Practice Essentials
In the United States, acute otitis media (AOM), defined by convention as the first 3 weeks of a process in which the middle ear shows the signs and symptoms of acute inflammation, is the most common affliction necessitating medical therapy for children younger than 5 years. [1, 2, 3] See the image below.
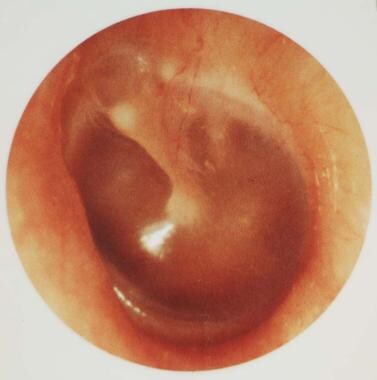
 Tympanic membrane of a person with 12 hours of ear pain, slight tympanic membrane bulge, and slight meniscus of purulent effusion at bottom of tympanic membrane. Reproduced with permission from Isaacson G: The natural history of a treated episode of acute otitis media. Pediatrics. 1996; 98(5): 968-7.
Tympanic membrane of a person with 12 hours of ear pain, slight tympanic membrane bulge, and slight meniscus of purulent effusion at bottom of tympanic membrane. Reproduced with permission from Isaacson G: The natural history of a treated episode of acute otitis media. Pediatrics. 1996; 98(5): 968-7.
Signs and symptoms
Although the history of AOM varies with age, a number of constant features manifest during the otitis-prone years, including the following:
-
Neonates: Irritability or feeding difficulties may be the only indication of a septic focus
-
Older children: This age group begins to demonstrate a consistent presence of fever and otalgia, or ear tugging
-
Older children and adults: Hearing loss becomes a constant feature of AOM and otitis media with effusion (OME); ear stuffiness is noted before the detection of middle ear fluid
Otalgia without hearing loss or fever is observed in adults with external otitis media, dental abscess, or pain referred from the temporomandibular joint. Orthodontic appliances often elicit referred pain as the dental occlusion is altered.
See Clinical Presentation for more detail.
Diagnosis
Pneumatic otoscopy is the standard of care in the diagnosis of acute and chronic otitis media. The following findings may be found on examination in patients with AOM:
-
Signs of inflammation in the tympanic membrane
-
Bulging in the posterior quadrants of the tympanic membrane may bulge; scalded appearance of the superficial epithelial layer
-
Perforated tympanic membrane (most frequently in posterior or inferior quadrants)
-
Presence of an opaque serumlike exudate oozing through the entire tympanic membrane
-
Pain with/without pulsation of the otorrhea
-
Fever
Testing
Testing in the acute phase is generally unhelpful, because all children with AOM have conductive hearing loss associated with the middle ear effusion. In addition, although tympanometry may assist in the diagnosis of middle ear effusion, this test is seldom necessary for the skilled pneumatic otoscopist.
Culture and sensitivity of a specimen from a fresh perforation or a tympanocentesis may be helpful.
Imaging studies
Radiologic studies are generally unnecessary in uncomplicated AOM. However, CT scanning may be necessary to determine if a complication has occurred. MRI might be more appropriate for diagnosing suspected intracranial complications.
Procedures
Tympanocentesis involves aspiration of the contents of the middle ear cleft by piercing the tympanic membrane with a needle and collecting that material for diagnostic examination.
Tympanocentesis should be performed in the following patients with AOM:
-
Neonates who are younger than 6 weeks (and therefore are more likely to have an unusual or more invasive pathogen)
-
Immunosuppressed or immunocompromised patients
-
Patients in whom adequate antimicrobial treatment has failed and who continue to show signs of local or systemic sepsis
-
Patients with a complication that requires a culture for adequate therapy
See Workup for more detail.
Management
Pharmacotherapy
Antibiotics are the only medications with demonstrated efficacy in the management of AOM; therefore, these agents are the initial therapy of choice. The antibiotic chosen should cover most of the common bacterial pathogens and be individualized for the child with regard to allergy, tolerance, previous exposure to antibiotics, cost, and community resistance levels. Duration of treatment may also be a consideration in the choice of antibiotic. [4]
Antibiotics used in the management of AOM include the following:
-
Amoxicillin
-
Amoxicillin/clavulanate
-
Erythromycin base/sulfisoxazole
-
Trimethoprim-sulfamethoxazole
-
Cefixime
-
Cefuroxime axetil
-
Cefprozil
-
Cefpodoxime
-
Cefdinir
-
Clindamycin
-
Clarithromycin
-
Azithromycin
-
Ceftriaxone
Surgery
Surgical management of AOM can be divided into the following 3 related procedures:
-
Tympanocentesis
-
Myringotomy
-
Myringotomy with insertion of a ventilating tube
Selection of the appropriate procedure results from evaluation of patient factors, surgeon factors, available resources, and urgency.
See Treatment and Medication for more detail.
Background
In the United States, acute otitis media (AOM) is the most common affliction necessitating medical therapy for children younger than 5 years. The total annual cost to society for this disease and for otitis media with effusion (OME) runs into the billions of dollars. Yet, despite research into prevention and therapy, the costs of this disease continue to rise while the incidence remains unabated.
AOM is defined by convention as the first 3 weeks of a process in which the middle ear shows the signs and symptoms of acute inflammation. OME is defined as the presence of fluid in the middle ear with accompanying conductive hearing loss and without concomitant symptoms or signs of acuity. OME is classified as subacute when it persists from 3 weeks to 3 months after the onset of AOM and is classified as chronic thereafter. [1, 2]
The emergence of antimicrobial-resistant bacteria requires reevaluation of traditional management. Nevertheless, there is still a consensus that antibiotics are the initial therapy of choice for AOM. Surgical management of AOM can conveniently be divided into 3 related procedures: tympanocentesis, myringotomy, and myringotomy with insertion of a ventilating tube.
For patient education resources, visit the Headache and Migraine Center and the Oral Health Center. See also the eMedicineHealth articles Sinus Infection and Teething.
Anatomy
Incision of the tympanic membrane is primarily governed by the relations of the structures behind the membrane (see the images below). The tympanic membrane can be divided into quadrants with an imaginary line drawn vertically along the long process of the malleus and extending to the inferior annulus, along with a horizontal line at the umbo. Generally, it can safely be incised in all quadrants except the posterior superior section, behind which lie the incus and stapes, structures that might be injured inadvertently by incision in this area. The area above the pars tensa, the pars flaccida, should be avoided.
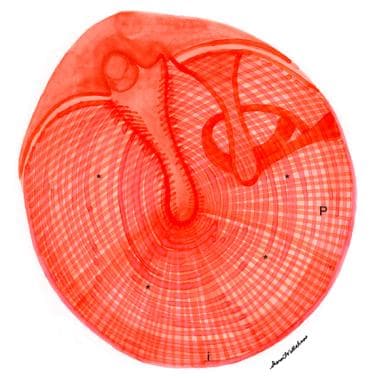 Drawing of normal right tympanic membrane. Note outward curvature of pars tensa (*) of eardrum. Tympanic annulus is indicated anteriorly (a), inferiorly (i), and posteriorly (P). M = long process of malleus; I = incus; L = lateral (short) process of malleus.
Drawing of normal right tympanic membrane. Note outward curvature of pars tensa (*) of eardrum. Tympanic annulus is indicated anteriorly (a), inferiorly (i), and posteriorly (P). M = long process of malleus; I = incus; L = lateral (short) process of malleus.
Two other structures, the facial nerve and the round window, are generally protected from any but the clumsiest of surgeons, the former by its high position in the middle ear and the latter by the overhanging niche.
Tubes are generally placed anteriorly, either superiorly or inferiorly. Because the posterior segments are deeper and have more vibratory motion, posterior placement gives a greater dampening effect. Anteriorly, any incision should avoid exposure of the malleus, the malleolar ligament, and the annulus; such exposure creates a greater tendency for perforations to persist after extrusion of the tube.
Pathophysiology
Obstruction of the eustachian tube appears to be the most important antecedent event associated with AOM. The vast majority of AOM episodes are triggered by an upper respiratory tract infection (URTI) involving the nasopharynx.
Viral and bacterial infection
The infection is usually of viral origin, but allergic and other inflammatory conditions involving the eustachian tube may create a similar outcome. Inflammation in the nasopharynx extends to the medial end of the eustachian tube, creating stasis and inflammation, which, in turn, alter the pressure within the middle ear. These changes may be either negative (most common) or positive, relative to ambient pressure.
Stasis also permits pathogenic bacteria to colonize the normally sterile middle ear space through direct extension from the nasopharynx by reflux, aspiration, or active insufflation.
The response is the establishment of an acute inflammatory reaction characterized by typical vasodilatation, exudation, leukocyte invasion, phagocytosis, and local immunologic responses within the middle ear cleft, which yields the clinical pattern of AOM.
In a minority of otitis-prone children, the eustachian tube is patulous or hypotonic. Children with neuromuscular disorders or abnormalities of the first or second arch are most likely “too open” and are therefore predisposed to reflux of nasopharyngeal contents into the middle ear cleft.
To become pathogenic in hollow organs, such as the ear or sinus, most bacteria must adhere to the mucosal lining. Viral infections that attack and damage mucosal linings of respiratory tracts may facilitate the ability of the bacteria to become pathogenic in the nasopharynx, eustachian tube, and middle ear cleft.
This theory might explain why viral antigens are commonly recovered from middle ear aspirates in children with AOM but the actual virus is only rarely isolated. Data have also been presented indicating that mucosal damage by endotoxins secreted by bacterial invaders may similarly enhance the adhesion of pathogens to mucosal surfaces.
Viral infection in the nasopharynx with subsequent inflammation of the orifice and mucosa of the eustachian tube has long been understood as part of the pathogenesis of AOM, although the complete role of the virus is not fully understood. Concurrent or antecedent URTIs are identified in at least a quarter of all attacks of AOM in children, but the virus itself seldom appears as the pathogen in the middle ear. Administration of trivalent influenza A vaccine has been shown to reduce the frequency of AOM during the influenza season. [5]
Viruses have been recovered with increasing frequency as techniques to identify them by direct culture and by indirect means (eg, enzyme-linked immunosorbent assay [ELISA]) have improved. On direct culture, the yield is less than 10%, with the respiratory syncytial virus (RSV) recovered most frequently; the influenza virus is a distant second. On ELISA, the presence of viral antigens is detected in approximately a quarter of middle ear aspirates; again, RSV is the virus most frequently detected by this method.
The presence of viruses in the middle ear effusion may influence the outcome of therapy for otitis media. Results of outcome studies have been mixed, ranging from no effect to evidence of prolongation of acuity and effusion when viruses are present in persons with AOM.
Immunologic factors
Immunologic activity may play a significant role in the frequency of AOM and its outcome. Although most research has focused on the immunologic aspects of OME, certain relations between AOM and the patient’s immune status have been demonstrated, as follows:
-
Production of antibodies may promote clearance of a middle ear effusion after an acute attack
-
Previous exposure or immunization may have a preventative role by suppressing colonization of the nasopharynx by pathogens
-
The formation of antibodies during an attack may prevent or modify future attacks; unfortunately, antibodies to both Streptococcus pneumoniae and Haemophilus influenzae are of the polysaccharide type and the ability to produce them develops late unless conjugated to proteins
-
Minor or transient immunologic defects may give rise to recurrent otitis media
Much attention has been focused on the immunoglobulins and the patient’s ability to form them. Immunoglobulin G2 (IgG2) and immunoglobulin G4 (IgG4) are responsible for immunity against polysaccharide antigens; deficiencies in the formation of these antibodies invariably lead to otitis media. Many patients with Down syndrome show decreased function of immunoglobulin A (IgA), IgG2, or IgG4, which partially explains their increased risk for chronic rhinitis and otitis media.
The immunologic aspects of AOM are not confined to the middle ear. The nasopharynx plays an important role in the pathogenesis of AOM, and immunologic modifications in this lymphoid tissue provide some protection from pathogens by preventing their adherence to mucosal surfaces. The presence of nasopharyngeal IgA antibodies to pneumolysin toxin released by pneumococcal autolysis appears to protect against invasion by healthy pneumococci.
On the other hand, not all immunoglobulins in the nasopharynx are protective. Bernstein describes the effects of immunoglobulin E (IgE) hypersensitivity or hyperimmune effects on the eustachian tube mucosa. [6] The allergic response in the nasopharyngeal end of the eustachian tube promotes stasis and the subsequent formation of a middle ear effusion.
A study by Pichichero et al indicated that children with recurrent AOM in the first years of life are more likely to suffer from viral and bacterial respiratory illnesses, with the investigators suggesting that innate and adaptive immune system dysfunction is the cause. Youngsters in the study were between age 6 months and 5 years. Those who were stringently defined as being prone to otitis had a frequency of lobar pneumonia, sinusitis, and influenza that was 6-fold, 2.1-fold, and 2.9-fold higher, respectively, than in non-prone children. Among the otitis-prone patients, the frequency of physician-diagnosed, medically attended respiratory infection illness visits was 2.42-fold, 2.20-fold, and 2.42-fold greater for patients aged 6-18 months, 18-30 months, and 30-42 months, respectively. [7]
Etiology
Viral pathogens
RSV is a large RNA paramyxovirus that is most commonly associated with bronchiolitis and pneumonia in very young persons, though it may cause acute respiratory disease in persons of any age group. [8, 9, 10] In northern climates, RSV is normally identified during annual epidemics in the winter and early spring, but it should be considered in any neonate with lethargy, irritability, or apnea, with or without otitis media. In older infants and children, respiratory symptoms are usually more prominent, making diagnosis easier.
RSV was identified early as a pathogen that appeared to create long-term pulmonary complications, primarily asthma, in as many as half of infants with bronchiolitis. RSV may be particularly lethal for children with congenital heart disease, cystic fibrosis, immunodeficiency, bronchopulmonary dysplasia, or prematurity of less than 37 weeks’ gestational age.
RSV-specific intravenous (IV) immunoglobulin prophylaxis is recommended only for high-risk children. When treating a child with concomitant pneumonia or other systemic disease and otitis media, the practitioner must ensure appropriate diagnosis and management of all aspects of the child’s illness. Drainage of the ear by tympanocentesis or myringotomy for culture and therapy may be necessary in some cases. Drainage is mandatory in neonates who are suspected to be in a septic state or in children who are immunosuppressed.
Bacterial pathogens
Pathogenic bacteria are recovered from the middle ear effusion in at least half the children with AOM, and bacterial DNA or cell wall debris is found in another quarter to a third of specimens previously classified as sterile. Four bacteria—namely, S pneumoniae, H influenzae, Moraxella catarrhalis, and Streptococcus pyogenes —are responsible for the majority of episodes of AOM in persons older than 6 weeks. Other bacteria recovered and implicated in AOM include Staphylococcus aureus, viridans streptococci, and Pseudomonas aeruginosa.
The emergence of resistance to antimicrobial agents is of increasing importance in the management of AOM and other bacterial illnesses. [11] The various mechanisms used by bacteria to confer this resistance will be delineated as the common pathologic agents linked to AOM are described.
Streptococcus pneumoniae
S pneumoniae is the most common etiologic agent responsible for AOM and for invasive bacterial infections in children of all age groups. [12] It is a gram-positive diplococcus with 90 identified serotypes (classified on the basis of the polysaccharide antigen), the frequency of which varies between age groups and geography. On direct culture, various studies have shown these bacteria to be responsible for 29-40% of isolates, but additionally pneumococcal antigens are recovered from approximately a third of those cultures classified as sterile.
Pneumococcal infections are probably responsible for at least 50% of AOM episodes. Serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F are responsible for most invasive pneumococcal disease in America; in ear aspirates from patients with AOM, serotypes 19 (23%), 23 (12.5%), 6 (12%), 14 (10%), 3 (8.5%), and 18 (6%) are isolated most commonly. The polyvalent pneumococcal vaccine confers immunity to approximately 85% of those serotypes responsible for AOM.
S pneumoniae was once susceptible to almost all common antibiotics, including penicillin G, erythromycin, and most sulfonamides. Alteration of the cell wall’s penicillin-binding protein (the antimicrobial target) has led to the appearance of multidrug-resistant S pneumoniae (MDRSP), which is resistant to beta-lactam compounds, macrolides, and sulfonamides. Resistance rates as high as 40% have been reported for these 3 antimicrobial groups. Serotypes 6B, 9V, 14, 19A, 19F, and 23F have the highest frequency of penicillin resistance.
Ceftriaxone, cefotaxime, rifampin, and vancomycin still appear to have therapeutic efficacy, as does immunization with polyvalent pneumococcal vaccine for prevention. Unfortunately, polysaccharide antigens are not immunogenic early in life. To overcome this problem, conjugated antigens, in which the polysaccharide antigen is attached to a protein carrier, may be administered to induce production of antibodies to these polysaccharides. Some conjugated antigens (eg, vaccinations for H influenzae type b [Hib]) are in widespread use.
A heptavalent vaccine for S pneumoniae is now in widespread use and appears to have made an impact on the number of cases of invasive pneumococcal disease. This vaccine confers long-term immunity to 7 of the most common and invasive strains. Emerging evidence suggests that other serotypes are beginning to be recovered more frequently in ear and sinus infections. This might render the vaccine less useful in future years. In North America, this vaccine has now been replaced by an updated 13-valent vaccine that contains conjugated antigenic material for 6 of those additional serotypes of the pneumococcus.
Haemophilus influenzae
In middle ear aspirates from patients with AOM, H influenzae is the second most frequently isolated bacterium and is responsible for approximately 20% of episodes in preschool children. [13] The frequency may be higher in otitis-prone children, older children, and adults who have received the pneumococcal vaccine.
A study by Martin et al looking at AOM cases between 1999 and 2014 in children aged 6-23 months found that, while nasopharyngeal colonization with S pneumoniae has reportedly decreased since pneumococcal conjugate vaccines (PCVs) were introduced, colonization with H influenzae in the study subjects initially increased before dropping back to levels seen prior to routine administration of 7-valent PCV (PCV7). The investigators obtained nasopharyngeal cultures from four cohorts of children with AOM. The first cohort was cultured in 1999-2000, before routine PCV7 use, while in the second (2003-2005) and third (2006-2009) cohorts, two or more doses of PCV7 were administered to 93% and 100% of children, respectively, and in the fourth cohort (2012-2014), 100% of the children received two or more doses of 13-valent PCV (PCV13). Nasopharyngeal colonization with H influenzae in cohorts 1, 2, 3, and 4 occurred in 26%, 41%, 33%, and 29% of children, respectively. [14]
The bacterium is a small, pleomorphic, gram-negative coccobacillus. Those bacteria encapsulated with a polysaccharide coating are classified into 6 distinct types (a-f); nonencapsulated types are referred to as nontypeable and are responsible for the great majority of AOM episodes. (The nonencapsulated strains have been subtyped biochemically and antigenically, but, to date, this classification has limited clinical application.)
Traditionally, Hib has been found responsible for most invasive illnesses attributed to these bacteria and for meningitis, epiglottitis, and septicemia. Hib accounts for only 10% of all episodes of AOM in which H influenzae is recovered. In areas of the world where the aforementioned Hib-conjugated vaccine is administered early in life, risks from this potentially lethal strain have greatly diminished.
Antimicrobial resistance in Hib is conferred almost exclusively (95%) by the formation of a single enzyme, triethylenemelamine 1 lactamase, which, in some series, is secreted by as many as 40% of all nontypeable strains. This resistance is overcome relatively easily by using blocking agents, extended-coverage cephalosporins, broad-spectrum macrolides, or sulfonamides.
H influenzae may participate more widely in head and neck infections than was once believed. One of the principal mechanisms is related to the ability of the bacterium to hide and recover from antibiotic action by forming a mucous complex known as a biofilm. Research has focused on enhancing penetration of or dissolving the protective biofilm.
Moraxella catarrhalis
In the mid-1970s, M catarrhalis was classified as nonpathogenic in middle ear infections, even though under its previous name, Neisseria catarrhalis, it constituted approximately 10% of all isolates from middle ear aspirates. At that time, M catarrhalis was almost universally susceptible to ampicillin-type penicillins. After 20 years and 2 name changes (from N catarrhalis to Branhamella catarrhalis to M catarrhalis), it is isolated in up to a quarter of children with AOM, and resistance to the ampicillin-type beta-lactams is almost universal.
M catarrhalis is a gram-negative diplococcus and is considered part of the normal flora of the human upper respiratory tract. Resistance is conferred by the secretion of multiple isoenzymes of lactamase, which may be plasmid or chromosomal in origin and which may be inducible (ie, present only in low levels until a substrate is provided). More than 1 isoenzyme may be secreted by a single bacterium.
At present, almost all forms are blocked by clavulanic acid, and most are still susceptible to sulfonamides, lactamase-stable cephalosporins, or broad-spectrum macrolides. M catarrhalis is often found to coexist with other airway pathogens. The lactamases (cephalosporinases) that M catarrhalis secretes may protect those other bacteria from antimicrobial agents to which the second target pathogen might ordinarily be susceptible.
A study by Chonmaitree et al of 367 infants (followed for 286 child-years) indicated that bacterial-viral interactions are associated with AOM, with such interactions between M catarrhalis and various respiratory viruses having been found in the report to affect the risk of upper respiratory tract infection and AOM. [15]
Streptococcus pyogenes
Although S pyogenes (a gram-positive coccus that constitutes the group A streptococci [GAS] in the Lancefield classification), is still the fourth most commonly isolated bacterial pathogen from ears with AOM, it has shown a steady decline in frequency of recovery from the ear and in virulence over the past half-century. Similarly, a substantial decline in the major complications of streptococcal infection, rheumatic fever, glomerulonephritis, and scarlet fever has occurred.
S pyogenes may be associated with streptococcal toxic shock syndrome, which may include coagulopathy, soft tissue necrosis or fasciitis, desquamating rash, and liver or renal involvement. [16] It is primarily a pathogen of the pharynx, with more than 80 distinct M-protein strains identified. Currently, with the improvement in primary care and the availability of rapid identification tests, early aggressive treatment is normally instituted against this bacterium, which has shown minimal ability to develop resistance to antimicrobial agents.
Acute necrotic otitis media was associated with scarlet fever in the early 1900s; however, the condition was also associated with measles, pneumonia, and influenza. Generally, the patient was extremely ill with the systemic component of the disease and presented with a spontaneous perforation shortly after the onset of otalgia.
Early inspection of the ear would show the perforation to be moderate to large; within days, significant evidence of tissue necrosis would be observed, perhaps including the entire tympanic membrane, ossicles, the tympanic mucoperiosteum, or the bone of the mastoid air cells. The patient would demonstrate a marked conductive hearing loss, although sensorineural loss was not uncommon.
Pathologically, the ear showed a marked paucity of the normal vascular proliferation associated with an inflammatory reaction. Instead, a complete loss of the vascularity normally associated with vasculitis or toxin exposure occurred. Healing was never normal; tissue was replaced by epithelial invasion or scar tissue formation.
In industrialized societies, acute necrotic otitis media is now primarily of historic interest. The disease is still reported in aboriginal populations living in areas where modern medicine has not yet penetrated.
In the preantibiotic era, S pyogenes also appeared to be the organism most commonly recovered from patients with acute coalescent mastoiditis. In the 1990s, S pyogenes relinquished this distinction to S pneumoniae, but it remains a prominent pathogen when this disease is encountered in very young persons.
Other aerobes
Except in neonates and children with chronic disease, few other pathogens have been demonstrated in aspirates from the middle ears of immunologically intact individuals.
S aureus is rarely recovered, except in Japan, where studies indicate a somewhat higher incidence (up to 10%). Mycobacterium tuberculosis is most often associated with chronic otitis media but should be considered when a patient presents with painless otorrhea as an initial complaint and/or has multiple tympanic perforations. Any patient with a compromised immune system may be at risk for this opportunistic infection. Chlamydia pneumonia is an uncommon but significant pathogen in persons with AOM and responds only to macrolide therapy.
Anaerobes
Anaerobic bacteria have been recovered from the middle ears of children with AOM, but the data do not support a prominent role for these microorganisms in persons with otitis media, at least in the acute form. They may, however, play a greater role in chronic inflammation of the adenoid bed and biofilm formation. When recovered from ears of children with AOM, the anaerobic pathogen most often is not the sole pathogen cultured.
Common bacterial pathogens in neonatal period
In the perinatal period, the Escherichia coli, Enterococcus species, and group B streptococci are the etiologic agents most commonly responsible for sepsis and meningitis. These agents are often recovered from the middle ear, though the total percentage is probably less than 10% of neonates with AOM.
S pneumoniae remains the most common pathogen responsible for AOM in all age groups, including neonates. The nonencapsulated H influenzae and nontypeable varieties may be invasive in these infants and constitute the second most common pathogens recovered from the ear.
Because bacteremia is common in all neonates with AOM, tympanocentesis should be performed for both diagnosis and therapy in any infant with signs of AOM or generalized sepsis and any middle ear effusion. This should be part of any septic workup in neonates.
Risk factors
The following are proven risk factors for otitis media:
-
Prematurity and low birth weight
-
Young age
-
Early onset
-
Family history
-
Race - Native American, Inuit, Australian aborigine
-
Altered immunity
-
Craniofacial abnormalities
-
Neuromuscular disease
-
Allergy
-
Day care
-
Crowded living conditions
-
Low socioeconomic status
-
Tobacco and pollutant exposure
-
Use of pacifier
-
Prone sleeping position
-
Fall or winter season
-
Absence of breastfeeding, prolonged bottle use
Epidemiology
In the United States, 70% of all children experience one or more attacks of AOM before their second birthday. A study from Pittsburgh that prospectively followed urban and rural children for the first 2 years of life determined that the incidence of middle ear effusion episodes is approximately 48% at age 6 months, 79% at age 1 year, and 91% at age 2 years. [17]
The peak incidence of AOM is in children aged 3-18 months. Some infants may experience their first attack shortly after birth and are considered otitis-prone (ie, at risk for recurrent otitis media). A study by Megged et al found that 30% of pediatric patients who had neonatal AOM suffered from recurrent AOM later in childhood, compared with 10% of controls. [18]
In the Pittsburgh study, the incidence of AOM was highest among poor urban children. Differences in incidence between nations are influenced by racial, socioeconomic, and climatic factors.
Using 2011-2018 National Health Interview Survey (NHIS) data, a study by Cwalina et al indicated that children in the United States with recurrent AOM are more likely to be experiencing food insecurity if they are older, Black, in a lower-income household, and on public insurance or uninsured, and if they have a self-reported health status of only poor to fair. The investigators suggested that food insecurity screening might be incorporated by otolaryngologists into patient visits for recurrent AOM. [19]
Age-, sex-, and race-related demographics
Children aged 6-11 months appear particularly susceptible to AOM, with frequency declining around age 18-20 months. The incidence is slightly higher in boys than in girls. A small percentage of children develop this disease later in life, often in the fourth and early fifth year. After the eruption of permanent teeth, incidence drops dramatically, although some otitis-prone individuals continue to have acute episodes into adulthood. Occasionally, an adult with an acute viral URTI but no previous history of ear disease presents with AOM.
Definite racial differences exist in the incidence of AOM. Native Americans and Inuits have very high rates of acute and chronic ear infection, whereas African Americans appear to have a slightly lower rate than white children living in the same communities.
Prognosis
Death from AOM is rare in the era of modern medicine. With effective antibiotic therapy, the systemic signs of fever and lethargy should begin to dissipate, along with the localized pain, within 48 hours. Children with fewer than 3 episodes are 3 times more likely to resolve with a single course of antibiotics, as are children who develop AOM in nonwinter months. Typically, patients eventually recover the conductive hearing loss associated with AOM.
Middle ear effusion and conductive hearing loss can be expected to persist well beyond the duration of therapy, with up to 70% of children expected to have middle ear effusion after 14 days, 50% at 1 month, 20% at 2 months, and 10% after 3 months, irrespective of therapy.
In most instances, persistent middle ear effusion can merely be observed without antimicrobial therapy; however, a second course of either the same antibiotic or a drug of a different mechanism of action may be warranted to prevent a relapse before resolution.
-
Healthy tympanic membrane.
-
Tympanic membrane of a person with 12 hours of ear pain, slight tympanic membrane bulge, and slight meniscus of purulent effusion at bottom of tympanic membrane. Reproduced with permission from Isaacson G: The natural history of a treated episode of acute otitis media. Pediatrics. 1996; 98(5): 968-7.
-
Drawing of normal right tympanic membrane. Note outward curvature of pars tensa (*) of eardrum. Tympanic annulus is indicated anteriorly (a), inferiorly (i), and posteriorly (P). M = long process of malleus; I = incus; L = lateral (short) process of malleus.