Overview
Shortly after Roentgen discovered radiographs in 1895, their clinical usefulness as a means of cancer treatment was first appreciated. Since that time, radiation therapy has developed into a recognized medical specialty. The Curie family discovered radium in 1898. Alexander Graham Bell suggested its use in brachytherapy for direct implantation in malignant tumors.
The lack of a precise method of measuring dosage limited the early use of radiation therapy. An early prescription typically called for an erythema dose as the standard unit of radiation. One of the limiting factors in early treatment was skin tolerance.
This hurdle was overcome by Coutard’s recognition of the usefulness of fractionation—that is, dividing the dose into several smaller increments rather than administering a single, massive dose. At the same time, high-energy supervoltage was developed, and later, megavoltage-dedicated radiotherapy units were introduced.
A study by Atun et al reported that there is currently a large gap worldwide between the need for radiation therapy and the ability to access it, with the investigators estimating that global expansion of such access between 2015 and 2035 in low- and middle-income countries could save 26.9 million life-years and produce a net economic benefit over 20 years of $278.1 to $365.4 billion. [1, 2]
Biologic Basis
The exact mechanism of cell death due to radiation is still an area of active investigation. A large body of evidence supports double-stranded breaks of nuclear DNA as the most important cellular effect of radiation. This breakage leads to irreversible loss of the reproductive integrity of the cell and eventual cell death.
Radiation damage can be directly ionizing; however, in clinical therapy, damage is most commonly indirectly ionizing via free-radical intermediaries formed from the radiolysis of cellular water.
Radiation can also affect the processes of the cell cycle necessary for cell growth, cell senescence, and apoptosis (programmed cell death). Many of these processes are only now beginning to be elucidated and manipulated in order to make radiation therapy more effective.
The therapeutic mechanism for radiation is based on the intrinsic ability of cells to repair damage and the ability of the radiation oncologist to take advantage of any geometric separation between malignant and nonmalignant tissues.
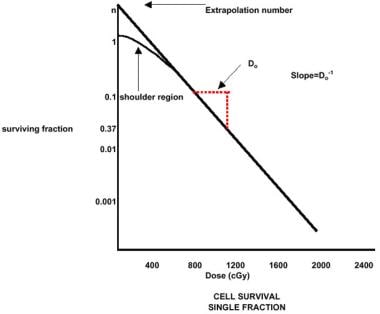
Cell survival after exposure can be expressed in terms of a logarithmic curve of survival versus dose. The curve forms an initial shoulder followed by a logarithmic decline in survival, which varies with the dose (see the image below). Sublethal damage, which must be overcome with each fraction of radiation therapy, is thought to cause the initial shoulder.
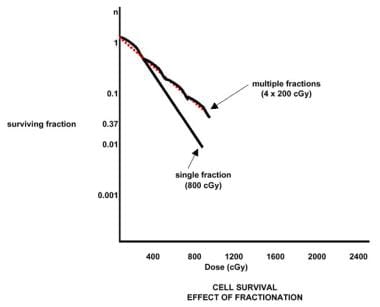
Repeated small doses of radiation are less damaging to a sensitive cell than a single fraction containing an equivalent total dose (see the image below). Manipulation of the cellular environment can alter the shape of the survival curve.
Besides being related to intrinsic cellular radiosensitivity, cell survival is also related to oxygen tension, the position of the cell in the mitotic cycle, and dose rate. These features are responsible for the 4 Rs of radiobiology—namely, R epair, R edistribution, R epopulating, and R eoxygenation.
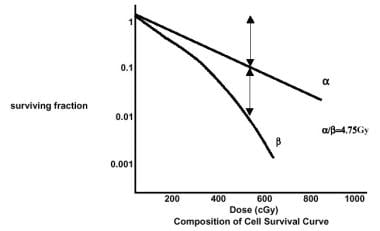
Several models have been used to conceptualize radiation-induced cell death and to explain the cell survival curve. The cell survival curve can be interpreted to follow a linear-quadratic model, according to which the surviving fraction is equivalent to e(αD - βD2), where α and β represent the alpha and beta components, respectively (see the image below).
In the linear-quadratic model, 2 components of cell injury are present. The linear alpha component is responsible for the initial shoulder on the cell survival curve and is caused by repairable damage to the target. The quadratic beta component represents irreparable damage. The linear component is proportional to the dose, whereas the quadratic component is proportional to the dose squared.
Early-responding tissues and tumors have a relatively larger alpha component and a larger α/β ratio; late-responding tissues have smaller α/β ratios. This difference between tumor and late-responding tissues is useful in designing therapeutic schemes that use multiple daily fractions rather than the conventional once-daily treatments.
Values for α/β in early-responding normal tissue are as follows:
-
Skin – Erythema, 10.6; desquamation, 11.2
-
Oral mucosa – Mucositis, 10.8
Values for α/β in early-responding tumor tissue are as follows:
-
Nasopharynx - 16
-
Oropharynx - 16
-
Vocal cord - 13
-
Tonsil - 7
-
Skin (squamous or carcinoma) - 8.5
Values for α/β in late responding normal tissue are as follows:
-
Skin – Telangiectasia, 2.7; fibrosis, 1.7
-
Spinal cord – Myelitis, 3.3
-
Cartilage – Fibrosis, 4.5
Basic Physics
X-rays and gamma ray photons are part of the electromagnetic spectrum. The dual nature of electromagnetic radiation is used to explain its wave and particulate behavior.
A photon is a packet of energy that can be characterized by the equation E = hv, where h is Planck’s constant (6.62 × 10-34 J-sec) and v is the frequency of the photon. Frequency is equivalent to the quotient of the speed of light (3 × 108 m/sec) divided by the wavelength. Thus, high-energy radiations have a short wavelength and a high frequency.
The interaction of a photon beam with matter results in the attenuation of the beam. Five major types of interactions typically occur:
-
Coherent scattering
-
Photoelectric effect
-
Compton scattering
-
Pair production
-
Photodisintegration
The particular type of interaction is related to photon energy. In radiation therapy, the photoelectric effect, the Compton effect, and pair production are of interest, with the Compton effect being the predominant interaction.
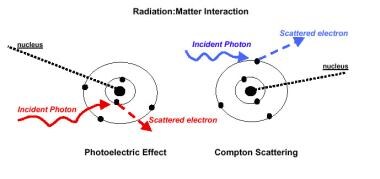
The photoelectric effect involves the interaction of the photon with the tightly bound inner electrons and is proportional to the cube power of the absorbing matter’s atomic number. This interaction is responsible for the different radiographic densities seen on diagnostic radiographs.
The Compton effect involves interaction with outer electrons that are bound more loosely. This effect is related to electron density and therefore results in much more uniform tissue absorption than lower-energy photons. In radiation therapy, the Compton effect predominates; therefore, the contrast observed on therapy port films is inferior to that observed on diagnostic radiographs (see the image below).
Pair production involves the interaction of the photon with the atomic nuclear electromagnetic field. This interaction becomes significant at high energies (> 10 MeV) and is proportional to the atomic number of the absorbing matter.
Radioactive isotopes occur naturally and can be generated artificially. The activity of a radioisotope decays over time. The energy spectrum, decay spectrum, and half-life are the key characteristics (see Table 1 below). A particular isotope may decay through production of alpha particles or beta particles (positive or negative charge), electron capture, internal conversion, or a combination of these reactions. Likewise, a nuclear transformation occurs when a chemical reaction takes place following a nuclear reaction and results in the generation of a new species.
Table 1. Physical Characteristics of Commonly Used Isotopes (Open Table in a new window)
Isotope |
Half-life |
Energy (MeV) |
Half-Value Layer (mm lead) |
Exposure Rate Constant (R/cm2/mCi-hr) |
Ra-226 |
1600 years |
0.047-2.45 (0.83 avg) |
8.0 |
8.25 |
Co-60 |
5.26 years |
1.17,1.33 |
11.0 |
13.07 |
Cs-137 |
30 years |
0.662 |
5.5 |
3.26 |
Ir-192 |
74.2 days |
1.36-1.06 (0.38 avg) |
2.5 |
4.69 |
I-125 |
60.2 days |
0.028 avg |
0.025 |
1.46 |
Radiation dose or exposure is measured in units of absorbed radiation per unit of tissue. The Gray (Gy) represents 1 J/kg of tissue. In older literature, the unit of measure was the rad, which is equivalent to 1 cGy (0.01 Gy). The exposure and dose rate of radiation decrease according to an inverse square law, so that the exposure decreases by 4 when the distance increases by 2.
External beam therapy is commonly delivered via a medical linear accelerator or cobalt-60 unit. The introduction of these megavoltage units ushered in the modern age of radiation therapy. These units deposit the maximum dose beneath the surface; therefore, external beam therapy is considered skin sparing. Photons traverse the entire tissue thickness but deposit less of the dose as the depth increases.
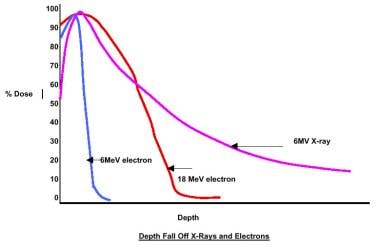
Many modern linear accelerators are also capable of producing electrons, which can be used for treatment situations where the limited depth penetration of these particles is useful, as in cases where the contralateral parotid gland must be spared (see the image below).
Delivery of Therapy
The process of treatment planning calls for integration of the physical findings and diagnostic imaging information with knowledge of the pertinent anatomy, pathology, and natural history of the particular tumor type. [3]
The radiation oncologist and other members of the multidisciplinary team must decide whether radiation will play a role in the treatment of the patient. Once the decision is made to employ radiation, consideration must be given to whether the radiation will be prescribed as definitive, palliative, or adjuvant therapy and whether it will be integrated with surgery and chemotherapy.
The radiation oncologist may recommend additional studies in the workup of the patient or may choose to coordinate the patient’s care. A detailed knowledge of the various modalities of radiation therapy in the physician’s armamentarium, together with their individual physical characteristics and unique toxicities, is essential.
Planning of radiation fields
Contemporary treatment-planning computers allow the incorporation of 3-dimensional anatomic data into the planning of radiation fields. With beam’s-eye-view technology, radiation delivery can be planned so as to ensure that the radiation field adequately covers the target and spares or minimizes the dose to the nontarget healthy tissues (see the image below). Complex beam arrangements can be used in the knowledge that a geometric miss can be avoided. An entire lexicon of treatment-planning terminology has been created as a result.
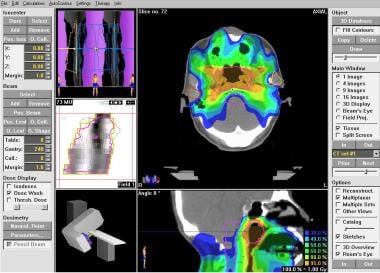 Radiation therapy, general principles. Intensity-modulated radiation therapy (IMRT) plan for nasopharynx.
Radiation therapy, general principles. Intensity-modulated radiation therapy (IMRT) plan for nasopharynx.
The physician uses the clinical and radiographic findings to determine the gross tumor volume (GTV). Next, the clinical tumor volume (CTV), including microscopic extension of the disease, is determined. The planning treatment volume (PTV) allows for day-to-day variation.
To minimize any variation in patient positioning, meticulous immobilization is essential. Thermoplastic masks and other similar positioning devices are frequently used in the treatment of head and neck cancer.
Fractionation
Conventional fractionation in the United States is considered to be 1.8-2 Gy/day, administered 5 days each week for 5-7 weeks, depending on the particular clinical situation. Alteration to this scheme has been considered for various reasons, including time constraints, staff constraints, machine availability, and patient convenience.
A variety of methods have been used to correlate different dose-fractionation schemes. These methods are rooted in an understanding of the dose-response curves and the association (or lack thereof) between acute reaction and long-term sequelae of treatment.
Strandquist produced the first clinical isoeffect curve, calculating the relations between time, dose, cure, and skin reactions in the treatment of skin cancers. Later, Ellis recognized that the time factor was actually dependent on both the overall treatment time and the number of fractions. He devised the nominal standard dose (NSD) formula:
D = NSD×N0.24 ×T0.11
where D = dose, N = number of fractions, and T = overall time.
These formulas have been used in an attempt to adjust for the alterations in the standard fractionation schemes. Most contemporary isoeffect formulas make use of the linear-quadratic model as a basis for dose adjustments:
D1/D2 = ( α/β + d2) / ( α/β + d1)
where D is the total dose and d is the dose per fraction. This formula assumes a complete repair of sublethal damage between the fractions. The major deficiency with these formulas is the lack of precision in determining α/β for the various tissue types, tumor types, and individual variations.
One of the early attempts at altered fractionation was the use of split-course therapy. The aim was to achieve comparable or better levels of tumor control by allowing reoxygenation with reduced acute toxicity. A 2- to 3-week rest from the treatment was often used. However, this rest period resulted in poorer long-term control because repopulation and accelerated repopulation dominated.
Other fractionation schemes include hyperfractionation, hypofractionation, and accelerated fractionation. In hyperfractionated regimens, the goal is to deliver higher tumor doses while maintaining a level of long-term tissue damage that is clinically acceptable. The daily dose is unchanged or slightly increased while the dose per fraction is decreased, and the overall treatment time remains constant. The α/β for the tumor must be greater than that of the dose-limiting tissue.
An additional rationale for hyperfractionation is to allow radiosensitization through redistribution. With a greater number of fractions, it is more likely that the tumor will be in a sensitive phase of the cell cycle at some time during the treatment. This strategy invariably results in more intense acute reactions when compared to conventional treatment.
In the accelerated fractionation schemes, the dose per fraction is unchanged while the daily dose is increased, and the total time for the treatment is reduced.
Continuous hyperfractionated accelerated radiation therapy (CHART) is an intense schedule of treatment in which multiple daily fractions are administered within an abbreviated period. An intense acute reaction develops in most patients. This reaction usually limits the total dose.
In a concomitant boost technique, the first fraction of the day is administered in a larger volume, and the second fraction is targeted to a reduced-boost field of treatment. The boost may be administered early in the course of therapy or toward the end of treatment. This regimen is based on the recognition that the treatment can induce an accelerated repopulation of the tumor cells, so that reduction of the overall treatment time results in improved control.
Clinical trials are in progress with the goal of evaluating these various altered fractionation patterns and comparing them with conventional treatment. Results from the randomized trial RTOG-9003 supports the benefit of altered fractionation over conventional treatment for head and neck cancer. So far, the benefit seems to lie in local regional control. Further follow-up is necessary to determine if there is an overall survival benefit as well. [4]
A study in the Cochrane Database of Systematic Reviews found that altered fractionation (either hyperfractionated or accelerated fractionated) radiotherapy improves survival (3.4% absolute benefit at 5 years) in patients with head and neck squamous cell carcinoma, with hyperfractionation providing the greatest benefit (8% absolute benefit at 5 years). Accelerated fractionation, without total dose reduction, provided a 2% absolute benefit at 5 years. [5]
Effects of Irradiation
Radiation effects on normal tissues are divided into acute and chronic (late) effects. Acute effects occur during the course of therapy and during the posttherapy period (approximately 2-3 weeks after the completion of a course of irradiation). Chronic effects can manifest anytime thereafter, from weeks to years after the treatment.
Patients are usually most bothered by the acute effects, but physicians are at least equally concerned about the chronic effects. The acute effects can be quite uncomfortable, but they generally resolve. The chronic effects can be devastating, permanent, and progressive.
Acute effects
Much of the effort that goes into treatment planning has to do with minimizing the effects of treatment on normal tissue. The tissues that divide rapidly (eg, mucous membranes) respond acutely to radiation and are responsible for much of the acute morbidity of the treatment.
The mucous membranes of the oral cavity and the oropharynx respond early to fractionated radiation. Erythema is often evident after 1 week of treatment at conventional doses. This condition progresses over the next few weeks through various stages of mucositis, ranging from small patches to confluent or even ulcerated areas.
Mucositis represents caking of the dead epithelial cells, fibrin, and inflammatory cells. [6] When patients report oral or throat pain during treatment, superimposed infection with yeast or bacteria should be considered. Healing begins while the patient is still undergoing treatment but may continue for several weeks after radiation therapy is completed.
Loss of taste is a common acute effect of treatment. [7] Taste loss begins early and progresses rapidly during the second 2 weeks of treatment. Patients may report diminished acuity, odd sensation, or complete absence of taste. Xerostomia is often present and exacerbates this loss of taste. This condition is often accompanied by a poor or absent appetite and weight loss. Recovery of taste is a slow and, frequently, an incomplete process.
The major salivary glands (ie, parotid and submandibular glands) are responsible for nearly 80% of salivary production. The parotid glands primarily consist of serous acini, whereas the submandibular glands produce mucinous and serous secretions. The minor salivary glands, which are distributed throughout the oral mucosa, produce mostly mucinous secretions.
Radiation affects the volume and production of saliva, as well as its composition. [8] Decreased salivary pH and decreased volume are significant contributors to altered oral mucosal flora and predispose patients to caries. Salivary production decreases rapidly with treatment, declining by nearly 50% after a week of treatment. Patients frequently describe thickened, tenacious, ropy saliva, which may make speech difficult besides affecting swallowing and taste.
High-energy radiographs are skin-sparing. Skin reactions, which were at one time considered dose-limiting, are rarely a problem when high-energy radiographs are used. Whereas erythema is common after several weeks of radiation, severe skin reactions, such as those observed in the orthovoltage era, are uncommon.
When a need for skin irradiation exists (eg, when the tumor involves the skin or when the skin is the target in the treatment of basal cell cancers), the technique is adjusted so that a brisk skin reaction is produced. The use of less-penetrating orthovoltage radiographs, treatment with electrons, or the addition of tissue-equivalent bolus material placed over the radiation field can circumvent skin sparing.
Even with megavoltage treatment, skin tanning and dry desquamation can occur. The addition of certain chemotherapeutic drugs can enhance cutaneous and other effects. Radiation can induce melanin production, which is often first observed in the skin follicles because these skin invaginations receive a slightly higher dose as the beam enters tangentially to the surface. Skin sensitivity is increased because of the treatment.
In situations where the skin is denuded (ie, moist desquamation), the area must be kept clean to prevent superinfection. Reepithelialization moves centrally from the field edges and is generally completed within 3 weeks before the completion of treatment.
Sweat glands and sebaceous glands may cease functioning, but the in-field hair loss is usually temporary. Regrowth is evident within a few weeks after cessation of therapy.
Chronic (late) effects
Two major theories are used to explain late injury. One theory attributes chronic injury to a damaged microvasculature, and the other attributes injury to stem cell depletion. In either case, late effects can be a source of ongoing morbidity after a course of radiation therapy. Like the acute effects, the chronic effects are related to site, dose, volume, and time. Other therapies, such as surgery and chemotherapy, can increase the probability and severity of radiation-related morbidity.
High doses of radiation to the neck can result in fibrosis, especially in the postoperative setting, where the neck may develop a woody texture and have limited movement. Likewise, the masticatory muscles may develop fibrosis, which can result in trismus. Accordingly, patients should be instructed to begin stretching exercises of their jaw muscles as soon as possible after surgery.
A study by Pratson et al of indicated that in patients who undergo radiation therapy for head and neck cancer, the grade of neck fibrosis tends to be higher in those who smoke or use alcohol during radiation treatment, have recurrent disease, are younger than 60 years, or are non-Caucasian. [9]
Obstruction of the cutaneous lymphatics results in lymphedema, which may be associated with episodes of intermittent erysipelas. A study by Kim et al indicated that in patients with nasopharyngeal carcinoma, the likelihood of developing moderate to severe facial lymphedema following concurrent chemoradiation may be reduced by restricting the mean radiation dose to the level IV and level I-VII neck lymph nodes to less than 58.7 Gy and 58.6 Gy, respectively. [10]
Delayed wound healing can be a consequence of high-dose preoperative radiation.
Telangiectasis may appear some time after radiation therapy in the areas that were treated without skin-sparing techniques, such as the use of electron, tangential irradiation, and deliberate addition of bolus material.
Loss of salivary function is usually complete after modest doses of radiation to the parotid glands. Basal flow rates correlate with the response to radiation therapy. With doses of 40-60 Gy, fewer than 20% of patients have a measurable salivary flow. With doses lower than 30 Gy, some function may return after 6-12 months. However, at doses exceeding 50 Gy, xerostomia is usually irreversible. [11]
Dry mouth is probably the most common problem for patients who receive therapeutic doses of radiation. Numerous approaches have been tried in an effort to address this problem. Pilocarpine 5 mg 3 times daily has been shown to stimulate residual salivary function. Some patients use artificial saliva substitutes, but most patients find them inadequate. Many patients must carry bottles of water to provide some relief.
The US Food and Drug Administration (FDA) has recently approved the use of intravenous (IV) amifostine as a radioprotectant agent. Given in a daily dose, amifostine is to be used in the prevention of radiation-induced xerostomia in the postoperative setting. Concern about tumor protection appears to be unwarranted. Disadvantages (eg, nausea, hypotension, the need for daily injections, and substantial cost) may limit its wide acceptance. Subcutaneous administration, though not a labeled use, seems to be equally effective and is associated with less toxicity.
Intensity-modulated radiation therapy (IMRT) is an increasingly available approach to the prevention of xerostomia. At the time of treatment planning, the radiation oncologist uses an inverse-planning algorithm that allows selective avoidance of critical normal tissues without compromising the tumor doses. Other potential uses for IMRT include the ability to administer biologically higher doses to target tissues and biologically lower doses to nontarget tissues, thereby increasing the therapeutic ratio.
Several planning systems and systems of delivery are currently available or are in the testing phases. The initial reports are encouraging.
As a consequence of oral pain and altered diet during treatment and xerostomia after treatment, the oral flora and pH can be altered significantly. Without meticulous dental care during and after radiation therapy, patients are prone to accelerated caries and decay. Oral and gingival tissue may undergo atrophy after irradiation, which results in a thin pale layer with evidence of telangiectatic vessels.
Ulceration and bone exposure may develop. If serious injury to the underlying bone occurs, osteoradionecrosis may follow. Fortunately, this complication is uncommon. When it does occur, management of the condition may be difficult. Although patience is important, some cases clearly necessitate intervention, which includes antibiotic therapy, hyperbaric oxygen treatment, and resection.
Irradiation of the spinal cord may result in a self-limited transverse myelitis known as Lhermitte syndrome. The patient notes an electric shocklike sensation that is most notable with neck flexion. Rarely does this condition progress to a true transverse myelitis with associated Brown-Séquard syndrome. To avoid this devastating complication, the dose to the spinal cord must be limited.
A course of treatment often affects the thyroid gland either directly or secondarily via the hypothalamic-pituitary axis. Chemical hypothyroidism is often the only manifestation of an endocrinopathy and is readily treated with supplemental thyroid preparation. Other endocrinopathies are uncommon.
Other structures that must be considered during treatment planning are the visual apparatus, the auditory apparatus, and the apex of the lungs. Exceeding the tolerance of the lacrimal gland can result in dry-eye syndrome. The lens is highly sensitive to radiation. Direct irradiation of the lens with the most commonly used therapeutic doses results in cataract formation. To avoid visual loss, the tolerance of the retina and optic nerve must also be respected during treatment planning.
Irradiation of the auditory apparatus may result in serous otitis or possible sensorineural hearing loss at high doses of radiation.
Radiation-induced cancers are, fortunately, quite uncommon after therapeutic doses of radiation. A much greater risk of a second malignancy is posed by the same etiologic and genetic mechanisms that are responsible for the primary tumor.
Associated Chemotherapy
Chemotherapy can enhance the effects of radiation therapy. Numerous agents have been used either sequentially or concurrently with radiation. Most studies indicate that the best results can be obtained with concurrent radiation and platinum-based chemotherapy. The concurrent use of chemotherapy also intensifies the acute toxicities of treatment, and patients often require nutritional support during therapy.
Encouraging data have been accumulated that suggest a role for induction chemotherapy followed by concurrent chemoradiotherapy. [12, 13] Other agents that are used in head and neck cancer include fluorouracil, paclitaxel, docetaxel, and hydroxyurea.
Biologic agents are being studied that may reduce some of the toxicities of combined chemotherapy and radiation or may enhance the effect of radiation therapy. Keratinocyte growth factor (KGF) has been found useful in reducing mucosal toxicity in patients undergoing total-body irradiation and may be useful in other applications as well.
Targeted therapies that act to interrupt signal transduction pathways are also being studied. As a consequence of the dramatic results achieved with the epidermal growth factor receptor (EGFR) antibody cetuximab, the US Food and Drug Administration (FDA) has approved this agent for use in head and neck cancers. [14]
-
Radiation therapy, general principles. Intensity-modulated radiation therapy (IMRT) plan for nasopharynx.
-
Radiation therapy, general principles. Cell survival, single fraction.
-
Radiation therapy, general principles. Effect of fractionation.
-
Radiation therapy, general principles. Composition of cell survival curve.
-
Radiation therapy, general principles. Interaction of radiation with matter.
-
Radiation therapy, general principles. Variation of dose with depth (x-rays and electrons).