. The epidermis is regenerated and may be reharvested, but each harvesting removes a portion of dermis that is not regenerated. The initial epithelium that is regenerated is very delicate and easy to disrupt with tape or dressing changes. Finally, hyperpigmentation of the donor site may persist for many months following donor site healing and darker-skinned individuals may experience hypertrophic scarring at the site.
Graft Survival
After graft placement, an initial adherence to the wound bed via a thin fibrin network temporarily anchors the graft until definitive circulation and connective-tissue connections are established. [23] This adherence begins immediately and is probably maximized by 8 hours postgrafting. The period of time between grafting and revascularization of the graft is referred to as the phase of plasmatic imbibition. The graft imbibes wound exudate by capillary action through the spongelike structure of the graft dermis and through the dermal blood vessels. This prevents graft desiccation, maintains graft vessel patency and provides nourishment for the graft.
This process is entirely responsible for graft survival for 2-3 days until circulation is re-established. During this time, the graft typically becomes edematous and increases in weight by 30-50%. Revascularization of the graft begins at 2-3 days postgrafting by a mechanism that is not completely understood. Inosculation is the establishment of direct anastomoses between graft and recipient blood vessels.
Alternatively, some investigators have demonstrated vascular ingrowth of recipient bed vessels into the graft along the channels of previous graft vessels. Still others propose that random new vascular ingrowth of recipient bed vessels into the graft occurs without regard for previous graft vessels. Regardless of the true mechanisms, full circulation to the graft is restored by 6-7 days postgrafting. Without initial adherence due to poor technique, hematoma, or shear, plasmatic imbibition and revascularization will not take place and the graft will slough. If the wound bed is properly prepared and the graft is bolstered and the wound spared from shear, split-thickness skin graft (STSG) survival should be near uniform. [36]
Several important aspects of skin graft healing deserve further discussion. [11] Wound contraction may present serious functional and cosmetic concerns, depending on the location and severity. Wound contraction on the face may produce ectropion, retraction of the nasal ala, distortion of the vermilion border, or loss of facial symmetry. Over joints, it may limit functional range of motion. Contraction probably begins shortly after initial wounding. [22] progressing slowly over 6-18 months following grafting. Myofibroblasts are believed to cause contraction. [37] The spectrum of wound contraction from least to most is as follows:
-
Full-thickness skin grafts (FTSG) – Least contraction
-
Thick STSG
-
Thin STSG
-
Open wounds – Most contraction
The ability of a skin graft to resist contraction is related to the thickness of the deep dermal component included in the graft, [22] not just the absolute thickness of the graft. This deep dermal component is able to suppress myofibroblast function by an unknown mechanism. [37] Contraction can be ameliorated by splinting or compression devices (facial masks, Jobst garments). These devices should be worn as much as tolerated each day for at least the first 6 months postgrafting and often even longer.
Epithelial appendages must be regenerated following grafting. Hair rarely grows from STSG as the follicles are not transferred with the graft. Sweat glands and sebaceous glands initially degenerate following grafting. Because only a portion of the gland is transferred, the remaining portion may not regenerate. Sweat gland regeneration is dependent on reinnervation of the skin graft with recipient bed sympathetic nerve fibers. Once this ingrowth has occurred, the skin graft assumes the sweating characteristics of the recipient site. Sebaceous gland regeneration is independent of graft reinnervation and retains the characteristics of the donor site.
Prior to regeneration, the skin graft is lacking the normal lubrication of sebum produced by these glands, making the grafts more susceptible to injury. [38] The grafts may appear dry and scaly during this period. Patients frequently complain of pruritus. Recommend bland creams (lanolin, cocoa butter) to moisturize the graft and reduce itching. Unfortunately, this condition often persists in thin STSGs. These glands also may regenerate on the deep surface of skin grafts and present as milia. When encountered, they should be unroofed with a needle.
Reinnervation of the graft occurs from the recipient bed and from the periphery along the empty neurolemma sheaths of the graft. Sensation returns to the periphery of the graft and proceeds centrally. Usually, this process begins during the first month but is not complete for several years following grafting. STSGs reinnervate more quickly, but FTSGs reinnervate more completely. Reinnervation is always incomplete and some degree of permanent derangement persists. Generally, the patient develops protective sensation but not normal perception. Pain is usually the first perceived sensation, followed later by touch, heat, and cold. STSGs may remain pale or white or may become hyperpigmented with exposure to sunlight. A general recommendation is to keep the graft be protected from direct sunlight for at least 12 months postgrafting. Hyperpigmentation has been treated with dermabrasion and laser resurfacing.
Graft Failure
Skin grafting may be unsuccessful for numerous reasons. The most common reason for skin graft failure is hematoma beneath the graft. Similarly, seroma formation may prevent graft adherence to the underlying wound bed, preventing the graft from receiving the necessary nourishment, as detailed above. Movement of the graft or shear forces may also lead to graft failure through disruption of the fragile attachment of the graft to the wound bed. This often occurs when the graft is placed over a flexor or extensor surface or over a mobile tendon sheath.
Another common source of failure is a poor recipient site. The wound may have poor vascularity, or the surface contamination may have been too great to allow graft survival. Bacteria and the inflammatory response to bacteria stimulate release of enzymes and other harmful substances at the wound interface that disrupt the fibrin adherence of the graft. Technical error may also yield graft failure. Most importantly, applying the graft dermis side superficial results in complete graft loss. Applying excess pressure, stretching the graft too tightly, or handling the graft in other traumatic ways may lead to partial or complete graft failure.
Biologic Skin Substitutes
No discussion of skin grafting would be complete without mention of currently available alternative substances. Biologic skin substitutes may be intended for permanent replacement or as a temporary biologic dressing until a permanent solution is available or normal skin regeneration and healing occur. [39] These substitutes serve multiple functions. They decrease bacterial counts and promote sterile wounds. They also slow the loss of water, protein, and electrolytes. They reduce pain and fever, help restore function, and facilitate early motion. They provide coverage of vessels, tendons, and nerves to prevent desiccation. The ideal skin substitute is one with little or no antigenicity and with tissue compatibility, lack of toxicity, and lack of disease transmission.
Cadaveric grafts and pig skin grafts are the historical skin substitutes with which most surgeons are familiar. Cadaveric grafts are termed allografts, or homografts, because they are transplanted from one organism to another within the same species. Pig skin grafts are termed xenografts, or heterografts, because they are transplanted from one organism to another of a different species.
These grafts may be prepared for use in several ways. They may be treated with glycerol and rapidly frozen with liquid nitrogen, or they may be lyophilized and freeze-dried. Although graft processing does not ensure cell viability, the structural details, proteins, and enzymes remain intact. Eventually rejected by the body, these grafts may be used as temporary biologic dressings, especially in extensive burns where skin graft donor sites are limited. These dressings must normally be changed every 3 days to prevent a rejection response, but patients with burns, who tend to be relatively immunosuppressed, may require removal only every 5 days.
A theoretical risk of disease transmission exists with cadaveric grafts. Cultured epithelial cells have also been developed, both as autografts and allografts. Cultured epithelial autografts require biopsies of the patient, followed by growth of these cells in culture. For this reason, they are not available for several weeks until they have grown to confluent sheets. Currently, this culturing process is quite costly and yields an extremely fragile sheet of cells that are very sensitive to infection. Allograft sheets are available immediately but share the structural weaknesses of autografts and the theoretical risk of disease transmission. Allografts are eventually rejected as well but, in the interim, can serve as a biologic dressing.
Allograft dermis has also been developed and effectively used for surgical wound coverage with adequate functional recovery and equivalent cosmesis. [40] This structure is not actually rejected by the body because it is rendered immunologically inert during processing. The body instead remodels and replaces it with a native dermal substitute. Cultured epithelial sheets or thin split-thickness skin grafts (STSGs) may be placed over this dermal substitute once it has become incorporated.
Bilayer collagen matrices are the latest development in this explosive field. [41] These are a porous, sponge-like lattice of bovine collagen, chondroitin-6-sulfate, and glycosaminoglycans that serve as the dermal substitute. The dermal substitute layer serves as a scaffold that facilitates ingrowth of native fibroblasts and blood vessels with its eventual replacement. An overlying silastic membrane simulates the epidermis and serves to seal the surface to reduce insensible fluid loss. This membrane is transparent, allowing wound inspection and progressively becomes less adherent to the dermal layer as it is incorporated into the body. At about 3 weeks the silastic layer may be peeled off and replaced with cultured epithelial cells or a thin STSG.
Current research in molecular biology, wound healing, and immunology will likely yield even better skin substitutes with which to treat patients in the future. One day, a synthetic bilayer membrane of quality equal to that of skin may be available off of the shelf for application as simple as that of a dressing change.
A study by Lee et al indicated that the use of split-thickness skin grafts (STSGs) in combination with acellular dermal matrix (ADM) grafts produces better scar results in full-thickness skin defects than does the use of STSGs alone. The study, of patients who underwent grafting to cover an anterolateral thigh free-flap donor site, found that with regard to the Vancouver Scar Scale, the STSG/ADM group had a significantly lower vascularity subscore and total score. In addition, the pain and stiffness subscores and total score for the Patient and Observer Scar Assessment Scale were also significantly lower in the STSG/ADM patients. [42]
A literature review by Dunn et al suggested that the use of platelet-rich plasma (PRP) is an effective adjunct to split-thickness skin grafting. The report indicated that STSG patients treated with PRP have decreased healing time, length of hospital stay, and scarring and do not require sutures/staples. [43]
-
Dermatome.
-
Dermatome blade guards.
-
The length of a split-thickness skin graft is dictated by the surgical technique.
-
A split-thickness skin graft passed through a meshing device.
-
Split-thickness skin graft.