Practice Essentials
Acoustic neuromas are intracranial, extra-axial tumors that arise from the Schwann cells, investing either the vestibular or cochlear nerve. As acoustic neuromas enlarge, they eventually occupy a large portion of the cerebellopontine angle and cause hearing loss, dizziness, and tinnitus. Acoustic neuromas account for approximately 80% of tumors found within the cerebellopontine angle. The remaining 20% are principally meningiomas. In rare cases, a facial nerve neuroma, vascular tumor, lipoma, or metastatic lesion is found within the cerebellopontine angle. The definitive diagnostic test for acoustic tumors is gadolinium-enhanced magnetic resonance imaging (MRI). Acoustic neuromas are managed through microsurgical excision, by arresting tumor growth using stereotactic radiation therapy, or through serial observation.
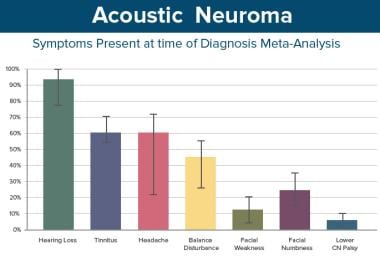 This table shows the distribution of presenting symptoms, ie, the symptom that brought the patient to a physician and that constituted the patient's chief ailment.
This table shows the distribution of presenting symptoms, ie, the symptom that brought the patient to a physician and that constituted the patient's chief ailment.
Signs and symptoms of acoustic neuromas
Unilateral hearing loss is overwhelmingly the most common symptom present at the time of diagnosis and is generally the symptom that leads to diagnosis. Other symptoms can include the following:
-
Tinnitus - Especially unilateral, non-pulsatile
-
Vertigo and disequilibrium - Uncommon
-
Headaches - Present in 50-60% of patients at the time of diagnosis
-
Facial numbness - Occurs in about 25% of patients
Though acoustic neuromas are generally slow-growing tumors and their associated hearing loss is usually progressive, they may also present with sudden sensorineural hearing loss (SNHL). All patients with sudden SNHL should be imaged for work up of acoustic neuroma, even if they respond to steroids or their hearing spontaneously recovers.
Diagnosis and management of acoustic neuromas
A study by Foley et al of 945 persons with acoustic neuroma reported unilateral hearing loss to be the most common presenting system (80% of patients). Unilateral tinnitus was the next most frequent presenting symptom, occurring in 6.3% of patients, with ataxia, vertigo, and headache being the presenting symptoms in 3.8%, 3.4%, and 2% of cases, respectively. Patients with larger tumors were more likely to suffer from headaches, facial weakness, and abnormalities in tandem gait and facial sensation. [1]
The definitive diagnostic test for patients with acoustic tumors is gadolinium-enhanced MRI. Gadolinium contrast is critical because nonenhanced MRI can miss small tumors. If suspicion is high and MRI is contraindicated, high-resolution computed tomography (CT) scanning with contrast can be used, although this may also miss smaller tumors.
Acoustic neuromas are managed in one of the following 3 ways: (1) microsurgical excision of the tumor, (2) arresting tumor growth using stereotactic radiation therapy, or (3) careful serial observation.
Observation involves monitoring diagnosed patients with serial imaging and audiologic evaluation without therapeutic intervention unless growth occurs that necessitates therapy.
Simple observation without any therapeutic intervention has been used in the following groups of patients [2, 3] :
-
Elderly patients
-
Patients with small tumors, especially if their hearing is good
-
Patients with medical conditions that significantly increase the risk of operation
-
Patients who refuse treatment
-
Patients with a tumor on the side of an only hearing ear
Stereotactic radiotherapy uses radiation delivered to a precise point or series of points to maximize the amount of radiation delivered to target tissues while minimizing the exposure of adjacent normal tissues. It is commonly delivered as a single dose or, less commonly, as multiple, fractionated doses.
Microsurgical removal remains the treatment of choice for tumor eradication. Various surgical approaches can be used to remove acoustic tumors, including the translabyrinthine approach, the retrosigmoid approach, and the middle cranial fossa approach. Decision on type of access depends on tumor location, tumor size, hearing status, facial nerve status, and surgeon preference.
History of the Procedure
The operative mortality rate has dropped dramatically, from 40% in the early 20th century to less than 1%. With current microsurgery, postoperative facial paralysis, once the rule, is now an uncommon permanent sequelae. Attempts at hearing conservation, unimaginable at the beginning of the 20th century, are increasingly successful.
These very dramatic improvements are the result of the convergence of several factors. Vastly improved imaging techniques permitting early diagnosis, adaptation of the microscope, development of facial and auditory nerve–monitoring techniques, improved anesthesia, and improved perioperative management have all contributed to improved outcomes.
Epidemiology
Frequency
Clinically diagnosed acoustic neuromas occur in 0.7-1.0 people per 100,000 population. The incidence may be rising, a reflection of the increasing frequency with which small tumors are being diagnosed with the more widespread use of MRI. A 2005 study by Lin et al suggested the prevalence of incidental acoustic neuromas to be 2 in 10,000 people. [4] Careful autopsy studies can detect small vestibular schwannomas in a higher percentage of elderly patients, which suggests that many acoustic neuromas never become clinically apparent.
Etiology
Most patients diagnosed with an acoustic neuroma have no apparent risk factors. Exposure to high-dose ionizing radiation is the only definite environmental risk factor associated with an increased risk of developing an acoustic neuroma. Multiple studies have determined cell phone use is not associated with an increased risk of developing an acoustic neuroma, although data on the effects of long-term cell phone use are still pending. [5]
Neurofibromatosis type II occurs in individuals who have defective tumor suppressor gene located on chromosome 22q12.2. The defective protein produced by the gene is called merlin or schwannomin. Bilateral acoustic tumors are a principle clinical feature of neurofibromatosis type II, although other manifestations, including peripheral neurofibromata, meningioma, glioma, and juvenile posterior subcapsular lenticular opacities, are often present as well. Many patients with neurofibromatosis type II present in late adolescence or early adulthood but occasionally may present later in the fifth to seventh decade with slowly growing tumors.
Pathophysiology
The vast majority of acoustic neuromas develop from the Schwann cells investing the vestibular portion of the vestibulocochlear nerve. Less than 5% of tumors arise from the cochlear nerve. The superior and inferior vestibular nerves appear to be the nerves of origin with about equal frequency. Overall, three separate growth patterns can be distinguished within acoustic tumors, as follows: (1) no growth or very slow growth, (2) slow growth (ie, 2 mm/y), and (3) fast growth (ie, ≥ 1.0 cm/y on imaging studies). Although most acoustic neuromas grow slowly, rarely, a tumor may grow quickly and may double in volume within 6 months to a year.
Although some tumors adhere to one or another of these growth patterns, others appear to alternate between periods of no or slow growth and rapid growth. Tumors that have undergone cystic degeneration (presumably because they have outgrown their blood supply) are sometimes capable of relatively rapid expansion because of enlargement of their cystic component. Because acoustic tumors arise from the investing Schwann cell, tumor growth generally compresses vestibular fibers on the surface. Destruction of vestibular fibers is slow; consequently, many patients experience little or no disequilibrium or vertigo. Once the tumor has grown sufficiently large to fill the internal auditory canal, it may continue growth either by expanding bone or by extending into the cerebellopontine angle. Growth within the cerebellopontine angle is generally spherical, which is different than the sessile growth pattern seen in a meningioma of the cerebellopontine angle.
Acoustic tumors, like other space-occupying lesions, produce symptoms by any of four recognizable mechanisms: (1) compression or distortion of the spinal fluid spaces, (2) displacement of the brain stem, (3) compression of vessels producing ischemia or infarction, or (4) compression and/or attenuation of nerves.
Because the cerebellopontine angle is relatively empty, tumors can continue to grow until they reach 3-4 cm in size before they contact important structures. Growth is often sufficiently slow that the facial nerve can accommodate to the stretching imposed by tumor growth without clinically apparent deterioration of function. Tumors that arise within the internal auditory canal may produce early symptoms in the form of hearing loss or vestibular disturbance by compressing the cochlear nerve, vestibular nerve, or labyrinthine artery against the bony walls of the internal auditory canal.
As the tumor approaches 2 cm in diameter, it begins to compress the lateral surface of the brain stem. Further growth can occur only by compressing or displacing the brain stem toward the contralateral side. Tumors greater than 4 cm often extend sufficiently far anteriorly to compress the trigeminal nerve and produce facial hypesthesia. As the tumor continues to grow beyond 4 cm, progressive effacement of the cerebral aqueduct and fourth ventricle occurs with eventual development of hydrocephalus.
Indications
Treatment depends on multiple factors including the age and medical status of the patient, tumor size and location, hearing status, and patient preference. In older patients with small tumors, careful observation may be elected consisting of serial MRIs. In older patients with a growing tumor, radiosurgery may be an appropriate option. Young patients, large tumors (greater than 2.5 to 3 cm) and patients with small tumors and intact hearing may choose surgery. See Surgical therapy.
Relevant Anatomy
The cerebellopontine angle is a space filled with spinal fluid. It has the brain stem as its medial boundary, the cerebellum as its roof and posterior boundary, and the posterior surface of the temporal bone as its lateral boundary. The floor of the cerebellopontine angle is formed by the lower cranial nerves (IX-XI) and their surrounding arachnoid investments. The flocculus of the cerebellum may lie within the cerebellopontine angle and may be closely associated with cranial nerves VIII and VII as they cross the cerebellopontine angle to enter the internal auditory canal.
The facial nerve arises 2-3 mm anterior to the root entry zone of the vestibulocochlear nerve. The foramen of Luschka (ie, the opening of the lateral recess of the fourth ventricle) is located just inferior and posterior to the root entry zones of the facial and vestibulocochlear nerve. A tuft of choroid plexus can frequently be observed extruding from it. Inferior and a bit anterior to the foramen of Luschka is the olive, and just posterior to the olive lie the rootlets of origin for cranial nerves IX, X, and XI. The hypoglossal nerve exits the brain stem through a series of small rootlets anterior to the olive.
Vascular structures within the cerebellopontine angle
The most important vascular structure within the cerebellopontine angle is the anterior inferior cerebellar artery (AICA). It arises most commonly as a single trunk from the basilar artery but can arise as two separate branches. In rare cases, it originates as a branch of the posterior inferior cerebellar artery (PICA). As the AICA moves from anterior to posterior, it first follows the ventral surface of the brain stem, but within the cerebellopontine angle it takes a long loop laterally to the porus acusticus. In 15-20% of cases, the AICA actually passes into the lumen of the internal auditory canal before turning back on itself toward the posterior surface of the brain stem. (These AICA loops are not symptomatic.) The AICA can thus be divided into the premeatal, meatal, and postmeatal segments.
The main branch of the AICA passes over cranial nerves VII and VIII in only 10% of cases. The remainder of the time, it either passes below the VII and VIII cranial nerves or, in 25-50% of individuals, actually passes between them. Three branches that regularly arise from the meatal segment of the AICA can be identified. Small perforating arteries supply blood to the brain stem. The subarcuate artery passes through the subarcuate fossa into the posterior surface of the temporal bone, and the third regular branch is the internal auditory artery (labyrinthine artery). Cranial nerves VII and VIII receive their blood supply from small branches of AICA.
Two venous structures must be kept in mind during surgical procedures involving the cerebellopontine angle. The petrosal vein (of Dandy) brings returning venous blood from the cerebellum and lateral brain stem to the superior or inferior petrosal sinus. It is generally encountered in the area of the trigeminal nerve anterior to the porus acusticus. The petrosal vein often carries enough venous blood that its obstruction can lead to venous infarction and cerebellar edema, and it should be preserved if at all possible. Additional venous blood reaches the superior petrosal sinus through a series of bridging veins that cross the cerebellopontine angle. Although every attempt should be made to preserve these veins, their sacrifice is generally inconsequential.
The vein of Labbé carries returning venous blood from the inferior and lateral surface of the temporal lobe to the superior petrosal sinus, tentorial venous lakes, or the transverse sinus. Its configuration and anatomy is quite variable. However, obstruction, obliteration, or occlusion of the superior petrosal sinus may, in some cases, result in occlusion of the vein of Labbé. Sudden occlusion of the vein of Labbé carries with it a high risk of venous infarction of the temporal lobe and rapid life-threatening cerebral edema.
Nerves
The facial nerve leaves the brain stem anterior to the foramen of Luschka. As it leaves the brain stem, the fibers are sheathed in oligodendroglia derived from the central nervous system. Within a few millimeters of leaving the brain stem, however, the nerve loses its oligodendroglial ensheathment and becomes ensheathed instead by Schwann cells. Throughout the remainder of its peripheral course, it remains within its Schwann cell investment. It passes directly across the cerebellopontine angle for about 15 mm, accompanied by the vestibulocochlear nerve. It consistently enters the internal auditory canal by crossing the anterior superior margin of the porus acusticus.
The vestibulocochlear nerve arises from the brain stem slightly posterior to the facial nerve. It remains sheathed in oligodendroglia for approximately 15 mm (almost to the point at which it passes into the internal auditory canal). It has the longest oligodendroglial investment of any peripheral nerve. The junction between oligodendroglia and Schwann cells (ie, the Obersteiner-Redlich zone) occurs just medial to the porus acusticus. Because acoustic neuromas arise from Schwann cells, they arise most commonly within the most lateral portions of the cerebellopontine angle or the internal auditory canal.
The nervus intermedius (nerve of Wrisberg) leaves the brain stem together with the vestibulocochlear nerve. At some point within the cerebellopontine angle, the nervus intermedius crosses over to become associated with the facial nerve. It may do so as several separate rootlets. The point where the nervus intermedius crosses to become associated with the facial nerve shows considerably variation, but in 22% of individuals, it is adherent to the vestibulocochlear nerve for 14 mm or more. As the vestibulocochlear and facial nerve reach the porus acusticus (medial opening of the internal auditory canal) they pass together with the nervus intermedius and sometimes a loop of AICA.
Internal auditory canal
The internal auditory canal is approximately 8.5 mm in length (range 5.5-10.5 mm), lined with dura, and filled with spinal fluid. Its medial end is oval in shape and is referred to as the porus acusticus. Its lateral end is a complicated structure referred to as the fundus or lamina cribrosa. The fundus is divided into a superior and inferior half by the transverse crest. The upper half is further subdivided into an anterior and posterior segment by a vertical crest, often referred to as Bill’s Bar, named after William House, who popularized its importance as a surgical landmark. The vertical crest separates the macula cribrosa superior, a series of very small openings through which the terminal fibers of the vestibular nerve pass in order to reach the cupula of the superior semicircular canal, from the meatal foramen, which marks the point at which the facial nerve leaves the internal auditory canal and enters the fallopian canal as the labyrinthine segment.
Because the most lateral portion of the internal auditory canal is 4-5 mm inferior to the level of the geniculate ganglion, the labyrinthine segment of the facial nerve must take a vertically oriented course upward to reach it. The labyrinthine segment may be less than a millimeter wide as it passes between the cochlea and the anterior end of the superior semicircular canal. The inferior portion of the fundus is a single oval-shaped space, the anterior portion of which is occupied by a rounded depression (tractus spiralis foraminosus) filled with small openings to accommodate the terminal branches of the cochlear nerve. The posterior portion is filled with a macula crista inferior through which pass the terminal ends of the inferior vestibular nerve.
Temporal bone
The anatomy of the superior surface of the temporal bone must be mastered if middle fossa approaches are to be undertaken successfully. Laterally, the irregular superior surface of the temporal bone transitions relatively smoothly to the temporal squamosa. The free edge of the tentorium and the superior petrosal sinus attach to the medial edge of the superior surface of the temporal bone. The arcuate eminence, a bony prominence that is perpendicular to the petrous ridge and lies two centimeters medial to the squamous temporal bone, often overlies the superior semicircular canal. The arcuate eminence is often difficult to identify, especially in well-pneumatized temporal bones.
The geniculate ganglion usually lies within the substance of the temporal bone just medial to and a few millimeters anterior to the head of the malleus. The geniculate ganglion may be dehiscent, or alternatively, it may lie several millimeters beneath the superior surface of the bone. The head of the malleus is generally easy to identify if the thin bone of the tegmen tympani is removed so as to enter into the middle ear space. In difficult surgical situations, the head of the malleus can be used to identify the geniculate ganglion. The greater superficial petrosal nerve originates from the geniculate ganglion and courses anteromedially, passing over the superior surface of the temporal bone at the facial hiatus. The facial hiatus is generally 4-8 mm anterior to the geniculate ganglion. The greater superficial petrosal nerve can generally be identified in this area. It can then be followed retrograde to the geniculate ganglion.
The middle meningeal artery and associated veins traverse the foramen spinosum, which is located approximately 1 cm anterolaterally to the greater superficial petrosal nerve. The mandibular division of the trigeminal nerve traverses the foramen ovale, which lies a few millimeters anterior and medial to the foramen spinosum. The horizontal portion of the carotid canal courses through the anterior temporal bone medial to the foramen spinosum and foramen ovale. The cochlea cannot be identified from the surface appearance of the superior temporal bone. It lies just anterior and inferior to the labyrinthine segment of the facial nerve but is deep to the geniculate ganglion.
Contraindications
Few absolute contraindications to the surgical removal of an acoustic tumor exist. Serious medical illness may make surgical removal in some patients too risky. Surgery must often be performed for large tumors with brain stem shift and obstructive hydrocephalus, even in the presence of significant medical illness. The translabyrinthine approach is contraindicated in a patient with chronic otitis media.
The decision to operate should be carefully considered when the tumor is within the internal auditory canal of a patient's only hearing ear. In some cases, observing the tumor until hearing has been lost is best, while in other cases, attempting surgical removal with hearing conservation is more prudent.
-
This table shows the distribution of presenting symptoms, ie, the symptom that brought the patient to a physician and that constituted the patient's chief ailment.
-
A small acoustic neuroma within the internal auditory canal is easily observed on postgadolinium MRI.
-
These large bilateral acoustic neuromas are easily observed on MRI. This patient has neurofibromatosis II. Both tumors were eventually removed, leading to anacusis. Facial nerve function remained entirely normal bilaterally.
-
The nerves of the internal auditory canal as observed in a cadaveric dissection are shown. The posterior wall of the internal auditory canal has been removed. F indicates the facial nerve. S is the superior vestibular nerve. VIII indicates the statoacoustic nerve as it leaves the brain stem, and P indicates the posterior ampullary nerve. The hollow arrow points to the posterior lip of the boney porous acusticus, and the solid arrow indicates the position of the vestibule. C indicates the cochlear aqueduct.
-
The bone that must be removed for a middle cranial fossa approach is indicated in yellow. The tumor is in orange.
-
The bone that must be removed for a translabyrinthine approach is indicated in yellow. The tumor is in orange.
-
The bone that must be removed for a posterior fossa approach is indicated in yellow. The tumor is in orange.






