History of the Procedure
In 1954, Smith reported the first anterior craniofacial resection in a patient presenting with a tumor arising in the frontal sinus. [1] Ketcham et al subsequently reported the first series of patients treated with an anterior craniofacial resection for tumors arising in the ethmoid sinuses. [2] This seminal report included the indications, morbidity, and outcome of the procedure and included a systematic description of the surgical technique.
The oncologic principles of anterior craniofacial resection as described by Ketcham involves an en bloc resection of the tumor, including the ethmoid sinuses, superior nasal septum, and floor of the anterior cranial fossa, corresponding to the interorbital area (ie, anterior craniofacial resection) or extended laterally to include part of the bony orbit or its soft tissue contents (ie, anterolateral craniofacial resection). [3]
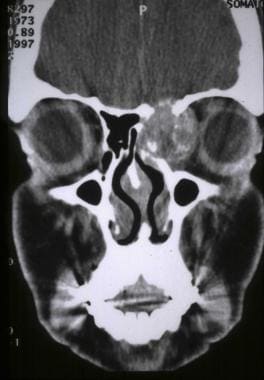 CT scan coronal view demonstrating an osteosarcoma of the ethmoid sinuses extending to the orbit and anterior cranial fossa.
CT scan coronal view demonstrating an osteosarcoma of the ethmoid sinuses extending to the orbit and anterior cranial fossa.
Subsequent reports included larger series of patients and multiple modifications of Ketcham's original surgical technique. These latter reports incorporated technical advances brought to craniofacial surgery by Tessier, such as the use of the subfrontal technique adopted for oncologic surgery by Derome. [4] These modifications improved the visualization of the tumor, facilitating its total removal, and decreased, although did not eliminate, the morbidity related to brain retraction. Recently, endoscopic and endoscopic-assisted techniques have been adopted to extirpate selected tumors that traditionally have been resected using a subfrontal approach.
As opposed to a craniofacial resection, the endoscopic resection does not involve an en bloc resection. However, it is still founded on a complete resection of the tumor, preferably corroborated by intraoperative frozen section analysis. In well-selected patients, endoscopic endonasal resection, however, should yield oncologic outcomes that are equivalent to those of traditional subfrontal approaches. [5]
Epidemiology
Frequency
From the head and neck surgeon's standpoint, the anterior skull base is affected most commonly by tumors arising in the paranasal sinuses; the neurosurgical experience will be biased toward tumors that arise from the anterior cranial fossa. In either case, these tumors may be resected using a completely endoscopic or an endoscopic-assisted approach according to their extension. Tumors that extend lateral to the meridian of the orbit, invade the soft issues of the orbit, the maxillary sinus, the anterior wall of the frontal sinus, or the facial skin require an open approach. Other tumors, such as those arising in the skin or orbits, may also require an anterior craniofacial resection via a subfrontal approach.
Malignant tumors of the sinonasal tract comprise approximately 3% of the malignancies that arise in the upper aerodigestive tract. Overall, sinonasal tumors are most commonly diagnosed in white males (the male-to-female ratio is 2:1) during the fifth to the seventh decades of life. Approximately 10% of tumors that arise in the sinonasal tract originate in the ethmoid and/or frontal sinuses and are likely to involve the anterior cranial base.
Most series of patients with malignancies arising in the ethmoid sinuses demonstrate a preponderance of squamous cell carcinoma (SCCA). However, in the authors' experience, the histopathology of the ethmoid sinuses is equally divided between SCCA and other malignancies, such as adenoid-type carcinomas, sarcomas, and melanomas. This may be a reflection of the referral base, as European series demonstrate a preponderance of intestinal-type adenocarcinomas. Malignancies of the frontal sinuses, which are extremely rare, comprise an equal number of SCCA and adenoid cystic carcinoma. The sellar and parasellar areas are affected by tumors such as chordomas, chondrosarcomas, invasive pituitary adenomas, and meningiomas that, as previously stated, also may require a subfrontal approach.
Etiology
Several reports have associated malignant tumors of the paranasal sinuses with exposure to industrial fumes, mining industry pollutants, and wood dust. Specifically, individuals who work with nickel demonstrate an incidence of SCCA and anaplastic carcinoma that is 250 times higher than in the general population. These tumors have a latent period of 18-36 years.
Woodworkers exposed to hardwood dust show an increased incidence of adenocarcinoma of the ethmoid sinuses, while woodworkers exposed to softwood dust demonstrate an increased incidence of SCCA, anaplastic carcinoma, and adenocarcinoma. Workers in the leather tanning industry also demonstrate an increased incidence of epithelial malignancies of the sinonasal tract. Other industrial exposures with an increased incidence of sinonasal cancer include mineral oils, chromium and chromium compounds, isopropyl oils, lacquer, paint, soldering and welding materials, and radium dye paint.
Tobacco smoking, which is a recognized pathogenetic factor for SCCA of the upper aerodigestive tract, does not appear to play a significant role in the genesis of these tumors. However, some have suggested the possibility of an additive effect between softwood dust and tobacco smoking.
Presentation
In general, the most common symptoms and signs produced by tumors arising in the sinonasal tract are similar to the symptoms produced by inflammatory disease (ie, chronic sinusitis). Nasal obstruction, anosmia, facial pressure or pain, epistaxis, and nasal discharge are the most common complaints. Thus, the diagnosis commonly is delayed as the patient seeks attention and/or receives treatment for sinusitis for an extended period. In addition, approximately 10% of patients with these tumors are asymptomatic. Even patients with advanced sinonasal tumors invading the anterior cranial fossa and frontal lobe may have subtle neurologic symptoms that may be easily overlooked.
A detailed physical examination should pay special attention to the sinonasal tract, orbits, and cranial nerves and include a nasal endoscopy. Sensory dysfunction of the first or second division of the trigeminal nerve is more common in patients presenting with malignant neoplasms of the paranasal sinuses than in patients with inflammatory disease of the paranasal sinuses, providing an important clue regarding the nature of the patient's problem.
Orbital findings, such as deficits of extraocular muscle movements, chemosis, or proptosis, should alert the physician to the possibility of a neoplasm. Similarly, loose dentition or a mass effect in the cheek, nose, forehead, palate, or gingivobuccal sulcus, which commonly presents as ill-fitting dentures, also should raise suspicion for presence of a sinonasal neoplasm. Although tumors of the paranasal sinuses are commonly advanced at the time of their diagnosis, they usually are confined to the local site and rarely present with cervical lymphadenopathy. Nonetheless, a detailed examination of the neck is mandatory.
Indications
A surgery that involves the resection of the skull base is usually performed with curative intent. A subfrontal approach is the preferred open technique to resect the anterior cranial base because it diminishes the retraction of the frontal lobes, thus decreasing the possibility of edema, contusion, or necrosis of the brain.
As previously mentioned, tumors that do not involve the skin, the soft tissues of the eye, or the anterior table of the frontal sinus and those that do not require an orbital exenteration or total maxillectomy and do not extend lateral to the optic nerves or the meridian of the orbit are amenable to an endoscopic resection.
Relevant Anatomy
Thorough understanding of the surgical anatomy of the nasal cavity, paranasal sinuses, orbits, and anterior cranial cavity is critical to predict the pattern of spread of tumors arising in the frontal and ethmoid sinuses and to enable their safe and complete removal. This article offers a brief summary of the anatomy of the sinonasal tract and skull base pertinent to the subject. For more information about the relevant anatomy, see Nasal Anatomy, Nasolacrimal System Anatomy, Paranasal Sinus Anatomy, Olfactory System Anatomy, Facial Bone Anatomy, Facial Nerve Anatomy, and Facial Nerve Embryology. The reader is also encouraged to review the cited references and anatomy textbooks for further details.
Embryology
The midfacial skeleton first appears at week 2.5 of embryonal development, when a mass of neural crest cells starts to migrate around the optic capsule and enter the first branchial arch to form the maxilla. A second mass of cells travels over the frontonasal process into the medial regions of the face to form the framework for the developing nose and paranasal sinuses. Once the bony framework is fully developed, aeration of the sinuses starts as invaginations arising from the middle meatus and sphenoid bone. Thus, the sinonasal tract develops in close association with structures derived from the first branchial arch, the orbits, and the anterior skull base. The ethmoid and maxillary sinuses are the first to develop, followed by the sphenoid sinus and, lastly, by the frontal sinus.
Nasal cavity
The nasal cavity can be visualized as a quadrangular corridor that is narrower at the top and divided into right and left compartments by a midline septum. It communicates with the exterior through an anterior opening, the nares, ie, nostrils. Posteriorly, it opens into the nasopharynx through the posterior choanae. Its external pyramidal shape reflects its skeletal support, which is composed of the paired nasal bones and the upper and lower lateral nasal cartilages as they surround the pyriform aperture.
The most anterior part of the nasal cavity, namely, the vestibule, is covered by skin that contains adnexal structures. Except for the most superior third, the rest of the nasal cavity is lined by pseudostratified ciliated epithelium (ie, respiratory epithelium). Olfactory epithelium (containing the sensory endings of the olfactory nerve) covers the nasal vault.
The walls of the nasal fossae include the nasal septum medially, the horizontal portion of the maxillary bone and palatine bone inferiorly, and the inferior turbinates and ethmoid bones laterally. Superiorly, the nasal fossae are confined by the cribriform plate and the rostrum of the sphenoid sinus as it slants posteroinferiorly toward the nasopharynx. The nasal septum is formed by the perpendicular plate of the ethmoid bone posterosuperiorly, the vomer posteroinferiorly, and the septal or quadrangular cartilage anteriorly. The suture formed by the junction of the perpendicular plate of the ethmoid bone and the vomer leads to the rostrum of the sphenoid sinus, thus providing a useful surgical landmark.
The cartilaginous septum (ie, anterior septum) is attached to the upper lateral cartilages and nasal bones along the dorsum of the nose. Thus, the posterior end of the nasal septum abuts the rostrum of the sphenoid sinus superiorly and has a free edge exposed to the nasopharynx inferiorly. Superiorly, the perpendicular plate of the ethmoid bone is continuous with the cribriform plate. Inferiorly, the nasal septum articulates with the maxillary crest in a tongue-in-groove fashion, stabilized by a dense layer of fibrous adhesions.
The lateral nasal wall separates the nasal fossae from most of the paranasal sinuses and contains their drainage ostia. It is formed superiorly and anteriorly by the nasal bones, the nasal process of the frontal bone, and the frontal process of the maxilla. Superiorly and posteriorly, it is formed by the ethmoid bone. Posteriorly, the wall is completed by the vertical process of the palatine bone and the medial plate of the pterygoid process. The sphenopalatine foramen, which is the passage for the corresponding neurovascular bundle and the posterior nasal artery, is formed by the most cephalic junction of these 2 bony areas. During surgery, the sphenopalatine foramen can be identified posteriorly in line with the posterior tip of the middle turbinate, just posterior to the back wall of the maxillary sinus and at a height level that is equivalent to that of the superior third of the back wall of the antrum.
The lateral wall supports 3-4 projecting scrolls of bone, ie, the conchae or turbinates. The inferior turbinate is an independent bone, whereas the middle and superior turbinates are part of the ethmoid bone. A thick mucous membrane containing a venous plexus with erectile properties covers the inferior and middle turbinates, while the superior and (infrequently found) supreme turbinates are covered by olfactory epithelium. The air spaces beneath them, the meati, are named according to the corresponding turbinate. The anterior third of the inferior meatus contains the ostium of the nasolacrimal duct. The anterior area of the middle meatus contains the frontonasal recess.
Inferior and posterior to the frontal recess, the surgeon can find the uncinate process, the ethmoid bulla, and the semilunar hiatus, which is located between these 2 structures. The nasofrontal recess receives the drainage of the frontal sinus, whereas the infundibulum receives the drainage of the anterior ethmoidal and maxillary sinuses. The superior meatus is short, and its mucous membrane (medial aspect) is mostly olfactory epithelium containing the sensory cells of the olfactory nerves. Posterior ethmoid cells usually drain into the superior meatus.
Sensory afferent fibers to the mucous membranes of the nasal cavities are supplied by the anterior ethmoidal and infratrochlear nerves coming from the nasociliary nerve (V1), the infraorbital nerve, the anterior superior alveolar nerve, pterygopalatine nerves (branches from V2), and the greater petrosal nerve from cranial nerve [CN] VII. Sympathetic innervation derives from branches traveling along the internal carotid artery through the deep petrosal nerve, then via the vidian nerve. Parasympathetic nerves travel in the nervus intermedius of CN VII, then in the greater petrosal nerve to reach the nose through branches of the pterygopalatine ganglion.
Blood to the posteroinferior nasal area is supplied by the internal maxillary artery and the anteroinferior area by branches of the facial artery, both branches from the external carotid artery, while the superior nasal area is supplied by the ethmoid arteries, which are terminal branches from the internal carotid artery. Venous drainage begins as plexuses in the turbinates that form veins that parallel the pathway of the arterial irrigation to then connect with the pterygoid venous plexus or the superior ophthalmic vein.
Lymph drains out of the nasal cavity running posteriorly from the vestibule toward the choanae, passing over the lateral nasal wall to a pretubal plexus, and then passes above and below the eustachian tube toward the posterior pharyngeal wall (ie, retropharyngeal nodes). These nodes drain into the deep jugular chain at the level of the carotid bifurcation. Small lymphatic branches may run upward from the lateral nasal wall toward the upper jugular lymph nodes. Lymphatics of the nasal vestibule run toward the submental and submandibular areas.
Ethmoid sinus
The ethmoid labyrinth consists of 3-18 cells per side that are connected in the midline by the cribriform plate. Each side is divided into an anterior and posterior group of cells based on the attachment of the middle turbinate to the lateral nasal wall (ie, basal lamella). Anterior cells drain into the anterior portion of the hiatus semilunaris. Posterior cells drain into the superior meatus. The lateral wall of the ethmoid sinuses is contiguous to the orbital cavity, and anterior ethmoid cells frequently communicate with the orbit through dehiscences in the lamina papyracea.
Furthermore, posterior ethmoid cells may "invade" the sphenoid sinus to be in close relation to, or even surround, the optic canal (ie, Onodi cells), which also may be dehiscent. Anterior, posterior, and, frequently, middle ethmoid neurovascular bundles pierce the medial orbital wall (ie, lamina papyracea) in their route to the ethmoid cells and then into the vertical lamina of the cribriform plate to reach the anterior cranial fossa. Their foramina can be identified close to the level of the frontoethmoidal suture, which therefore constitutes a reliable surgical landmark.
The anterior ethmoidal artery exits the orbit at a retrobulbar level and between the superior oblique and medial rectus muscles just posterior to the anterior wall of the bulla ethmoidalis. The posterior artery exits the orbit just anterior to the rostrum of the sphenoid. The lacrimal sac and duct and the soft tissues of the anterior and inferior area of the medial orbit may be in close relation to the most anterior ethmoid cells (ie, agger nasi cells).
The roof of the ethmoid sinuses is contiguous to the frontal sinus laterally and anteriorly, to the anterior cranial fossa laterally and posteriorly (ie, fovea ethmoidalis), and to the cribriform plate medially. Although variable, the bottom of the cribriform plate is located approximately just above the level of an imaginary horizontal line joining the medial canthi. Intranasally, it can be identified as the level of the highest attachment of the middle portion of the middle turbinate. Posteriorly, the ethmoid cells may extend into the floor of the middle cranial fossa and may extend lateral and/or superior to the sphenoid sinus.
Blood to the ethmoid sinuses is supplied by the posterolateral nasal artery (a branch of the sphenopalatine artery) and by the anterior and posterior ethmoidal arteries. Sensory innervation of the anterior and middle cells comes from the anterior ethmoidal nerve (V1). Posterior cells are supplied by branches of the pterygopalatine nerve (V2).
Frontal sinus
The frontal sinuses are paired cavities within the diploic frontal bone with frondlike pneumatization and asymmetric shape and size. Their anterior wall is contiguous with the soft tissue of the forehead. The posterior wall is shared with the anterior cranial fossa. The floor corresponds to the roof of the orbit and anterior ethmoid cells. The supraorbital neurovascular bundle supplies sensory and vascular supply and drainage to the frontal sinuses.
Maxillary sinus
The maxillary antrum is the largest of the paranasal sinuses. It is a pyramidal cavity with its apex extending into the body of the zygoma and its base at the lateral wall of the nose. The maxillary sinus walls are contiguous with other anatomic regions of the mid face, and the sinus shares its roof with the orbital cavity. This bony wall is pierced by the infraorbital canal, which communicates with the inferior orbital fissure. Its floor, which is formed by the alveolar process of the maxilla, lies 1-1.5 cm inferior to the floor of the nose and is in close relation with the first to third molars. The anterior wall is contiguous with the soft tissues of the mid face. The posterior and posterolateral walls of the maxillary sinus are contiguous with the pterygopalatine and infratemporal fossae, respectively. The medial wall of the antrum corresponds to the lateral wall of the nasal cavity and contains the drainage ostium at its highest aspect.
Arterial supply to the antrum comes from branches of the sphenopalatine artery, the terminal branch of the internal maxillary artery. Sensory innervation derives from the infraorbital and pterygopalatine nerves of V2. Venous and lymphatic drainage routes travel through the drainage ostium to join their nasal and ethmoidal counterparts at the lateral nasal wall as they travel toward the posterior choana.
Sphenoid sinus
The sphenoid sinus usually is divided into two asymmetric cavities by a bony intersinus septum. Its anterior wall bulges into the nasal cavity and contains the drainage ostium, which may be found at the sphenoethmoidal recess above and posterior to the middle turbinate. The anterior wall of the sphenoid sinus is approximately 9 cm from the anterior nasal spine. However, the overlap between the range of the distances between the nasal spine and its anterior and posterior walls is significant. Thus, distance is an unreliable anatomic reference.
Inferiorly, the sphenoid sinus is contiguous with the nasal and nasopharyngeal cavities. Posteriorly, it abuts the clivus, which separates it from the pons and the basilar artery. Superiorly, it borders the middle cranial fossa, the hypophysis, and the optic chiasm. The lateral wall abuts the cavernous sinus, the optic nerves, and CN V1, III, IV, and VI, which are en route to the superior orbital fissure. Dehiscences of the lateral wall may leave any of these structures exposed to the interior of the sinus. The posterior ethmoid arteries and branches from the sphenopalatine vessels provide arterial blood supply and venous drainage to the sphenoid sinus. Sensory innervation comes from the pterygopalatine ganglion.
Orbital cavity
The orbit shares three of its walls with the paranasal sinuses. Its medial wall corresponds to the lateral wall of the ethmoid sinus. It bears, from anterior to posterior, the nasolacrimal sac lying on the lacrimal bone (ie, lacrimal fossa), the anterior and posterior ethmoidal arteries, the trochlea, and the optic nerve with its foramen at the apex of the orbital cavity. Its inferior wall corresponds to the roof of the maxillary sinus.
The orbital cavity bears the infraorbital fissure and infraorbital neurovascular bundle. The infraorbital fissure is continuous with the pterygomaxillary fissure, thus providing access to the infratemporal fossa laterally or to the pterygopalatine fossa medially. The superior wall is contiguous with the ethmoid or frontal sinuses and with the anterior cranial fossa. As previously stated, the superior orbital fissure serves as a passage for CN V1, III, IV, and VI and represents a potential pathway to the middle cranial fossa. The lateral wall is contiguous with the temporal fossa anteriorly to laterally. Posteriorly and medially, it is contiguous to the middle cranial fossa.
Scalp
The scalp comprises six different layers, which include the skin, subcutaneous tissue, frontalis muscle, galea, areolar tissue, and periosteum. By convention, the term pericranium refers to the combination of the periosteum and the areolar tissue. Vessels and sensory nerves supplying the scalp are superficial to the galea.
Anterior cranial fossa
The floor of the anterior cranial fossa comprises the frontal, ethmoid, and sphenoid bones. Laterally, the floor of the anterior cranial cavity corresponds to the roof of the orbits, while, centrally, it corresponds to the vault of the nasal cavity and the fovea ethmoidalis. The floor of the anterior cranial cavity is concave, with the medial or central area (ie, cribriform plate) located at a lower level than the lateral area. A slant from anterior (higher) to posterior (lower) is noted.
At the central anterior skull base, the most prominent structure is the cribriform plate, which contains multiple foramina, through which the olfactory filaments pass into the nasal cavity. Branches of the anterior ethmoid artery penetrate the vertical wall of the cribriform plate. This area is the weakest point of the anterior skull base. Anterior to the cribriform plate and just posterior to the foramen cecum is a tooth-shaped prominence, the crista galli. In children, the foramen cecum, anterior to the crista galli, may contain a vein that connects to the superior sagittal sinus. The planum sphenoidale denotes the area posterior to the cribriform plate, and its posterior aspect marks the posterior boundary of the anterior cranial fossa. The average distance between the foramen cecum and the tuberculum sella ranges from 28-50 mm, with an average distance of 42.5 mm.
Contraindications
Hematogenous metastasis, invasion of the cavernous sinus or internal carotid artery, bilateral invasion of the orbits (ie, soft tissues), optic nerves, or optic chiasm by a high-grade malignant tumor are contraindications for a craniofacial surgery under most circumstances. Lymphoreticular lesions, such as lymphoma and plasmacytoma, are best treated with nonsurgical therapies. Other contraindications include patients who refuse surgery or patients who are poor surgical candidates due to comorbidities.
-
CT scan coronal view demonstrating an osteosarcoma of the ethmoid sinuses extending to the orbit and anterior cranial fossa.
-
MRI axial view demonstrating extensive invasion of the frontal lobes by an adenocarcinoma.
-
MRI sagittal view demonstrating invasion of the fontal lobes by an osteosarcoma.
-
Markings for a bicoronal incision.
-
Markings for a lateral rhinotomy incision.
-
Intraoperative demonstration of a degloving approach.
-
Exposure of the superior orbital rims and glabella. The supraorbital neurovascular bundles have been freed from their canal and are being retracted to facilitate the exposure.
-
Craniotomy bone graft including the orbital rims and glabella as a monobloc.
-
Craniotomy and supraorbital block removed as separate bone grafts.
-
Surgical specimen after en bloc resection of anterior cranium, ethmoids, septum, and cribriform plate (posterior view).
-
Neurosurgical view after bifrontal craniotomy. The osteotomies to remove the supraorbital block have been performed, but the rims are still in place.
-
Neurosurgical view after the supraorbital rims and glabella have been removed, enhancing access to the cribriform plate area.
-
Defect after resection of the cribriform plate.
-
Elevation of a pericranial flap.
-
Fixation of the cranial bone grafts with titanium adaptation plates. Titanium mesh is used to cover bone gaps caused by the loss of bone associated with the craniotomy.
-
Reconstruction of the glabellar area using titanium mesh.
-
CT scan axial view demonstrating a tension pneumocephalus. In the author's experience, this is the most common major complication after an anterior craniofacial resection.
-
CT scan axial view of a frontal lobe contusion after an anterior craniofacial resection. A subfrontal approach was not used, resulting in the need for brain retraction with the subsequent trauma.






