Overview
The use of intraoperative facial nerve monitors has resulted in objectively demonstrable improvement in facial nerve outcome for patients undergoing posterior fossa surgery for tumor removal.
The importance of such monitors is borne out by the devastating complications that can result from facial nerve injury during surgery, including grotesque alteration of facial appearance, exposure of the eye to vision-threatening desiccation and infection, and impairment of competence of the oral sphincter, resulting in drooling and alterations in vocal quality.
Individuals who have had severe facial nerve injury experience degraded self-image and loss of self-confidence and self-esteem. Most experience at least transient depression, and social interaction and occupational status can be affected.
Many occupations cannot be successfully pursued with distorted facial features. Newscasters, actors, and other public personalities are obvious examples, although people in sales and public service positions may also find achieving occupational success more difficult because of facial distortion that is off-putting to potential clients and customers. Facial paralysis, therefore, can have a disastrous effect not only on socialization but also on career and income.
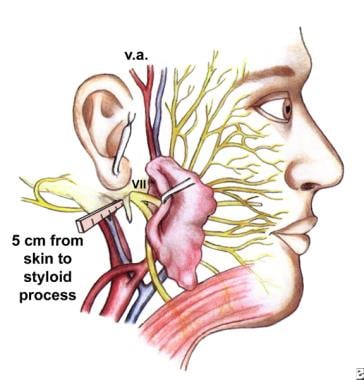
Consequently, surgeons who operate in the anatomic areas traversed by the facial nerve (see the image below) welcome and accept any adjunctive technique that potentially reduces the incidence of facial paralysis. Few surgeons would remove an acoustic neuroma without a functioning facial nerve monitor. [1]
However, although, as stated, intraoperative facial nerve monitoring has resulted in demonstrably improved facial nerve outcomes in posterior fossa tumor surgery, objective documentation of improved results in mastoid and middle ear surgery is not yet forthcoming. [2] Nonetheless, many surgeons are convinced, despite the absence of objective data, that the facial nerve monitor is helpful for otologic surgery and regularly use it for routine otologic operations. [3, 4]
Limitations
Facial nerve monitoring is not a panacea, and it does not substitute for anatomic knowledge. As Prass stated in 1996, "Improper set up, equipment failure, or misuse may lead to worse outcomes than if facial monitoring was not used." [5] Consequently, all surgeons who use this technology must understand the principles that underlie it and must become thoroughly familiar with its use.
Sources of nerve trauma
The facial nerve can be injured by direct mechanical disruption from a rotating burr, transection with a sharp instrument, accidental evulsion (eg, from traction), or a crushing injury. A rotating surgical burr can produce thermal injury without directly contacting the facial nerve. Thermal injury is more likely when diamond burrs are used than when cutting burrs are employed.
Principles of Electrophysiologic Monitoring
Some enthusiasm was evident in the early 1980s for mechanical monitoring techniques that relied on sensing actual facial movement. These techniques have since fallen into disfavor principally because actual movement requires a large suprathreshold response; consequently, mechanical techniques are less sensitive to facial nerve stimulation than are electrophysiologic techniques. Only a few surgeons continue to use them.
In 1979, Delgado became the first person to use electrophysiologic monitoring of the facial nerve. He used evoked electromyography and monitored the electromyographic responses, as is generally practiced today.
Electromyography depends on noting the difference in electrical potential that occurs within the muscle associated with a depolarizing current. As the electrical potential moves past the first of the two paired electrodes, that electrode becomes negative with respect to the second. As the wave reaches the second electrode, a deflection in the opposite direction occurs. The electromyographic electrical response is biphasic, ie, it has both positive and negative components. [6, 7]
As a practical matter, neurophysiologic monitoring of the facial nerve continuously evaluates the electromyographic activity in the monitored facial muscles. A graphic signal, which can be observed on an oscilloscope screen, and an acoustic signal, which can be heard throughout the operating theatre, are generated. Prass has distinguished between 2 types of potential responses. [5, 8]
Repetitive responses
Repetitive responses occur as a result of repetitive depolarizations that follow the cessation of surgical manipulation. Such responses may indicate increased irritability as a result of injury. They sometimes are referred to as "training". They cannot be used to locate the nerve but can warn the operating surgeon that injury has occurred or is imminent. Repetitive responses can occur as a result of thermal stimulation, trauma, or traction. Occasionally, cold irrigation alone suffices to produce brief trains of repetitive responses.
Nonrepetitive responses
Nonrepetitive responses are produced by direct mechanical or electrical stimulation of the facial nerve and occur in close temporal association with the stimulus. These nonrepetitive responses are much more important in defining the boundaries of the nerve. Because they are single responses that are directly associated with the stimulus, nonrepetitive responses allow the surgeon to locate the edge of the nerve and to map its anatomic contour.
Experienced surgeons who have used facial nerve monitors for many years agree that the significance of evoked electromyography can be assessed only within the context of the surgical events that occur during the response. [9]
Safety
Occasionally, questions about the safety of facial nerve monitoring have been raised, including the following:
-
Does repetitive intraoperative stimulation injure the nerve
-
Can frequently repeated stimulation produce metabolic exhaustion (a permanent injury)
-
Are low-stimulation intensities safer than high-stimulation intensities
To address such questions, Hughes et al utilized a mouse sciatic nerve model to examine pulsed and direct current stimuli. [10] A pulsed current produced no evidence of injury. Direct current produced some mild injury and, occasionally, axonal degeneration. Virtually all available monitors use a pulsed current technology.
Babin et al developed a cat facial nerve model to assess the safety of continuous facial nerve stimulation. [11] They applied 3 stimulations per second to the cat facial nerve at 1 milliampere (mA) for 1 hour. The nerve had no permanent changes in sensitivity, although a transient decrease in sensitivity occurred for several minutes after cessation of stimulation.
Especially during the last decade, many thousands of patients have been monitored using electrophysiologic techniques, including intraoperative stimulation. This wide clinical experience has not produced any evidence that nerve monitoring can be harmful.
Equipment Setup
Several variables can be selected when setting up facial nerve monitoring. Some can be changed during the operation, although others cannot be changed. Selesnick pointed out that reports indicate no standardization across the following variables [12] :
-
Pulse duration
-
Monopolar versus bipolar stimulation
-
Constant current versus constant voltage
-
Stimulus intensity
-
Threshold
Pulse duration
The actual charge transmitted to the nerve is the product of the amount of current times the duration over which the current is delivered. Consequently, a pulse duration of 100 microseconds delivers twice the amount of charge than does a pulse deviator of 50 microseconds, assuming a constant current.
The default setting for most physiologic monitors is 100 microseconds. Selesnick showed that 50 microseconds provides a more sensitive stimulus. Some surgeons use a pulse duration of 200 microseconds.
Monopolar versus bipolar stimulation
When a monopolar probe is used, the current flows from the stimulation probe in all directions. Whether a response is obtained depends on the distance of the nerve from the tip of the probe, the impedance of the tissue between the tip of the probe and the nerve, and the strength of the stimulus.
Bipolar stimulation allows current flow only from one tip directly to the other; therefore, the facial nerve is stimulated only if it lies directly between the 2 tips.
Bipolar stimulation is more precise because less opportunity exists for shunting of current away from the site of stimulation. However, it is much more prone to false-negative results because no stimulation occurs unless the nerve lies directly in the current's path. Monopolar stimulation is more common than bipolar, but both techniques are used.
Constant current versus constant voltage
Ohm's law indicates that if constant voltage stimulation is used, voltage increases to compensate for current shunting.
In a study, Prass did not detect any difference between constant voltage and constant current systems in either efficacy or safety. [8] Nonetheless, constant current systems are used more commonly.
Stimulus intensity
Stimulus intensity is variable on most commercially available facial nerve monitors and must be adjusted by the operating surgeon. The appropriate stimulus intensity may vary considerably. Unless injured, the unsheathed facial nerve in the subarachnoid space reliably responds to a stimulus intensity of as low as 0.05mA, although the sheathed facial nerve in the mastoid segment often does not respond to stimulus intensities of less than 0.15mA. The operating surgeon must be familiar with this variability.
Using an inappropriate setting can be highly misleading. If a stimulus setting of 0.2mA is used within the internal auditory canal, almost everything the probe touches is likely to result in a facial nerve response. Consequently, this setting would not help to identify, isolate, and map the course of the facial nerve.
On the other hand, direct stimulation on the sheath of the facial nerve using a stimulus intensity of 0.05mA in the mastoid does not generally produce a response. If the operating surgeon is not aware of these differences, he or she may transect a normal facial nerve in the mastoid.
Threshold
The threshold at which the monitor produces an audible response can be set for most facial nerve monitors and is generally set at 100 microvolts. While varying the threshold setting is possible, it is generally held constant at this level.
The threshold setting may be altered if prognostic information about facial nerve recovery is sought at the end of the procedure.
Needle versus surface electrodes
A study by Park et al indicated that in patients undergoing parotidectomy, facial nerve monitoring can be effectively accomplished with adhesive surface electrodes as opposed to those inserted by needle. Mean amplitudes were derived “after electrical facial nerve stimulation at 1 mA after specimen removal.” The surface electrodes had mean amplitudes of 226.50 μV (orbicularis oculi muscle) and 469.6 μV (orbicularis oris muscle), while the needle electrodes used in the study had mean amplitudes of 449.85 μV and 654.66 μV, respectively. The investigators considered the surface electrodes, despite their lower amplitudes, to be safe and feasible for use in parotid surgery. [13]
Electrode Placement
Paired electrodes are placed in 2 facial muscle groups. (Two separate muscles are usually monitored, principally to assure redundancy.) The orbicularis oris and the orbicularis oculi are usually selected. They are relatively large facial muscles and are easily identified. The portion of the electrode not within the muscle itself should be insulated.
No known topographic organization of the facial nerve exists, either intratemporally or within its extratemporal portions; accordingly, a single lead theoretically suffices.
Commercial electrodes are available from a variety of manufacturers. The electrode leads must not touch each other but must not be too far apart. Some systems are self-checking and alert the surgeon to inadequate electrode placement.
A ground electrode for the intramuscular electrodes and a special remote ground electrode for the stimulation probe must be placed. Thus, a total of 6 electrodes are placed. Each electrode must be correctly connected to the facial nerve monitor.
Once all the electrodes are properly positioned and placed, tapping gently on the face to determine whether a faint response can be heard from the facial nerve monitor is useful. A response indicates that the system is functioning properly.
Monitoring
The amount of electromyographic feedback that can be expected during a given operative procedure depends on the following variables:
-
The adequacy of intramuscular electrode placement
-
The number of independent electromyographic channels
-
The effectiveness of nerve stimulation
-
The level of nerve irritability
-
The conduction status of the nerve distal to the point of stimulation
Silverstein pointed out that a conduction block in the distal portion of the nerve causes a loss in the monitoring value of the facial nerve monitor proximal to that point. [14] Prass, therefore, suggested that, when possible, dissection should proceed from proximal to distal, so that a fresh nerve distal to the area of dissection transmits a stimulated impulse.
The facial nerve monitor can then be used during the course of dissection for the following purposes:
-
To identify the facial nerve
-
To map its contour
-
To identify potentially injurious actions during surgery
-
To obtain prognostic information about postoperative facial nerve function
Identification of the facial nerve
The facial nerve can be identified on the basis of spontaneous activation of the facial nerve monitor due to mechanical stimuli (eg, traction, compression, abrasion). Generally speaking, such mechanical stimuli occur when the surgeon deliberately manipulates the nerve or tissues around the nerve or touches some portion of the nerve with a dissecting tool.
Surgeons must recognize that the facial monitor cannot be relied upon to invariably identify the nerve prior to serious injury. The information provided by facial nerve monitoring must be used to help interpret information gained by the operating surgeon during the ongoing course of the dissection. Severely injuring or transecting the facial nerve with no warning is possible. Such silent transections have been reported.
The second method by which the facial nerve can be identified is through the use of the stimulating tip or probe. Most stimulating probes now have a tip that follows Kartush's design recommendations; ie, the tip is insulated flush to the flat stimulating surface of the probe. This minimizes current shunting.
Low-impedance materials, such as spinal fluid, blood, and irrigation fluid, can divert current from the stimulating tip of the probe. The operating surgeon must always keep this caveat in mind. When possible, fluid should be aspirated away from the site of stimulation so that the tip can encounter the nerve in a relatively dry field. This minimizes false-negative responses.
Facial nerve mapping
Once the nerve is identified, the facial nerve monitor can be used to map its course. [15] Some surgeons prefer to use a bipolar tip for this purpose in areas where important structures are small and in close proximity. The bipolar tip provides much more precise information about the exact anatomic location of the edge of the nerve; however, it is prone to false-negative results. As a general rule, using the stimulating probe to confirm a visual identification of the facial nerve is best. The use of the facial nerve monitor to map the facial nerve in the absence of the nerve's visual identification is unreliable and fraught with hazard.
Ashram et al pointed out that stimulation of the nervus intermedius can result in a long-latency, low-altitude response recorded only in the orbicularis oris channel. [16] The nervus intermedius lies in an entirely different position from the main trunk of the facial nerve. The operating surgeon must be aware of this potential pitfall in intraoperative facial nerve monitoring and should be aware of whether both the orbicularis oris and the orbicularis oculi channels are activated with intraoperative stimulation.
Identification of injury risk
The monitor can be used to determine that certain types of surgical manipulations are potentially injurious to the nerve. Tugging, torsion of the nerve, or scraping tumor from the nerve may result in facial nerve stimulations, which, in turn, can indicate to the surgeon that he or she is at risk of causing facial nerve injury. The surgeon can then adjust his or her technique accordingly. Its ability to alert surgeons to potential injury may be the principal reason why the facial nerve monitor produces improved outcomes in posterior fossa surgery.
Prognostic assessment
At the end of the surgical procedure, the facial nerve monitor can be used to assess the likelihood of postoperative facial nerve weakness. Two principle variables have been used to make this determination: (1) determination of the size of the stimulated compound action potential and (2) the nerve's stimulus threshold near the brainstem.
Compound action potential
The size of the compound action potential is the less frequently used variable. Its magnitude depends critically on electrode placement. Consequently, consistent electrode placement is required if the sizes of the compound action potentials are to be compared between individuals. Such constancy of electrode placement is difficult to maintain.
Various authors have indicated that a compound action potential at the end of the procedure that exceeds 500-800 microvolts indicates a high likelihood of a House-Brackmann (HB) I or II facial nerve outcome. The incidence of permanent facial weakness increases as the amplitude of the compound action potential drops below 500 microvolts.
The maximum compound facial nerve amplitude at the end of the procedure depends somewhat on stimulus intensity. For lower stimulus intensities, the amplitude of the compound action potential varies as stimulus intensity increases. Sobottka demonstrated that this effect plateaus with a stimulus intensity of 0.4mA. [17] Consequently, if postdissection amplitude is used as a prognostic indicator, the stimulus intensity should be set to at least 0.4mA. Using this technique, Sobottka showed that 15 of 16 individuals whose compound electromyographic amplitude was more than 800 microvolts in response to proximal stimulation of the nerve had HB nerve outcomes of I or II. [17]
Stimulus threshold
The more commonly used method of prognosticating postoperative facial nerve outcome is to determine the threshold stimulus intensity required to generate a detectable response. Articles in the literature have identified several different breakpoints. As Prass pointed out, some of the variability may be due to the use of different stimulus durations.
Sobottka demonstrated that 16 of 16 individuals who required more than 0.3mA to stimulate the proximal facial nerve (around the brainstem) had HB postoperative facial nerve outcomes of III-V. [17] Conversely, when the stimulus intensity required to produce a response was less than 0.3mA, 19 of 22 individuals had HB I or II outcomes.
Presade showed that mean stimulus thresholds at the brainstem of 0.1mA correlate with HB I and II and that 0.725mA is the typical stimulus intensity for HBs III-VI.
Selesnick showed the following correlations at 1-year postoperative follow-up for a large number of patients who had cerebellopontine angle surgery with HB I or II facial nerve outcomes:
-
Stimulus intensity of 0.1mA - 90%
-
Stimulus intensity of 0.2mA - 58%
-
Stimulus intensity of 0.3mA - 41%
Selesnick evaluated the data in several different ways and determined that the statistically important breakpoint is 0.2mA for a P value of 0.01. In the study, a 0.2mA threshold had a P value of 0.01.
Nonetheless, recognizing that almost half of the subjects with poor intraoperative electrophysiologic results had good long-term outcomes is important. Consequently, although good electrophysiologic data are very helpful in reassuring patients postoperatively, poor electrophysiologic data do not appear to be reliable enough for use in planning postoperative facial nerve reanimation procedures. In other words, a stimulus threshold of 0.3mA at the end of the procedure is not sufficiently reliable to allow a direct move to facial nerve reanimation procedures within the first several postoperative months.
Bozord and Grayeli demonstrated that facial nerve outcome can be more reliably prognosticated with simulation intensities of less than 0.05mA. [18] At day 8, HB I/VI or II/VI function was observed in 93% of patients with stimulus thresholds of 0.01-0.04mA but in only 79% of patients with stimulus intensities of greater than 0.3mA.
Neff et al demonstrated that combining stimulus intensity with the amplitude of the induced response is a better predictor of HB grade at 1 year than is either function alone. [19]
Electromyographic activity during emergence from anesthesia
Prass discussed his preference for leaving the facial nerve monitor in position in patients emerging from anesthesia. [5, 8] The idea behind this is that if voluntary facial nerve electromyographic activity can be identified during emergence, the prognosis for a good long-term facial nerve outcome is excellent.
-
The surgical anatomy and landmarks of the facial nerve.