Overview
Cervical metastasis by a tumor is firm statement of its aggressive malignant nature. Nothing is more controversial than the management of cervical metastatic disease. This is not surprising considering the lack of knowledge of carcinogenesis, pathophysiology of metastases, and implications of tumor spread. Fortunately, great strides have been made in the understanding of the intricate processes related to metastatic disease. Proper understanding of anatomy and the detection of cervical metastatic disease is crucial to this process. Forthcoming techniques will also facilitate the detection of primary and metastatic disease.
For excellent patient education resources, visit eMedicineHealth's Cancer Center. Also, see eMedicineHealth's patient education article Cancer of the Mouth and Throat.
Anatomy of the Cervical Lymphatics
The lymphatic system has 3 components: the capillaries, vessels, and nodes.
Capillaries
Larger than arteriovenous capillaries, lymphatic capillaries are thin-walled, with a single layer of endothelial cells. Lymphatic capillaries are found in all tissues; however, they are more abundant in the upper respiratory and GI tracts. Pooled capillaries drain lymphatic fluid into lymphatic vessels, which have 3 layers.
Vessels
As in the capillaries, the vessels have a single layer of endothelial cells surrounded by an inner, longitudinal elastic layer. This first muscle layer is surrounded by a circular smooth muscle layer, which, in turn, is enveloped by an outer connective tissue layer. Lymphatic vessels contain many more valves than the venous system, with the lymph circulation entirely dependent on compression by surrounding muscles. Lymphatic vessels drain into lymph nodes.
Nodes
These nodules of tissue are of variable size. Typically, as many as 75 nodes are located on each side of the neck. Nodes contain a subcapsular sinus below a prominent capsule, into which lymphatic fluid drains. This capsule is often the first site of metastatic growth. The fluid permeates the substance of the node (composed of a cortex and a medulla) and exits through the hilum to enter more lymphatic vessels. These nodes are located between the superficial cervical and prevertebral fascia and, thus, are very amenable to surgical removal. The lymphatic fluid eventually enters the venous system at the junction of the internal jugular and subclavian veins. Many nodal descriptions exist today; Rouvière's is the classic model. The following describes the main cervical node groups:
The occipital nodes are in the superficial group, which includes 3-5 nodes. This group of nodes is localized between the sternocleidomastoid (SCM) and trapezius muscles, at the apex of the posterior triangle. These nodes are superficial to the splenius capitis.
The deep posterior cervical group includes 1-3 nodes. This group of nodes is located deep to the splenius capitis and follows the course of the occipital artery. These nodes drain the scalp, the posterior portion of the neck, and it's the deep muscular layers of the neck.
The postauricular nodes vary in number from 2 to 4; they are located in the fibrous portion of the superior attachment of the SCM muscle to the mastoid process. Postauricular nodes drain the posterior parietal scalp and the skin of the mastoid region.
The parotid nodes can be divided into intraglandular and extraglandular groups. The extraglandular parotid nodes are located outside but adjacent to the parotid gland, where they drain the frontolateral scalp and face, the anterior aspects of the auricle, the external auditory canal, and the buccal mucosa. Embryologically, the lymphatic system develops before the parotid gland, which surrounds the intraglandular nodes as it develops. This explains why the parotid gland contains lymphoid tissue. The intraglandular nodes drain the same regions as the extraglandular nodes, to which they interconnect and then drain into the upper jugular group of lymph nodes. As many as 20 parotid nodes may be found.
The submandibular nodes are divided into 5 groups: preglandular, postglandular, prevascular, postvascular, and intracapsular. The preglandular and prevascular groups are located anterior to the submandibular gland and facial artery, respectively. The postglandular and postvascular groups are posterior to these structures. Differing from the parotid gland in embryological development, there is no true intraglandular node; however, occasionally, a node has been identified inside the capsule of the gland. The submandibular nodes drain the ipsilateral upper and lower lip, cheek, nose, nasal mucosa, medical canthus, anterior gingiva, anterior tonsillar pillar, soft palate, anterior two thirds of the tongue, and submandibular gland. The efferent vessels drain into the internal jugular nodes.
For the submental nodes, 2-8 nodes are located in the soft tissues of the submental triangle between the platysma and mylohyoid muscles. These nodes drain the mentum, the middle portion of the lower lip, the anterior gingiva, and the anterior third of the tongue. The efferent vessels drain into both the ipsilateral and contralateral submandibular nodes or into the internal jugular group.
The sublingual nodes are located along the collecting trunk of the tongue and sublingual gland and drain the anterior floor of the mouth and ventral surface of the tongue. These nodes subsequently drain into the submandibular or jugular group of nodes.
The retropharyngeal nodes are divided into a medial and lateral group, located between the pharynx and the prevertebral fascia. The lateral group, located at the level of the atlas near the internal carotid artery, consists of 1-3 nodes, which may extend to the skull base. The medial group extends inferiorly to the postcricoid level. This group drains the posterior region of the nasal cavity, sphenoid and ethmoid sinuses, hard and soft palates, nasopharynx, and posterior pharynx down to the postcricoid area. Management of these nodes must be considered if any malignancy arises from the mentioned drainage areas.
The anterior cervical nodes are divided into the anterior jugular chain and the juxtavisceral chain of nodes. The anterior jugular chain nodes follow the anterior jugular vein, located superficial to the strap muscles. These nodes drain the skin and muscles of the anterior portion of the neck, and the efferent vessels empty into the lower internal jugular nodes.
The juxtavisceral nodes are separated into the prelaryngeal, prethyroid, pretracheal, and paratracheal nodes. Prelaryngeal nodes are located from the thyrohyoid membrane to the cricothyroid membrane and drain the larynx and the thyroid lobes. A single delphian node is often found overlying the thyroid cartilage.
The pretracheal group consists of nodes between the isthmus of the thyroid gland down to the level of the innominate vein. Varying from 2-12 in number, these nodes drain the region of the thyroid gland and the trachea and receive afferent flow from the prelaryngeal group. The pretracheal efferents empty in the internal jugular group and the anterior superior mediastinal nodes.
The paratracheal nodes lie near the recurrent laryngeal nerve and drain the thyroid lobes, parathyroid glands, subglottic larynx, trachea, and upper esophagus. The efferent vessels travel to the lower jugular group or directly toward the junction of the internal jugular vein and the subclavian vein. The anterior nodes drain bilaterally because the midline of the neck has no division. Treatment must be planned accordingly when a tumor is located in subjacent draining areas.
The lateral cervical nodes are divided into superficial and deep groups. The superficial group follows the external jugular vein and drains into either the internal jugular or transverse cervical nodes of the deep group.
The deep group forms a triangle bordered by the internal jugular nodes, the spinal accessory nodes, and the transverse cervical nodes. The transverse cervical nodes, forming the base of the triangle, follow the transverse cervical vessels and may contain as many as 12 nodes. These nodes receive drainage from the spinal accessory group and from collecting trunks of the skin of the neck and upper chest. The spinal accessory chain follows the nerve of the same name and may account for as many as 20 nodes. This chain receives lymph from the occipital, postauricular, and suprascapular nodes and from the posterior aspect of the scalp, nape of the neck, lateral aspect of the neck, and the shoulder.
The internal jugular chain consists of a large system covering the anterior and lateral aspects of the internal jugular vein, extending broadly from the digastric muscle superiorly to the subclavian vein inferiorly. As many as 30 of these nodes may exist, and they have been arbitrarily divided into upper, middle, and lower groups. The efferents of these nodes eventually pass into the venous system via the thoracic duct on the left and multiple lymphatic channels on the right. These nodes drain all the other groups mentioned. Direct efferents may be present from the nasal fossa, pharynx, tonsils, external and middle ear, eustachian tube, tongue, palate, laryngopharynx, major salivary glands, thyroid, and parathyroid glands.
Although fairly consistent, these drainage patterns are subject to alteration with malignant involvement or after radiotherapy. In such cases, rerouting is possible, with metastases arising in unusual sites. Metastases have also been shown to skip first-echelon nodes and manifest in the lower internal jugular group.
Classification of Cervical Node Groups
Spread patterns of cancer from various primary sites in the head and neck to the cervical nodes have been documented in retrospective analyses of large groups of patients undergoing neck dissections. Since the first descriptions of nodal groups, various classification systems have been described.
To address surgical management of early-stage neck metastases via neck dissection, various authors have proposed a number of classification schemes. This lack of uniformity and standardization results in redundancy, misinterpretation, and confusion among clinicians. The most widely accepted terminology was originally described by a group of head and neck surgeons at Memorial Sloan-Kettering Hospital. This classification uses neck levels or zones and divides each side of the neck into 6 separate regions. This system is still used today.
-
Level I is bordered by the body of the mandible, anterior belly of the contralateral digastric muscle, and anterior and posterior bellies of the ipsilateral digastric muscle. Two nodal subgroups are found. The submental group (Ia) is found in the submental triangle (anterior belly of the digastric muscles and the hyoid bone), and the submandibular group (Ib) is found within the submandibular triangle (anterior and posterior bellies of the digastric muscle and the body of the mandible).
-
The nodes found in level II are located around the upper third of the internal jugular vein, extending from the level of the carotid bifurcation inferiorly to the skull base superiorly. The lateral boundary is formed by the posterior border of the SCM muscle; the medial boundary is formed by the stylohyoid muscle. Two subzones are also described; nodes located anterior to the spinal accessory nerve are part of level IIa, and those nodes posterior to the nerve are located in level IIb.
-
The middle jugular lymph node group defines level III. Nodes are limited by the carotid bifurcation superiorly and the cricothyroid membrane inferiorly. The lateral border is formed by the posterior border of the SCM muscle; the medial margin is formed by the lateral border of the sternohyoid muscle.
-
Level lV contains the lower jugular group and extends superiorly from the omohyoid muscle to the clavicle inferiorly. The lateral border is formed by the posterior border of the SCM muscle; the medial margin is formed by the lateral border of the sternohyoid muscle.
-
The lymph nodes found in level V are contained in the posterior neck triangle, bordered anteriorly by the posterior border of the SCM muscle, posteriorly by the anterior border of the trapezius, and inferiorly by the clavicle. Level V includes the spinal accessory, transverse cervical, and supraclavicular nodal groups.
-
Level VI lymph nodes are located in the anterior compartment. These nodes surround the middle visceral structures of the neck from the level of the hyoid superiorly to the suprasternal notch inferiorly.
A complete understanding of these anatomic relationships allows various practitioners to exchange information in an unbiased fashion and is critical in the decision-making processes involved in management of nodal metastases.
Mechanisms of Lymph Node Metastasis
The current hypotheses on the development of malignancies relate to alterations in the normal mechanisms of cellular proliferation and differentiation and a failure of cell death (apoptosis). This loss of growth control is the result of genetic mutations, including the activation of proto-oncogenes and/or inactivation of tumor suppressor genes. The resulting phenotypic changes provide cancer cells a growth advantage, including loss of response to normal growth controls, defects in response signals for programmed cell death, resistance to cytotoxicity, and defects in terminal differentiation.
Proposed by Fidler, the concept of tumor heterogeneity suggests that tumors are composed of heterogeneous subpopulations of cells differing in immunogenicity, invasiveness, cellular growth kinetics, sensitivity to cytotoxic drugs, and ability to metastasize. The local tumor environment may favor the development of more aggressive clones in the formation of metastases. Although the size of individual clones with metastasizing potential in a given tumor is significant, only a very small percentage of circulating cells lead to the development of metastatic colonies.
The events surrounding the initiation of local tumor invasion by epithelial tumors include a loss of cellular adhesion to surrounding tumor cells and basement membrane, invasion by malignant cells of the subjacent connective tissues by the production of cellular enzymes and growth mediators, cellular attachment to extracellular membrane molecules, neovascularization, and entry or exit from the circulation through the attachment to endothelial cell ligands. A repeat of these events occurs at metastatic sites.
In the case of head and neck squamous cell carcinomas, malignant cells may progress from carcinoma in situ, to microinvasive carcinoma, to a deeply invasive tumor with lymphatic metastases. Interestingly, a head and neck squamous cell carcinoma has the ability to manifest at both extremes of histopathological development in the same anatomic location. The critical step in the transition from carcinoma in situ to microinvasive and invasive carcinoma is the destruction of the basement membrane. This destruction is accomplished by the production of specific proteolytic molecules by tumor cells, including matrix metalloproteinases, collagenases, and plasminogen activators.
Angiogenesis is the growth of new capillaries by sprouting from established vessels. In normal tissues, self-limiting angiogenesis is part of reproduction and organogenesis in addition to wound repair and healing. Conversely, pathological angiogenesis is not autoregulated, but results from alterations in growth-control mechanisms of disease processes (eg, malignant transformation). Various tumor-derived factors (eg, prostaglandin E2, platelet-derived growth factor, transforming growth factor-beta, transforming growth factor-alpha, beta-fibroblast growth factor) are still being investigated for their propensity to facilitate endothelial cell proliferation.
Recent research looking specifically at the production of cytokines regulating immune, inflammatory, and angiogenetic responses in patients with laryngeal squamous cell cancer has revealed higher serum concentrations of the cytokines interleukin-6, interleukin-8, and vascular endothelial growth factor. These agents may be important in proinflammatory and proangiogenetic responses of tumor cells.
The ability of a tumor to stimulate an angiogenic response should directly determine the capability of a tumor to metastasize and ultimately kill the host. A clear correlation between tumor angiogenesis and nodal metastasis has been demonstrated in early and invasive breast carcinoma, ovarian and endometrial carcinoma, non–small-cell carcinomas, prostatic carcinoma, adenocarcinoma of the colon, and squamous cell carcinoma of the esophagus.
The literature notes conflicting reports regarding microvessel density and nodal metastasis in head and neck squamous cell carcinomas. Tumor sites of varying origins with different vascularization patterns at their primary sites may behave differently. Malignancies of the head and neck, especially head and neck squamous cell carcinomas, are the result of a series of genetic misadventures of squamous epithelial cells leading to malignant transformation. Variable genetic susceptibility, prolonged tobacco and alcohol exposure, viruses, and immune suppression all can facilitate these genetic derangements.
Tumors invade local connective tissues by the production of proteinases and the expression of surface markers that facilitate attachment to extracellular matrix components. Tumor growth and size being limited by available nutrients from the surrounding milieu, recruitment of host capillaries leads to the formation of an intratumoral blood supply. Capillary and lymphatic invasion by tumor cells allow malignant cell dissemination and the establishment of histologically identical tumors at distant sites.
Most recently, the expression of vascular endothelial factor-D in a mouse tumor model was found to lead to the lymphatic spread of tumor cells, tumor angiogenesis, and tumor growth. Further research in this area will likely provide more details in the multiple steps involved in the lymphatic spread of squamous cell cancer.
The dissemination of tumor cells beyond the primary site unfortunately remains the most significant factor in prognosis and needs further study.
Evaluation of the Neck for Cervical Metastases: Physical Examination
Evaluating neck metastases based on physical examination findings has been the classic method for patients with new tumors in the head and neck. The single most important factor in determining prognosis is whether nodal metastasis is present. Survival rates decrease by 50% when nodal metastases are present. Furthermore, the presence of cervical adenopathy has been correlated with an increase in the rate of distant metastasis.
During the clinical evaluation, careful palpation of the neck, with specific attention to location, size, firmness, and mobility of each node, is noted. Attention is particularly directed to nodes that appear fixed to underlying neurovascular structures, visceral organs, or nodes that demonstrate skin infiltration. The description of each node becomes an important part of the medical record, which can be used to assess the response to treatment or the progression of the disease.
Unfortunately, clinical palpation of the neck demonstrates a large variation of findings among various examiners. Although both inexpensive to perform and repeat, palpation findings are generally accepted as inaccurate. Both the sensitivity and specificity are in the range of 60-70%, depending on the tumor studied. Because of the known low sensitivity and specificity of palpation, a neck side without palpable metastases is still at risk of harboring occult metastasis, with the risk determined by the characteristics of the primary tumor. The incidence of false-negative (occult) nodes based on physical examination findings varies in the literature from 16-60%. Before the introduction of diagnostic imaging, particularly CT scan, clinical palpation was shown to be inadequate for detecting cervical metastasis. Soko et al reported that only 28% of occult cervical metastases were found by clinical palpation. Martis reported a 38% prevalence of occult metastasis based on clinical examination findings.
Detection of Cervical Metastasis: Radiological Investigations
Debate persists over the relative merits of imaging in the evaluation of the neck for metastatic disease. [1] Studies that correlate radiologic and histopathologic findings show that early microscopic metastases can be present in nodes smaller than 10 mm that demonstrate no stigmata of neoplasia (ie, central necrosis, extracapsular spread). Evidence of early metastatic disease in clinically occult nodes is minimal and may evade the efforts of the pathologist and radiologist.
Ultrasound
Ultrasound is reported superior to clinical palpation for detecting lymph nodes and metastases. The advantages of ultrasound over other imaging modalities are price, low patient burden, and possibilities for follow-up. [2, 3]
Sonographs of metastatic lymph node disease characteristically find enlargement with a spherical shape. Commonly, nodes are hypoechoic, with a loss of hilar definition. In cases of extranodal spread with infiltrative growth, the borders are poorly defined. Common findings of metastases from squamous cell carcinoma are extranodal spread and central necrosis together with liquid areas in the lymph nodes. Lymph node metastases from malignant melanoma and papillary thyroid carcinoma have a nonechoic appearance that mimics a cystic lesion. Sonography may also be useful for assessing invasion of the carotid artery and jugular vein.
Because lymph nodes of borderline size cannot be reliably diagnosed using ultrasound alone, ultrasound-guided fine-needle aspiration and cytologic examination of the nodes in question can be easily performed. The result of the aspirate examination depends on the skill of the ultrasonographer and the quality of the specimen (ie, harboring an adequate number of representative cells). Using this technique, most studies report that a sensitivity of up to 70% can be obtained for the N0 neck.
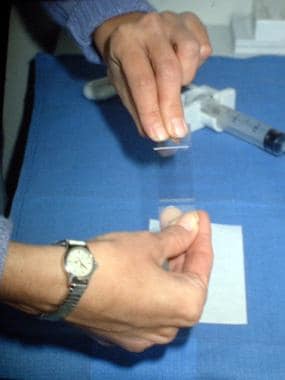 Photograph showing the smear technique for plating a sample aspirate. After a small drop of fluid is placed on a glass slide, a second slide is used to smear the aspirate evenly over the surface of the slide. The slide is then prepared for cytologic evaluation.
Photograph showing the smear technique for plating a sample aspirate. After a small drop of fluid is placed on a glass slide, a second slide is used to smear the aspirate evenly over the surface of the slide. The slide is then prepared for cytologic evaluation.
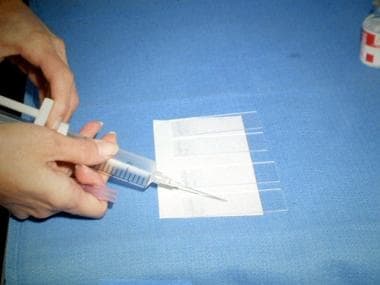 Photograph showing an aspirate being placed on a glass slide. After the 20-mL disposable syringe with an attached 21-gauge needle is placed under the skin surface and the mass is aspirated, a small drop of aspirated fluid is placed on a glass slide.
Photograph showing an aspirate being placed on a glass slide. After the 20-mL disposable syringe with an attached 21-gauge needle is placed under the skin surface and the mass is aspirated, a small drop of aspirated fluid is placed on a glass slide.
CT scan
Since its debut in the 1970s, CT scans have been an invaluable tool in all fields of medicine, including the evaluation of head and neck cancer. Since the advent of high-resolution systems and specific contrast media, fine-cut CT scanning has allowed the detection of pathological cervical nodes of smaller size that may be missed by clinical examination. CT scanning is now used routinely for the preoperative evaluation of the neck because, presumably, it helps decrease the incidence of occult cervical lymphadenopathy. [4]
Introduced in 1998, multiple-spiral CT scanning promises further improvement of temporal and spatial resolution (in the longitudinal axis). This technique permits rapid scanning of large volumes of tissue during quiet breathing. The volumetric helical data permit optical multiplanar and 3-dimensional reconstructions. Improvement of the assessment of tumor spread and lymph node metastases in arbitrary oblique planes is another advantage of the spiral technique.
Criteria for the identification of questionable nodes are also evolving as technology advances. Central necrosis remains the most specific finding suggestive of nodal involvement, but its absence does not exclude metastasis. Unfortunately, metastasis is usually quite rare or not visible in small lymph nodes, where detection would be crucial. Because of the higher imaging resolution, various studies have reduced the traditional values of 10-15 mm for a node to be suggestive. Many authors have proposed a minimal axial diameter of 11 mm for the submandibular triangle and 10 mm for the rest of the neck. Other criteria include the presence of groups of 3 or more borderline nodes and the loss of tissue planes.
An imaging-based classification has also been proposed by Som et al, [5] and due to its specificity, has also been endorsed by clinicians managing head and neck cancer. The boundaries of the nodal levels were easily discerned by radiologists and yielded consistent nodal classifications.
For an evaluation of the diagnostic abilities of Ga-SPECT, see Kotani et al. [6]
Magnetic resonance imaging
The value of MRI is its excellent soft tissue resolution. MRI has surpassed CT scanning as the preferred study in the evaluation of cancer at primary sites such as the base of the tongue and the salivary glands. The sensitivity of MRI exceeds that of clinical palpation in detecting occult cervical lymphadenopathy. Size, the presence of multiple nodes, and necrosis are criteria shared by CT scanning and MRI imaging protocols. [7, 8]
Many reports indicate that CT scanning still has an edge over MRI for detecting cervical nodal involvement. Advances in MRI technology (eg, fast spin-echo imaging, fat suppression) have not yet surpassed the capacity of CT scanning to identify lymph nodes and to define nodal architecture. Central necrosis, as evaluated by unenhanced T1- and T2-weighted images, has been shown to provide an overall accuracy rate of 86-87% compared with CT scanning, which has an accuracy rate of 91-96%. [9] The use of newer contrast media, especially supramagnetic contrast media agents, hopefully will improve the sensitivity of MRI.
Positron emission tomography imaging
This new imaging modality has been increasingly studied in the staging of head and neck cancer. [10, 11] The technique relies on the uptake of 2-fluoro-2-deoxy-D-glucose (FDG) in metabolically-active lesions. The study may also be fused to a corresponding CT scan to facilitate the localization of the lesion of concern.
In comparing their usefulness in the detection of cervical metastasis, PET/CT fusion images have been found to be superior and more accurate for the detection of cervical metastasis, compared with PET alone or with conventional imaging modalities. In addition, PET can contribute to the detection of residual or early recurrent tumors, leading to the institution of earlier salvage therapy. [4]
A study by Bae et al found that in 130 clinically node-negative (cN0) patients with oral cavity squamous cell carcinoma, pretreatment 18F-FDG-PET/CT scanning detected pathologic cervical metastasis in 29 (22.3%) individuals. Such node positivity was reported to be significantly associated with age, tumor differentiation, lymphovascular invasion, and T classification, while maximum standardized uptake value (SUVmax) and total lesion glycolysis were found to be independently associated with overall survival. [12]
A study by Tan et al indicated that FDG-PET/CT scanning is more sensitive than contrast-enhanced multislice helical CT (MSCT) imaging in detecting paraesophageal lymph node metastases in patients with esophageal cancer. The study involved 115 patients with esophageal cancer, in whom a total of 946 lymph node groups were resected; metastases were confirmed histopathologically in 221 of these groups. Although no significant differences were found between FDG-PET/CT scanning and enhanced 64-slice helical CT imaging with regard to specificity and accuracy in detecting lymph node metastases, the sensitivity of FDG-PET/CT scanning was 74.7%, compared with 64.7% for enhanced MSCT scanning. The sensitivity difference was even greater for paraesophageal lymph node metastases, being 72% and 57.7%, respectively. [13]
The investigators’ findings also indicated, however, that enhanced MSCT imaging can effectively distinguish false-negative lymph node metastases visualized on FDG-PET/CT scans. Tan and colleagues concluded that combining FDG-PET/CT scanning with MSCT imaging should increase accuracy in staging lymph node metastases in esophageal cancer. [13]
A study by Jung et al suggested that in papillary thyroid carcinoma, a primary tumor with a high avidity for FDG and a high SUVmax is predictive for cervical lymph node metastasis. The study included 193 patients with papillary thyroid carcinoma who underwent FDG-PET/CT scanning prior to treatment, with the FDG-avid tumors being larger than the nonavid tumors (0.93 cm vs 0.59 cm, respectively) and the incidence of cervical lymph node metastasis being greater in the avid tumors than in the nonavid ones (49.2% vs 33.3%, respectively). Moreover, among the avid tumors, the SUVmax was higher in the ones that metastasized than in those that did not. [14]
Conclusions
None of the currently available imaging techniques can help depict small tumor deposits inside lymph nodes. Characteristics of metastatic lymph nodes that can be depicted are the size and presence of noncontrast-enhancing parts inside metastatic lymph nodes caused by tumor necrosis, tumor keratinization, or cystic areas inside the tumor. Only rarely does tumoral tissue enhance more than reactive lymph node tissue; in these rare cases, the tumor can be visualized within a reactive lymph node.
Patients who need an evaluation for a possible nodal malignancy require a comprehensive multidisciplinary evaluation of all potential sites of drainage to that node to identify its primary source. This includes a thorough evaluation of potential primary sites using endoscopic techniques. When appropriate, include laryngoscopy, esophagoscopy, bronchoscopy, and examination of the nasopharynx. If no primary source is identified, taking blind mucosal biopsy samples of the most likely head and neck subsites is essential. [15]
PET/CT techniques have a promising role; however, greater clinical experience is needed prior to making this modality the standard for the detection of metastasis in head and neck cancer.
Complete documentation of nodal characteristics by clinical examination and palpation guide the examiner in using adjunctive radiological tools to exclude occult nodal metastasis.
-
Photograph showing the smear technique for plating a sample aspirate. After a small drop of fluid is placed on a glass slide, a second slide is used to smear the aspirate evenly over the surface of the slide. The slide is then prepared for cytologic evaluation.
-
Photograph showing an aspirate being placed on a glass slide. After the 20-mL disposable syringe with an attached 21-gauge needle is placed under the skin surface and the mass is aspirated, a small drop of aspirated fluid is placed on a glass slide.










