Background
Cleft palate is a congenital deformity that causes a multitude of problems and represents a special challenge to the medical community. Special care is needed for patients with cleft palate. Speech production, feeding, maxillofacial growth, and dentition are just a few important developmental stages that may be affected. [1, 2]
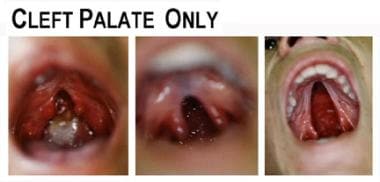
Multiple specialists make up the team that works together to improve the quality of life for patients with cleft palate. Otolaryngologists, oromaxillofacial surgeons, plastic surgeons, nutritionists, and speech pathologists are just a few of the members of the team. Psychological effects on both the patient and the parents are important aspects that also need to be addressed. A significant number of patients with cleft palate have associated syndromes that may result in cardiac, limb, or other system defects. Although cleft palate deformity was described hundreds of years ago, to this day, no agreed-upon management algorithm exists for patients with cleft palate. See the image below.
History of the Procedure
The first recorded operation on a palate was performed in 500 AD for inflammation of the uvula. For centuries, literature and interest in clefts were lacking because the deformity was thought to be due to syphilis.
Misconception also existed regarding the genetic predisposition. In 1887, the following passage was published in Lancet:
George Williamson stated that "[the fourth] law of heredity that [imposes] hideous physical impressions on the mind of a mother are [sic] capable of producing deformity and monstrosity in the offspring." As evidence, he introduced a report by Dr Child that described "a mother attending a penny show, where a trained horse pulled the trigger of a pistol, pretending to shoot a rabbit. A dummy was thrown out; back of its head was bleeding, having all the appearance been shot off." The woman bore a child resembling a rabbit.
Pare first described the use of obturators for palatal perforations in 1564. In 1552, Jacques Houllier proposed that the cleft edges be sutured together. However, it was not until 1764 that LeMonnier, a French dentist, performed the first successful repair of a cleft velum. Dieffenbach closed both the hard palate and the soft palate in 1834. von Langenbeck first described cleft palate closures with the use of mucoperiosteal flaps in 1861. In 1868, Billroth thought that fracturing the hamulus would enable better outcomes in surgery. Further modifications of the von Langenbeck technique came from Gillies, Fry, Kilner, Wardill, Veau, and Dorrance. The debate over the timing of closure led to a short break in early surgical repair. However, in 1944, Schweckendiek again began closing cleft defects in young patients.
Epidemiology
Frequency
Cleft deformities of the palate are among the most common congenital malformations. A cleft palate can be diagnosed as early as the 17th week of gestation by means of ultrasonography. Although many studies exist, the exact environmental and genetic factors that play a role are still largely unknown. [3]
Among the total number of clefts, 20% are an isolated cleft lip (18% unilateral, 2% bilateral), 50% are a cleft lip and palate (38% unilateral, 12% bilateral), and 30% are a cleft palate alone. The incidence of isolated cleft palate (without cleft lip) is 1 case in 2000 live births. Submucous cleft palate is more common, with an incidence of 1 case in 1200-2000 patients, depending on the study population. Bifid uvula occurs in 1 of 80 patients and often occurs in isolation, with no clefting of the palatal muscles.
No racial predilection exists for cleft palate, with an equal incidence among all races. Although cleft lip and palate together occur more commonly in males, isolated cleft palate is more common in females.
Etiology
Palate formation begins at the end of the fifth week of gestation. [4] At this stage, the palate consists of 2 parts, namely, the anterior (primary) palate and the posterior (secondary) palate. The medial nasal prominences form the intermaxillary (premaxillary) segment, which comprises the primary palate and incisor teeth. The primary palate extends posteriorly to the incisive foramen.
The secondary palate, which is formed by the lateral palatal processes, begins at the incisive foramen and contains a bony section and a muscular section. The lateral palatine processes appear at about the sixth week of gestation. They comprise the deep portions of the maxillary prominence that form 2 horizontal structures or palatal shelves, which ultimately are derivatives of the first branchial arch. These shelves are originally on either side of the tongue. As the tongue moves downward in the seventh week of gestation, the lateral processes grow medially. Fusion of the hard palate begins anteriorly and continues posteriorly in the eighth week of gestation. [5]
A number of processes are involved in the fusion of the 2 processes. Programmed cell death at the free edges and production of a sticky coat of glycoproteins and desmosomes provide an ideal bonding surface interface. The left side tends to lag behind the right side, leading to a propensity for left-sided clefts. The nasal septum subsequently grows downward into the newly formed palate. The process is completed between the 9th and 12th weeks of gestation.
Bone begins to form in the anterior palate first and extends posteriorly. The soft palate and the uvula, which make up the posterior portion of the secondary palate, develop during the eighth week of gestation. The tensor veli palatini develop, followed by the musculus uvula. These structures are completed by the 17th week of gestation.
The genetic basis of cleft deformity is most likely heterogeneous and multifactorial. [3] Autosomal recessive, autosomal dominant, and X-linked inheritance patterns have been described. For all parents, the odds of having a child with a cleft are 1 in 700. In families in which no first-degree relatives are affected, the recurrence rate for a cleft lip or palate in subsequent children is 2.5%. When one first-degree relative is affected, the rate of recurrence is 10%. Similar recurrence rates (10-12%) occur in offspring of persons born with cleft deformities. If the cleft is part of an autosomal dominant syndrome, the recurrence rate can be as high as 50%. A cleft deformity is associated with a syndrome in 30% of cases. More than 400 syndromes with a cleft deformity as one of the characteristics have been described.
As previously mentioned, the etiology of the cleft palate is not well understood; however, some evidence exists that external factors may play a role. Relatively few of the many recognized teratogens cause cleft palates. Alcohol consumption in the embryologic period does result in many infants with clefts. Other teratogens associated with cleft palates include phenytoin, retinoids, and illegal drugs (eg, cocaine). Mechanically induced clefts can occur in utero by means of direct impingement on the embryo.
Genetic mapping of families with inherited forms of cleft palate has resulted in the identification of genes involved in palate development. Cleft palate associated with ankyloglossia, an X-linked disorder, was shown to be caused by mutations of the TBX22 gene. TBX22 is a member of the T-box gene family, which are transcription factors in vertebrates involved with mesoderm direction. Specifically, TBX22 is expressed in the palatal shelves just prior to their elevation above the tongue. Mutations in this gene result in cleft palate due to loss of TBX22 function.
Presentation
Most overt clefts of the hard palate and/or the soft palate are discovered at birth and are often manifested by feeding difficulties. Suckling may be compromised by the loss of an oral seal on the nipple. Cleft palate, especially when associated with mandibular hypoplasia (as with a Pierre Robin sequence), may also cause airway difficulties because the tongue prolapses through the cleft into the nasal cavity and the posterior oropharynx.
Partial clefts of the soft palate or submucous clefts may be overlooked in neonates because they may be asymptomatic. Early manifestations include nasal reflux of liquids or food. Later, as speech develops, hypernasal speech or nasal emission may result.
Indications
Major clefts of the hard palate and/or the soft palate are repaired surgically before the patient is aged 1 year. Instances in which this does not occur include those with complicating medical conditions, such as congenital heart disease or airway compromise. Cleft repair is deferred for cardiac conditions that may be compromised by a change in upper airway resistance. When upper airway obstruction is a major problem, such as with a Pierre Robin sequence, a tracheotomy may be necessary. Cleft repair can then be accomplished with a secure airway.
When a submucous cleft is present, the indications for surgery concern velar competence. Often, the decision to repair a submucous cleft palate is deferred until the patient is aged 4-5 years, when speech development is sufficient to determine the degree of hypernasality and the effect of the cleft on intelligibility. Cleft repair at this age may involve a pharyngeal flap, depending on the amount of velopharyngeal incompetence present. [6]
Relevant Anatomy
The role of the palate is to provide a barrier between the nasal and oral portions of the respiratory tract. Velar actions with deglutition, respiration, and phonation are similar to those of a sphincter; hence, the velopharyngeal mechanism is often termed the velopharyngeal sphincter.
Familiarity with the anatomy of the palate is essential in understanding functional and surgical repair. Blood is supplied to the hard palate by the greater palatine artery, which enters via the greater palatine foramen. The lesser palatine artery and nerves pass through the lesser palatine foramen. Nerve supply originates from the maxillary branches from the trigeminal nerve, which forms a plexus that innervates the palatal muscles. Contributions from cranial nerves VII and IX enter posterior to the plexus.
The palatine aponeurosis is the principal structural element within the velopharynx. It provides an anchoring point for muscles, adding a degree of stiffness, and is continuous laterally around to the hamulus with the tensor veli palatini muscle. The aponeurosis is diamond shaped. More posterolaterally, the salpingopalatine ligament, the fascia of Tröltsch, and the internal fascia of the pharynx (which all form the membranous portion of the eustachian tube) contribute to the velopharynx.
The normal structure and function of the soft palate is dependent on the levator sling. This structure comprises portions of the tensor veli palatini, palatoglossal, palatopharyngeal, and uvular muscles. Functionally, the levator veli palatini, palatoglossus, and musculus uvulae muscles either elevate the soft palate or alter its shape. Other muscles, such as the superior constrictor, palatopharyngeus, palatothyroideus, and salpingopharyngeus muscles, are involved with movements of the lateral and posterior pharyngeal walls. The tensor veli palatini is involved mainly with middle ear aeration. In patients with cleft palate, the muscle attachments are directed anteriorly and attach onto the posterior portion of the bony palate. These fibers must be surgically reoriented to achieve proper palatal function.
-
Examples of cleft palate.
-
Submucous clef palate