Overview
Background
Hearing loss is one of the most common sensory impairments. An estimated 14.3% of Americans aged 12 years or older (about 38.2 million people) have bilateral hearing loss. [1] According to the US Centers for Disease Control and Prevention (CDC), of babies screened in the United States in 2019, 1.7 per 1000 had hearing impairment. [2] Depending on the degree of hearing loss, many affected individuals can be successfully fitted with hearing aids. For patients with hearing loss that is not mitigated by hearing aids, a cochlear implant may provide an opportunity for hearing. [3, 4, 5]
The cochlear implant is a surgically placed device that converts sound to an electrical signal. This electrical signal is transmitted via electrodes to the spiral ganglion cells in the cochlear modiolus. About 736,900 registered cochlear implant devices had, as of December 2019, been placed in patients worldwide. [6] However, many candidates for cochlear implants do not have access to this procedure, due to failure to recognize appropriate candidates or because of inadequate healthcare resources.
Although individual responses to cochlear implants are highly variable and depend on a number of physical and psychosocial factors, the trend toward improved performance with increasingly sophisticated electrodes and programming strategies has dramatically expanded indications for cochlear implantation.
An image depicting labyrinthitis ossificans can be seen below.
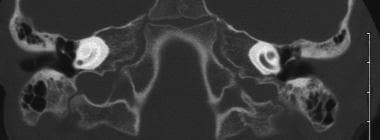 Labyrinthitis ossificans. Cochlea on the left is obliterated by bone after meningitis. Scala tympani of the cochlea on the right was patent, and the patient underwent successful implantation with complete electrode insertion.
Labyrinthitis ossificans. Cochlea on the left is obliterated by bone after meningitis. Scala tympani of the cochlea on the right was patent, and the patient underwent successful implantation with complete electrode insertion.
History
Throughout the 1970s, the US Food and Drug Administration (FDA) recommended that devices be implanted only in adults with profound hearing loss. In 1986, the FDA allowed children at least 2 years of age to be implanted, but the age limit has since been lowered to 9 months. [7] Over time, indications have been broadened to include adults with severe hearing loss who may also achieve some benefit from conventional amplification.
Preoperative Considerations
Cochlear implantation is a collaborative effort involving patients, families, schools, audiologists, speech/hearing therapists, and surgeons. A patient with hearing impairment does not simply have a surgical problem that responds only to the intervention of an implant surgeon. Because preoperative expectations affect the patient's postoperative satisfaction and use of the implant, all patients and families require attention and counseling from an implant team before they embark on the life-changing journey of cochlear implantation.
Pure-tone audiometry
The human ear is capable of hearing frequencies from 20-20,000 Hz. Pure-tone audiometry is used to assess a subject's response to a frequency at a specific intensity measured in decibels. In most cases, frequencies from 250 Hz to 8000 Hz are assessed, as these are most important for speech perception.
Speech audiometry
Although a number of speech-recognition tests are currently used for different reasons, one of the most common such tests is the hearing in noise test (HINT), which assesses speech recognition in the context of sentences. In determining cochlear implant candidacy, the HINT is performed without background noise, despite its name. The HINT measures word-recognition abilities to evaluate the patient's candidacy for cochlear implantation, in conjunction with conventional pure-tone and speech audiometry. The HINT consists of 25 equivalent 10-sentence lists, which may be presented in quiet or noise to assess the patient's understanding of sentences.
As noted earlier, when used to assist in the determination of cochlear implant candidacy, the HINT is currently performed in quiet. Administer the first test in quiet by using two lists of 10 sentences, which are scored for the number of words correctly identified.
Criteria
For adults and children who can respond reliably, standard pure-tone and speech audiometry tests are used to screen likely candidates. For children aged 12-23 months, the pure-tone average (PTA) for both ears should equal or exceed 90 dB. For individuals older than 24 months, the PTA for both ears should equal or exceed 70 dB. If the patient can detect speech with best-fit hearing aids in place, a speech-recognition test in a sound field of 55-dB HL sound pressure level (SPL) is performed. A number of speech-recognition tests are currently in use.
Labeling approved by the FDA with regard to speech-recognition indications varies by cochlear implant device. For Advanced Bionics’ HR90K Ultra 3D device, for example, the indication is open-set sentence recognition of 50% or less, as measured using HINT sentences. For Cochlear Limited’s Nucleus Profile and Nucleus Profile Plus devices, the indications are 50% or less in the ear receiving the implant or at most 60% in the best-aided condition, “on recorded tests of open-set sentence recognition.” [8]
Many centers have replaced HINT sentences with AzBio sentences, based on the findings of Gifford (2008). Gifford compared HINT sentences, AzBio sentences, and consonant-nucleus-consonant (CNC) words in hearing-impaired patients with and without cochlear implants. Unlike AzBio sentences, HINT sentences were found to have a poor correlation with CNC words and had a significant ceiling effect. [9]
Other tests
In children with prelingual deafness, cochlear implant candidacy is established when auditory skills fail to develop after amplification and aural rehab over a 3-month period. Progress is typically monitored with the Meaningful Auditory Integration Scale or the Early Speech Perception test. Other scales of auditory benefit may be used in older children.
A study by Verrecchia et al found that in infants and young children who were candidates for cochlear implants, vestibular evaluation could be successfully carried out with a test battery consisting of the head impulse test (HIT), the video head impulse test (vHIT), cervical vestibular-evoked myogenic potentials (cVEMP), and the mini–ice-water caloric test (mIWC), as adapted from previous test methods. Regarding valid responses, the investigators reported that substantial test agreement existed between HIT and vHIT, with moderate agreement determined to exist between vHIT/HIT and mIWC and no apparent agreement found between the canal tests and cVEMP. Resolution of test disagreement and improvement of protocol reliability were addressed with guidelines stating that confirmation of a pathologic response was required in at least two different canal tests and in at least three cVEMP trials and that canal/otolith disagreement pointed to a partial vestibular loss, as opposed to complete vestibular insufficiency. [10]
Imaging
Imaging with computed tomography (CT) scanning or magnetic resonance imaging (MRI) is performed prior to implantation to evaluate the inner ear, facial nerve, cochleovestibular nerve, brain, and brainstem. MRI may reveal hypoplasia or aplasia of the cochleovestibular nerve, whereas CT scanning may show a narrow internal auditory canal or absence of the bony cochlear nerve canal at the modiolus. Inner ear malformations ranging from rare cases of cochlear aplasia to the more common, enlarged vestibular aqueduct are easily visualized on CT or MRI scans. Such results may alter the choice of side of implantation or raise other issues such as electrode selection.
Patients with cochlear malformations are still candidates for cochlear implantation, but they may require a different type of electrode and a different surgical approach (ie, drill out), and they may be more at risk for meningitis or cerebrospinal fluid gusher. Surgeons should be prepared for a cerebrospinal fluid leak caused by incomplete partition between the cochlea and the internal auditory canal. Absence of the cochlea or the cochlear nerve can usually be confirmed with imaging and is an absolute contraindication for cochlear implantation.
Clinicians must take extra caution to identify the cochleovestibular nerve complex; auditory stimulation with a cochlear implant in patients with supposed cochlear nerve aplasia has been reported. These cases may be more appropriately labeled cochlear nerve hypoplasia. Families and patients must be adequately counseled and fully informed about the variable performance of patients with dysplastic cochleae and the potential risk of cerebrospinal fluid leakage and meningitis.
In pediatric or young adult patients with progressive hearing loss, exclude neurofibromatosis II by performing MRI before proceeding with implantation. MRI can visualize the inner ear, cochleovestibular nerve, brain, and brainstem. Also, unlike CT scanning, this modality is very useful in identifying early labyrinthitis ossificans, which typically begins with endoluminal fibrosis of the scala tympani at the basal turn.
Preoperative counseling and education
In addition to the purely audiologic criteria discussed above, pediatric candidates must be enrolled in an educational program that supports listening and speaking with aided hearing. For patients of all ages, no medical contraindications (eg, cochlear or auditory nerve aplasia, active middle-ear infection) may be present. Communication among patients, families, schools, audiologists, therapists, and surgeons is required.
Immunization
A study published in 2003 reported that pediatric and adult patients with cochlear implants are at increased risk for acquiring Streptococcus pneumoniae meningitis. [11]
Pneumococcal vaccination in children
According to the CDC, children under age 2 years who are receiving cochlear implants should undergo vaccination with 13-valent pneumococcal conjugate vaccine (PCV13), as specified by the childhood immunization schedule. In addition, vaccination with PCV13 may be needed in older children who are receiving cochlear implants if they were not previously immunized as recommended. Children aged at least 2 years should also undergo vaccination with 23-valent pneumococcal polysaccharide vaccine (PPSV23). [12]
All recommended pneumococcal vaccine shots should be administered 2 weeks or more prior to cochlear implant surgery. No extra presurgical pneumococcal shots are required by children whose vaccinations are current. [12]
Pneumococcal vaccination in adults with cochlear implants
According to the CDC, in adults who are receiving cochlear implants but who have never undergone pneumococcal vaccination, one shot of PCV15 or PCV20 should be administered. Those who receive PCV15 should subsequently receive one shot of PPSV23. All recommended shots should be administered 2 weeks or more prior to cochlear implant surgery. No extra presurgical pneumococcal shots are required by adults whose vaccinations are current. [12]
Etiologies of Severe to Profound Hearing Loss
Genetic
Genetic hearing loss is the most common etiology of childhood deafness (33-50%), and many of these cases can be attributed to single gene mutations. Seventy five to eighty percent of genetic deafness is secondary to autosomal recessive gene defects, and 18-20% is secondary to autosomal dominant gene defects, with the remainder being secondary to X-linked gene defects. Another potential cause of genetic deafness is mitochondrial gene defects. Genetic hearing loss is generally divided into nonsyndromic and syndromic, with the former being twice as common as the latter.
As of 2021, over 130 genes for nonsyndromic hearing loss had been identified, including 51 autosomal dominant genes, 78 autosomal recessive genes, and five X-linked genes. [13] The GJB2 gene encodes for the protein connexin 26, and mutations in GJB2 account for the most common cause of nonsyndromic autosomal recessive deafness, which is thought to be responsible for 20% of hereditary hearing loss in children. Connexins are transmembrane proteins that form gap-junction channels. These channels allow ion transport and cell-to-cell communication. Syndromic hearing loss conditions include but are not limited to Waardenburg, Stickler, brachio-oto-renal, Treacher Collins, neurofibromatosis, Usher, Jervell Lange-Neilsen, Alport, and Pendred syndromes.
Infectious
See the list below:
-
Nonmeningitic
Nongenetic or environmental causes generate 25-33% of childhood deafness. Congenital cytomegalovirus (CMV) is the most common environmental cause of hearing impairment in children. [14]
Ten to fifteen percent of patients with asymptomatic congenital CMV infections develop mild to profound sensorineural hearing loss. Other infectious conditions that can lead to hearing loss include rubella, syphilis, toxoplasmosis, mumps, and measles.
-
Meningitic
Meningitis causes approximately 9% of childhood deafness and can make implantation difficult. Organisms commonly causing meningitis (from most to least common) include Haemophilus influenzae, S pneumoniae, and Neisseria meningitidis.
The organism causing the highest incidence of hearing loss is S pneumoniae (31%). Patients with meningitis are predisposed to developing obstruction of the cochlear lumen (ie, labyrinthitis ossificans; see the image below), especially when S pneumoniae is the causative organism.
Although 9 months is the current age limit the FDA has established for implantation, various factors may cause the implant team to treat younger infants. In particular, a child with deafness due to meningitis may develop labyrinthitis ossificans, which can fill the cochlear duct or the entire cochlear labyrinth and usually the scala tympani starting near the round window with bone. In cases of labyrinthitis ossificans, special techniques may be needed for successful cochlear implantation. However, even with such techniques, this condition often leads to a suboptimal outcome.
The image above depicts a CT scan of a child with deafness due to meningitis whose left cochlea has ossified. In this patient, successful implantation of the patent right cochlea was accomplished. For patients at risk of labyrinthitis ossificans, implantation may be indicated soon after early ossification or fibrosis is diagnosed. Early implantation in the setting of labyrinthitis ossificans may allow a full electrode insertion and obviate the need for performing a cochlear drill-out procedure.
Using serial imaging, implant teams may monitor patients with new deafness due to meningitis and perform implantation at the first sign of replacement of the scala tympani with fibrous tissue or bone. Several reports have described spontaneous hearing improvement after meningitis in patients with residual hearing. In patients with profound hearing loss after meningitis, however, the chance of hearing improvement is unlikely, and cochlear implantation should proceed as soon as possible. Ossification can begin as early as 2 weeks after meningitis. In the setting of bilateral labyrinthitis ossificans, expeditious placement of bilateral implants should be considered, as these patients are not likely to benefit from future technology.
Ototoxicity
Numerous medications can cause hearing loss. Perhaps the most well known ototoxic medications are the aminoglycoside antibiotics. Other commonly cited ototoxic medications include loop diuretics, erythromycin, salicylates, vancomycin, cisplatin, and quinine.
Trauma
Hearing loss from temporal bone trauma is most commonly conductive secondary to hemotympanum, tympanic membrane perforation, or ossicular discontinuity. The otic capsule is fairly resistant to trauma, but fractures may occasionally involve the cochlea or labyrinth. Injuries to the otic capsule almost always result in profound sensorineural hearing loss. Bilateral otic capsule fractures are very uncommon but would be an indication for cochlear implantation. Intraluminal fibrosis or ossification may occur in the setting of otic capsule fractures, which can make electrode insertion more difficult. Preoperative imaging may provide additional information on fibrosis or ossification.
Hyperbilirubinemia
In the setting of neonatal jaundice, bilirubin may cross the blood-brain barrier. Bilirubin can deposit in the ventral cochlear nucleus and cause sensorineural hearing loss. Transient loss of wave IV and V on an auditory brainstem response (ABR) is seen in 33% of neonates with bilirubin levels of 15-25 mg/dL. Hyperbilirubinemia is also a risk factor for auditory neuropathy.
Auditory neuropathy/dyssynchrony
Patients with auditory neuropathy are characterized by intact outer hair cell function with abnormal or absent ABR, implicating the vestibulocochlear nerve as the site of this condition's pathology. Outer hair cell function can be assessed with otoacoustic emissions, the cochlear microphonic, or pure-tone audiometry. A defect in inner hair cells, spiral ganglion cells, or the synapse between the two is the cause of auditory neuropathy.
Auditory neuropathy occurs in adults and children. Pure-tone audiometry may reveal normal to profound hearing loss, but poor performance on speech discrimination testing is the common denominator in patients with auditory neuropathy. Anoxia, hyperbilirubinemia, and prematurity are potential risk factors for auditory neuropathy. Several reports have described hereditary forms of auditory neuropathy, including a nonsyndromic autosomal dominant condition that progresses to outer hair cell involvement, with eventual decline of pure-tone hearing. Some patients may benefit from FM devices or hearing aids, but in some cases a cochlear implant may provide the only means to communicate.
Ménière disease
Ménière disease is characterized by room-spinning vertigo, fluctuating hearing, tinnitus, or aural fullness. The diagnosis is made with a thorough history and physical examination, as well as imaging studies to rule out retrocochlear pathology. Histologic examination of postmortem temporal bones in subjects with Ménière disease has revealed dilation of the endolymph compartment. Histologic endolymphatic hydrops may be the end result of some other pathologic process that is causing the symptoms of Ménière disease or may be the actual cause of the symptoms.
A study showed that 11% of patients with Ménière disease presented with bilateral disease and that another 12% eventually developed symptoms in their unaffected ear. [15] The progression of hearing loss is difficult to predict but may result in bilateral profound loss. A series of 9 patients with bilateral Ménière disease who underwent cochlear implant surgery showed overall excellent performance results with at least 1 year of follow-up. Some patients in this series experienced fluctuations in their implant performance associated with episodic vertigo attacks.
Noise-induced hearing loss
A CDC study using data from the 2011-2012 National Health and Nutrition Examination Survey (NHANES) estimated that 39.4 million adults in the United States aged 20-69 years have unilateral or bilateral noise-induced hearing loss. [16] Hearing loss secondary to noise exposure can be temporary (temporary threshold shift) or permanent (permanent threshold shift). Acoustic trauma is the immediate, permanent onset of hearing loss after exposure to sounds louder than 140 dB, such as explosions or firearm discharge.
Outer hair cells are the most susceptible to noise exposure. The outer hair cells typically swell with excessive noise exposure but may normalize if the noise exposure ceases. A notch at 4000 Hz on pure-tone audiometry is fairly common in the setting of noise-induced hearing loss, but hearing loss may progress to a level where even amplification provides little benefit.
Presbycusis
Hearing loss associated with aging initially begins with loss of high frequencies, with eventual progression to include lower-frequency loss. Thirty to thirty-five percent of 65 year olds have some hearing loss; this figure rises to 40-50% in individuals over 70 years. Men are more commonly affected with age-related hearing loss. Hearing aids often provide meaningful benefit for patients with presbycusis, but only one in five individuals who would benefit from a hearing aid actually wear one. Patients with severe to profound hearing loss who are unable to hear with traditional amplification may benefit from a cochlear implant. In cases where residual low-frequency hearing is found, some individuals may benefit from a combined hearing aid /cochlear implant that provides both electrical and acoustic stimulation.
Bilateral Implants
Patients with unaided unilateral hearing loss often have difficulty in everyday listening situations. Significant disadvantages to unilateral hearing loss are well known and include the head shadow effect, difficulty with hearing in noise, and sound localization. Amplification for unilateral hearing loss is routinely recommended in children and adults.
A number of arguments have been made against bilateral implantation, including saving the ear for future technology or using a hearing aid with residual hearing. However, a multitude of studies have demonstrated significant performance improvement in patients with bilateral cochlear implants. Moreover, no one knows when future technologies (eg, gene therapy) will become available for clinical use, and a critical time period for auditory development remains. Indeed, a study showed that a second critical time period with regard to bilateral implantation may exist. Significant delay between the first and second implant may significantly decrease future benefit. [17] In 2008, a formal position statement supporting bilateral cochlear implants as accepted medical practice was issued by the William House Cochlear Implant Society. This position statement was released after pertinent literature regarding bilateral cochlear implants was reviewed. [18, 19]
A study by Anderson et al indicated that the ability to localize sounds in persons with bilateral cochlear implants is most predicted by the age at which deafness occurs and by the age of testing. The investigators suggested that these factors have a significant impact because the development of accurate localization abilities requires experience with binaural hearing, while temporal resolution worsens with aging. The results also implied that in patients with earlier onset of deafness (age 5 years or before), localization errors were particularly likely in association with medial target angles. [20]
Outcomes
Critical outcome factors for pediatric patients receiving implants include (1) age at onset of deafness and duration of deafness prior to implantation, [21] (2) progression of hearing loss, and (3) educational setting.
In general, early implantation facilitates rapid development of oral communication ability. Progressive hearing loss, which allows for the development of speech-reading skills, favors performance after implantation. Placement in a school setting that stresses oral versus signed communication is important for optimal implantation outcome. However, many variables remain unknown because approximately one half of the variance in performance after implantation cannot be predicted. [22]
Children should be receptive to wearing a hearing aid before cochlear implantation because all current implants require an external processor. A period of hearing aid use to ascertain development of aided communication ability is an important criterion in determining candidacy for young children.
After audiologic criteria have been met, parental expectations and attitudes must be assessed and addressed. Unrealistic expectations can frustrate the efforts of the child and the implant team. Families must be appropriately counseled about the need for long-term therapy, variable outcome of implantation, and the limitations of implantation.
With regard to revision cochlear implantation, a study by Dillon et al indicated that this procedure should not be contraindicated by older age. The study, of 14 adults under age 65 years and 15 patients aged 65 years or older, all of whom underwent revision cochlear implantation, found improvements in speech perception performance tests in both groups at 3 and 6 months post revision, compared with prerevision results, with no relationship found between age and performance scores. [23, 24]
Complications
A literature review by Terry et al found that severe complications following cochlear implant surgery are rare but nonetheless possible, even years after the operation. With follow-up ranging from 1 month to 17 years, the investigators determined that the rate of delayed complications was 5.7%. Vestibular complications were the most common (3.9%), with device failure (3.4%) and taste problems (2.8%) being the next most frequent. The report suggested that patients with cochlear implants receive lifetime follow-up. [25, 26]
According to a retrospective case-control study by Hatch et al of patients who underwent cochlear implantation, obesity increases the likelihood of perioperative and postoperative complications, with the odds ratios being 6.21 and 3.97, respectively, in comparison with nonobese patients. The investigators, who evaluated primary, single-sided implantation in adults, also found an association between obesity and longer total and surgical operating room times. [27]
A study by Patel et al indicated that in children who undergo cochlear implantation, the likelihood of postoperative overnight hospital admission is approximately twice as likely in patients with asthma or structural central nervous system abnormalities. The report evaluated 2012-2015 data from the American College of Surgeons National Surgical Quality Improvement Program-Pediatric. [28]
Tinnitus
Tinnitus is an abnormal perception of sound that currently affects approximately 10% of the population. A subset of patients will pursue active treatment for their tinnitus in cases in which it has significantly reduced their quality of life. Tinnitus retraining therapy is one of the few proven treatment options available to patients experiencing severe tinnitus. The expense, duration of therapy, and limited number of programs offering tinnitus retraining therapy leave a significant number of patients with severe tinnitus without a viable treatment option.
Several studies have revealed that adult cochlear implant recipients with preoperative tinnitus have noticed either reduction or abolition of their tinnitus while wearing their cochlear implant. [29, 30] One report showed a significant reduction or cessation of tinnitus in 20 out of 21 patients with single-sided deafness who were implanted for severe tinnitus. Three of the patients in this series displayed permanent residual inhibition (ie, no evidence of tinnitus even while not wearing their processor). To date, cochlear implantation appears to be one of the more successful treatment options for severe tinnitus. [29]
Unilateral deafness
Patients with unilateral deafness are unable to localize sound, have difficulty hearing in the presence of background noise, and must expend increased listening effort. Rehabilitative strategies for unilateral deafness have utilized the intact ear via contralateral routing of sound using a CROS (contralateral routing of signals) hearing aid or a bone-anchored hearing aid. The aforementioned therapies have provided significant benefit for a number of patients with unilateral deafness with respect to the head shadow effect and in select situations of background noise. Sound localization and hearing in significant background noise requires normal to near-normal binaural hearing. [31]
Several studies examined the outcomes of unilaterally deaf patients who underwent a cochlear implant for the primary indication of severe tinnitus. These patients did not have any evidence of improvement in summation effect but did have improved speech recognition with respect to the head shadow effect. Sound localization in patients with normal hearing in the intact ear showed improvement when assessed indirectly via a questionnaire. [30]
Based on the limited evidence previously available, cochlear implantation in patients with unilateral deafness without tinnitus was not considered to be indicated. However, research outcomes showing the benefit of cochlear implants in a wider range of hearing loss led to a 2019 change in FDA policy, making asymmetrical hearing loss in persons aged 5 years or older, including deafness in one ear and normal hearing in the other, an indication for cochlear implantation. [32, 33]
Residual hearing
A study by Kim et al indicated that even in the presence of significant preoperative residual hearing (as evidenced by Categories of Auditory Perception [CAP] scores of 5 or 6), hearing-impaired children who undergo cochlear implantation can experience substantial pronunciation improvement. This was evidenced, at 6 months post surgery, by significant improvement in the Urimal Test of Articulation and Phonation (U-TAP) scores. Infant-Toddler Meaningful Auditory Integration Scale (IT-MAIS) and word perception scores also substantially improved over this period. Pronunciation continued to improve past the 6-month point to 1-year follow-up, with the degree of improvement surpassing that of other speech parameters. [34]
Hybrid cochlear implants
The first hybrid cochlear implant system was approved by the FDA in March 2014 for patients aged at least 18 years. A hybrid implant has a short implant electrode array that reduces the risk of losing residual low frequency hearing in patients with profound high-frequency loss. A small cochleostomy is created, and the short electrode array is slowly inserted into the inferior basal turn of the cochlea. The use of a short electrode array reduces the risk of intracochlear trauma, which increases the likelihood of preserving residual hearing. The preserved low-frequency hearing can then be amplified with a hearing aid built into the speech processor.
This approach allows for the auditory rehabilitation of patients who are not candidates for conventional implants because their low-frequency hearing exceeds current guidelines. Hearing preservation after implantation of the hybrid device often results in improved melody and instrument recognition, better frequency resolution, and improved hearing in noise. [35]
-
Cochlear implant electrode passing through the facial recess to the scala tympani.
-
Cochlear malformations. Neural foramen on the right is absent. Right arrow indicates a rudimentary vestibule. On the left is a severe cochlear malformation (large arrow). Small arrow indicates the internal auditory canal.
-
Labyrinthitis ossificans. Cochlea on the left is obliterated by bone after meningitis. Scala tympani of the cochlea on the right was patent, and the patient underwent successful implantation with complete electrode insertion.
-
This axial FIESTA image of the basal turn of the cochlea demonstrates loss of T2 signal in the scala tympani. This patient has a history of pneumococcal meningitis with profound hearing loss.






