Background
Metastatic neck disease is the most important factor in the spread of head and neck squamous cell carcinoma from primary sites. The primary sites most commonly involved in the spread of this carcinoma are the mucosal areas of the upper aerodigestive tract, particularly the larynx, oropharynx, hypopharynx, and oral cavity.
Lymph node metastasis reduces the survival rate of patients with squamous cell carcinoma by half. The survival rate is less than 5% in patients who previously underwent surgery and have a recurrent metastasis in the neck. Therefore, the control of the neck is one of the most important aspects in the successful management of these particular tumors.
Radical neck dissection is an operation that was created in 1906 to solve the problem of metastatic neck disease. It is a well-designed operation that is relatively easy for the trained head and neck surgeon to learn and to perform. Classic radical neck dissection is still the criterion standard for surgical control of a neck metastasis.
See the image below.
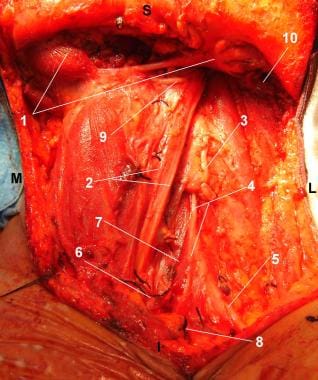 Patient in supine position and head turned to the right side. Radical Left Neck Dissection completed: The classical radical neck dissection encompasses the lymphatic nodes in levels I-V. View of the surgical wound after removal of the operative monoblock specimen. S = Superior. I = Inferior. M = Medial. L = Lateral. (1) Anterior and posterior bellies of the digastric muscle. (2) Carotid artery and vagus nerve. (3) Anterior cervical nerve root. (4) Phrenic nerve. (5) Brachial plexus. (6) Internal jugular vein, inferior aspect, cut and ligated. (7) Anterior scalene muscle. (8) External jugular vein, inferior aspect, cut and ligated. (9) Hypoglossal nerve. (10) Sternocleidomastoid muscle, superior aspect, cut.
Patient in supine position and head turned to the right side. Radical Left Neck Dissection completed: The classical radical neck dissection encompasses the lymphatic nodes in levels I-V. View of the surgical wound after removal of the operative monoblock specimen. S = Superior. I = Inferior. M = Medial. L = Lateral. (1) Anterior and posterior bellies of the digastric muscle. (2) Carotid artery and vagus nerve. (3) Anterior cervical nerve root. (4) Phrenic nerve. (5) Brachial plexus. (6) Internal jugular vein, inferior aspect, cut and ligated. (7) Anterior scalene muscle. (8) External jugular vein, inferior aspect, cut and ligated. (9) Hypoglossal nerve. (10) Sternocleidomastoid muscle, superior aspect, cut.
In the last 3 decades, progressive advances have occurred in the understanding of cervical fascial planes, lymphatic drainage patterns, preoperative staging, and extracapsular spread. A concern to maximize control and to minimize morbidity has prompted modifications to the classic neck dissection. One such modification is the preservation of 1 or more nonlymphatic structures (eg, spinal accessory nerve, internal jugular vein, sternocleidomastoid muscle). Further observations have indicated that the pattern of nodal disease depends on the primary site. Therefore, these findings led to another neck dissection modification, which is the selective preservation of 1 or several lymph node groups.
History of the Procedure
In 1906, George W. Crile was the first person to describe radical neck dissection, which encompasses the surgical removal of neck metastasis contained between the superficial and deep fascial layers of the neck. Hayes Martin routinely used radical neck dissection for the management of neck metastasis in the 1950s. The main goal of this procedure was to remove, en bloc, the entire ipsilateral lymphatic structures from the mandible superiorly to the clavicle inferiorly and from the infrahyoid muscles to the anterior border of the trapezius.
The resection included the spinal accessory nerve, the internal jugular vein, the sternocleidomastoid muscle, and the submandibular gland. The anatomic structures that remained were the carotid arteries, vagus nerve, hypoglossal nerve, brachial plexus, and phrenic nerve. This operation and its oncologic concept are still valid; however, the procedure has been modified to decrease its morbidity but to maintain its oncologic efficacy. In the 1960s, O. Suarez and E. Bocca independently described a more conservative operation that involved removing all the lymph nodes but sparing the spinal accessory nerve, sternocleidomastoid muscle, and internal jugular vein. Finally, further procedures were designed to remove only selected regional lymph groups involved, depending on the primary site of origin.
Multiple modifications to the radical neck operation brought about new terms to describe such changes, and the terms for the same modification varied from author to author. Many cases of unclear terminology created confusion among clinicians from different geographical areas and institutions. Therefore, standardization was necessary. In 1991, the American Academy of Otolaryngology-Head and Neck Surgery published an official report that standardized the terminology for the different types of neck dissection.
The report was updated and published in 2002, with only a few changes, which dealt with the application of various types of selective neck dissection procedures for oral cavity cancer, pharyngeal and laryngeal cancer, thyroid cancer, and cutaneous malignancies. In addition, 2 new neck sublevels, Va and Vb, were added for a total of 6 neck levels and 6 neck sublevels. (The 1991 version of the report listed only 4 neck sublevels.) With the exception of the 2 added neck sublevels, the terminology in the updated report is the same as that of the 1991 version. The authors refer the reader to the article "Neck Dissection Classification Update, Revisions Proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery." [1, 2]
Modifications to the radical neck dissection include the following:
-
Type I: The spinal accessory nerve is preserved.
-
Type II: The spinal accessory nerve and the internal jugular vein are preserved.
-
Type III: The spinal accessory nerve, the internal jugular vein, and the sternocleidomastoid muscle are preserved.
-
Extended radical neck dissection: The lymph node groups and/or additional structures not included in the classic neck dissection are resected.
The 2011 publication by Ferlito [3] states the rationale for a new neck dissection classification/nomenclature and uses the symbol “ND” for neck dissection. The levels and sublevels of nodes removed are identified by Roman numerals (I through VI) and the nonlymphatic structures removed are identified by abbreviations (SCM, IJC, CNXI, etc.). The purpose of this new classification is the unification of terminology used in neck dissection around the world.
Problem
Lymphatic metastasis is the most important mechanism of spread in head and neck squamous cell carcinoma. The risk of lymph node involvement by metastasis varies depending on the site of origin, size of primary tumor, histologic grade of the primary tumor, perineural invasion, perivascular invasion, and extracapsular spread. Management of the neck lymph nodes is an integral part of treatment of head and neck cancer. Conversely, no single standardized treatment for cervical metastasis exists.
The indications and type of neck dissection to be performed in the N+ neck and management of the N0 neck remain controversial. Management is based on personal experience and retrospective studies. Radical neck dissection was the first attempt at adequately treating metastatic cervical lymphatic spread. The classic operation was subsequently modified several times to decrease morbidity without decreasing oncologic control. The 2 procedures in common use today are the modified radical and the selective neck dissections.
Epidemiology
Frequency
The incidence of metastatic disease for the upper aerodigestive tract varies widely, from 1-85%, depending on the site, size, and differentiation of the tumor. The rate of ipsilateral metastatic disease in patients with stage T3-T4 squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, or supraglottis is approximately 50%. The rate of bilateral or contralateral metastatic disease in these patients varies from 2-35%.
Nasopharyngeal carcinoma appears as a neck mass in approximately 50% of patients. Metastatic neck disease in thyroid gland tumors occurs as follows: papillary, 55%; medullary, 50%; and follicular, 25%.
Tumors localized in the oral cavity, oral mucosa, oropharynx, hypopharynx, and supraglottis have a higher incidence of metastasis than tumors of the superior gingiva, hard palate, and glottis. Many other factors contribute to the risk of neck metastasis, including the following:
-
Anterior portions of the oral cavity are associated with smaller risk of neck metastasis than posterior portions.
-
Young patients with oral carcinoma have a higher risk of developing nodal metastasis than older patients.
-
Risk of neck involvement by metastasis increases with an increase in tumor size.
-
Perineural and perivascular invasion are associated with a high risk of nodal metastasis. The extracapsular spread of the nodes also carries a high probability for lymphatic spread.
-
Poorly differentiated tumors are associated with a higher risk of neck metastasis than well-differentiated tumors.
Etiology
Radical neck dissection is performed for the surgical control of metastatic neck disease in patients with squamous cell carcinomas of the upper aerodigestive tract, salivary gland tumors, and skin cancer of the head and neck (including melanomas). Radical neck dissection is also indicated for the surgical control of metastatic carcinoma to the neck when the nasopharynx and thyroid are the primary sites.
Pathophysiology
Metastasis occurs frequently in malignancies. The tumor grows at the primary site by dysfunction in cellular proliferation, differentiation, and death. Mutations due to chemical carcinogens, radiation, or viruses may cause activation of oncogenes by normal cells, multiple genetic mutations, activation of proto-oncogenes, and/or inactivation of tumor suppressor genes, which may cause alterations in growth control.
Tumor cells move through the basement lamina of the epithelium and the stroma into the lymphatic and vascular channels (ie, the tumor progresses from carcinoma in situ to microinvasive tumor). This process is related to the production of cytokines, enzymes, and growth factors that destroy the basement membrane and create abnormal angiogenesis, which, in turn, triggers neovascularization and growth. The tumor spreads into the regional lymph nodes by lymphatic and vascular channel invasion and may seed other parenchymal sites if tumor invasion is not controlled at the lymphatic level. The usual sites of secondary spread include the lungs, liver, bone, brain, and adjacent skin, as well as other sites, depending on the tumor histology.
Presentation
Most patients present with a unilateral or bilateral neck mass. Usually, the patient is already aware of the primary lesion, or it is found during physical examination of the upper aerodigestive tract.
In 15% of patients, a metastatic neck mass is present without an obvious primary lesion. In this situation, making every effort to identify the primary lesion is difficult. These patients require a careful evaluation of all mucous membranes of the upper aerodigestive tract with random biopsies and an ipsilateral tonsillectomy.
Nodal classification
The most important prognostic factor in patients with squamous cell carcinoma of the head and neck is the status of the neck nodes. The extent of cervical lymphadenopathy constitutes the N portion of the tumor, node, metastases (TNM) classification by the American Joint Committee on Cancer. [4] This committee assigns N1-N3 ratings to different degrees of cervical adenopathy, with subgroupings of a, b, and c for certain stages. The nodal classification is as follows:
-
NX - Cervical neck nodes not assessable
-
N0 - No cervical node metastasis
-
N1 - Single ipsilateral node metastasis, 3 cm or less in diameter
-
N2a - Single ipsilateral node, more than 3 cm but not more than 6 cm in diameter
-
N2b - Multiple positive ipsilateral nodes no more than 6 cm in diameter
-
N2c - Bilateral or contralateral positive nodes no more than 6 cm in diameter
-
N3 - Massive adenopathy more than 6 cm in diameter
For further details see the Quick Reference Guide to TNM Staging of Head and Neck Cancer and Neck Dissection Classifcation from the American Academy of Otolaryngology–Head and Neck Surgery. [2]
Palpation
The accuracy of nodal status relates to the physician's ability to detect cervical adenopathy. Palpation is the technique used most in the detection of neck metastasis. Although palpation is inexpensive and easy to perform, it is not totally reliable.
Sensitivity and specificity of the neck examination by palpation range from 60-70%. A short obese neck and/or previous radiation or surgery makes the physical examination more difficult. Therefore, negative palpation findings of the neck still indicate a risk for occult metastatic disease. This risk increases according to the site, size, and particular characteristics of the primary lesion.
Imaging
Imaging is an integral part of clinical diagnosis and staging, and the results are helpful in deciding treatment. Among these techniques are computed tomography (CT) scanning, magnetic resonance imaging (MRI), ultrasonography, and ultrasound-guided aspiration cytology.
CT reveals metastatic adenopathy by central necrosis and extracapsular spread by enhancement of the nodal capsule. MRI is less precise than CT scan in identifying tumor necrosis and extracapsular spread, but MRI is better in assessing enlarged lymph nodes that do not necessarily represent metastasis. Both techniques cannot detect lymph nodes smaller than 1 cm. which, on occasion, independently of the size, are involved in metastasis. Ultrasound-guided aspiration cytology has a higher specificity than CT and MRI in analyzing lymph nodes, particularly in smaller nodes of less than 10 mm. However, the yield of this technique is directly related to the experience of the ultrasonographer and the pathologist.
Positron emission tomography (PET) has recently been used to assist in the diagnosis of lymph node metastasis. PET provides information about the metabolic activity of the tissues. Tissues with squamous cell carcinoma cells capture [18 F] fluoro-2-deoxy-D-glucose (FDG) at increased rates compared with normal tissues. Therefore, a minimal amount of tumor tissue must be present for the finding to be positive. Thus, precision of PET is limited to about 5 mm. [5]
Recent literature has demonstrated the higher sensitivity and specificity of the FDG-PET compared with ultrasonography, CT scan, and MRI in the assessment of metastatic staging of neck carcinomas. This finding could signify a positive role for PET in the identification of metastatic disease in patients with a clinically N0 neck. PET findings could provide early diagnosis of recurrent head and neck cancer, as well as indicate the status of the neck after chemoradiotherapy.
New challenges have occurred in the last 20 years in the selection of candidates for neck dissection who were treated initially with organ preservation treatment protocols and who may have persistence of neck disease after the nonoperative management. This group of patients can benefit greatly from the routine use of posttreatment PET/CT during their assessment for subsequent surgical management of the neck. PET and PET/CT are discussed further in the Workup section.
Histologic examination
Finally, the criterion standard for the detection of lymph node metastasis in the neck is the careful histologic examination of all nodes by the pathologist after the neck dissection is completed. Detection and accurate staging of neck metastasis are extremely important because staging has major implications for prognosis and treatment.
The most widely accepted terminology to define the regions of involvement of the cervical lymph node groups is the one developed originally by head and neck surgeons at the Memorial Sloan-Kettering Hospital. The terminology is as follows:
-
Region/level I - Submental and submandibular nodes
Ia - Nodes in the submental triangle bound by the anterior belly of the digastric and the hyoid bone
Ib - Nodes in the triangle bound by the anterior and posterior bellies of the digastric and body of the mandible
-
Region/level II - Upper jugular lymph nodes, including the jugulodigastric nodes
IIa - Nodes in the region anterior to the spinal accessory nerve
IIb - Nodes in the region posterior to the spinal accessory nerve
-
Region/level III - Nodes from the carotid bifurcation to the omohyoid muscle
-
Region/level IV - Nodes of the lower jugular area that extend from the omohyoid to the clavicle
-
Region/level V - All lymph nodes within the posterior triangle of the neck
-
Region/level VI - Nodes in the anterior compartment group, including the lymph nodes that surround the midline structures of the neck (These nodes extend from the hyoid bone superiorly to the suprasternal notch inferiorly.)
The revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology Head and Neck Surgery Committee and published in 2002 recommended the use of 6 neck levels and 6 sublevels, which added 2 extra sublevels (a and b) at level V. The 6 sublevels are Ia (submental nodes), Ib (submandibular nodes), IIa and IIb (upper jugular nodes), Va (spinal accessory nodes), and Vb (transverse cervical and supraclavicular nodes). [2]
Indications
The use of radical neck dissection has decreased over the last 2 decades with the use of chemoradiation as a non-surgical option. [6] At present, the classical radical neck dissection represents less than 20% of all cervical dissections. [7]
Therefore, when oncologic principles are not compromised (ie, no gross evidence of extension to the spinal accessory nerve or the internal jugular vein), modified radical neck dissection is preferred. Furthermore, in selected patients with clinical neck metastasis, a selective neck dissection has been advocated in clinical trials. Therefore, in the case of positive neck metastasis, the option of Modified RND or Selective ND will remain a surgeon's judgment call, taking into consideration the intraoperative findings and the level of oncological experience. As a consequence, the indications for Modified RND and Selective Neck Disection are not well defined or standardized, and vary from surgeon to surgeon. A word of caution, never sacrifice surgical oncological principles for a less radical surgery.
In general terms, the present use of the classical radical and modified neck dissection applies to patients with advanced metastatic neck disease, including the following:
-
Multiple positive neck nodes clinically present in an untreated patient (N2b, N2c), in particular in the proximity of the accessory nerve and posterior neck triangle.
-
One or more positive neck nodes clinically present with extracapsular extension involving the spinal accessory nerve and internal jugular vein.
-
Imaging studies and/or clinical evidence of extranodal disease.
-
Neck metastasis with involvement of the platysma muscle and cervical skin.
-
Recurrent or persistent metastatic disease is present after previous conservative neck dissection, irradiation, chemoradiation, or a combination thereof.
Radical neck dissection is effective in controlling a postirradiation cervical metastasis from a nasopharyngeal carcinoma if the primary site is under control.
Indications (usually an intraoperative assessment) for a modified radical neck dissection include the following: [10]
-
Modified radical neck dissection type I - A clinically positive lymph node metastasis that does not include the spinal accessory nerve
-
Modified radical neck dissection type II - Metastatic tumor mass that involves the sternocleidomastoid muscle but not the internal jugular vein or spinal accessory nerve
-
Modified radical neck dissection type III - Indicated to remove clinically positive lymph node metastasis that does not infiltrate the nonlymphatic structures
Modified radical neck dissection type III is also indicated for patients with a palpable metastasis caused by a differentiated carcinoma of the thyroid.
According to some authors, modified radical neck dissection type III is indicated in patients with squamous cell carcinoma and an N0 neck when the original tumor is in the larynx, hypopharynx, or both. Other authors prefer further modification by sparing the submandibular triangle nodes because of low risk of metastasis to those nodes.
Relevant Anatomy
A comprehension of the relevant neck anatomy is mandatory to understand how to perform an adequate radical neck dissection. From the surgical point of view, each side of the neck is divided into 2 cervical triangles.
Cervical neck triangles
The neck is divided into an anterior and a posterior cervical triangle.
The borders of the anterior cervical triangle are the inferior border of the mandible, the sternocleidomastoid muscle, and the midline of the neck.
The anterior cervical triangle is further subdivided into 4 smaller triangles: the submandibular triangle, submental triangle, muscular triangle, and carotid triangle. Understanding and identifying each of these areas guide the surgeon in performing a complete removal of the entire contents of the anterior cervical triangle.
The inferior border of the mandible and the 2 bellies of the digastric muscle delineate the submandibular triangle. The mylohyoid and hyoglossus muscles form the floor. Contents are the submandibular gland, lymphatic structures, anterior facial vein, and facial artery. The lingual nerve is above the muscular floor and below the deep layer of the deep cervical fascia.
The anterior belly of the digastric muscle, the hyoid bone, and the midline of the neck delineate the submental triangle. The mylohyoid muscle forms the floor of the submental triangle. It contains a few lymph nodes and small tributaries of the anterior jugular vein.
The omohyoid muscle in the anterior cervical triangle delineates the muscular triangle below and the carotid triangle above.
The posterior cervical triangle is also referred to as the lateral cervical triangle and is limited by the anterior margin of the trapezius muscle, the posterior border of the sternocleidomastoid muscle, and the middle third of the clavicle.
The posterior aspect of the omohyoid muscle further subdivides the posterior cervical triangle into 2 smaller triangles, the occipital triangle, which is located above the omohyoid muscle, and the supraclavicular triangle, which is located inferiorly to the muscle.
Cervical lymph nodes
The cervical lymph nodes are divided into superficial and deep chains.
Superficial lymph nodes are involved in a late stage of cancer; therefore, they have less oncologic importance.
Deep cervical lymph nodes receive drainage from areas of the oral cavity, pharynx, larynx, salivary glands, thyroid, and the skin of the head and neck. These deep cervical (superior, middle, inferior) lymph nodes accompany the internal jugular vein and its branches. Oncologically, the superior jugular nodes (ie, the group that is near the anterosuperior aspect of the accessory nerve) are crucial. They represent the most difficult area in the resection of the deep jugular nodes.
Cervical lymph nodes localized in the posterior triangle of the neck are classified into the upper, middle, and inferior cervical nodes. Posterior triangle nodes are located beneath the upper portion of the sternocleidomastoid muscle and extend posteriorly along the course of the spinal accessory nerve. This group of lymphatics receives drainage from the nasopharynx and communicates directly with the upper deep nodes from the internal jugular vein. The posterior triangle nodes in the inferior aspect progress anteriorly to the supraclavicular area to join the internal jugular vein at the base of the neck.
The above groups are easier to understand if they are divided into levels or zones as recommended in the Neck Dissection classification update revision proposed by the American Head and Neck Society and the American Academy of Otolaryngology Head and Neck Surgery.(2002).
Cervical lymph node level and location is as follows:
-
Region/level I
Ia - Submental
Ib - Submandibular
-
Region/level II
Upper jugular lymph nodes, including the jugulodigastric nodes
IIa - Nodes in the region anterior to the spinal accessory nerve
IIb - Nodes in the region posterior to the spinal accessory nerve
-
Region/level III - Nodes from the carotid bifurcation to the omohyoid muscle
-
Region/level IV - Nodes of the lower jugular area that extend from the omohyoid to the clavicle
-
Region/level V
All lymph nodes within the posterior triangle of the neck
Va - Spinal accessory nodes
Vb - Transverse cervical and supraclavicular nodes.
-
Region/level VI - Nodes in the visceral compartment from the hyoid bone superiorly to the suprasternal notch inferiorly
Nonlymphatic structures
See the list below:
-
Platysma muscle: The rectangular and sheetlike platysma muscle extends obliquely from the upper chest to the lower face, from posteroinferior to anterosuperior. Its undersurface creates an ideal plane in which to elevate the skin flaps in a neck dissection. The platysma muscle is absent in the lower anterior midline of the neck and in the area posterior to the external jugular vein and greater auricular nerve.
-
Sternocleidomastoid muscle: The sternocleidomastoid muscle runs from its anteroinferior attachment to the sternum and medial clavicle, posterosuperiorly to the mastoid tip and surrounding skin. The greater auricular nerve and the external jugular vein cross the upper aspect of this muscle. These structures guide the surgeon to the right plane of dissection and should be left on the surface of the sternocleidomastoid during flap elevation. The fascial envelope of the muscle is a key structure for selective neck dissections.
-
Spinal accessory nerve
The spinal accessory nerve crosses over the internal jugular vein in approximately 70% of individuals. The nerve then passes medially to the posterior belly of the digastric and stylohyoid muscles. Anatomic variations include the spinal accessory nerve that runs medially to the internal jugular vein (approximately 30% of individuals) and that runs through the vein (approximately 3% of individuals).
The nerve then enters obliquely into the sternocleidomastoid muscle from superior to inferior with the exit at Erb point. The Erb point is near the greater auricular nerve at the posteroinferior edge of the sternocleidomastoid muscle.
-
Digastric muscle: The posterior belly of the digastric is an important landmark. This belly extends from the hyoid bone to the undersurface of the mastoid tip. Important and delicate structures are recognized medial to the muscle. Therefore, it is superficial to the external and internal carotid artery, the hypoglossal nerve, and the internal jugular vein. Lateral to the posterior belly of the digastric, the only structure to be preserved is the marginal mandibular nerve.
-
Marginal mandibular nerve
The marginal mandibular nerve is localized deep to the superficial layer of the deep cervical fascia, which covers the submandibular gland and is superficial to the anterior facial vein.
The best way to preserve the nerve is to identify it carefully at the above locations. Once the nerve is identified, the tissue lateral and inferior to the nerve can be divided for exposure of the posterior belly of the digastric.
-
Trapezius muscle: The trapezius muscle extends from the posterior occiput to the lateral third of the clavicle. The anterior border of the trapezius is the posterior edge of level V, or the posterior triangle, of the neck.
-
Omohyoid muscle
Like the digastric muscle, the omohyoid muscle has 2 bellies. The anterior belly is superficial to the internal jugular vein. The posterior belly is superficial to the brachial plexus, phrenic nerve, and transverse cervical artery and vein.
Like the digastric muscle, the omohyoid is a key anatomic landmark in radical neck dissection.
-
Hypoglossal nerve and vagus nerve
The vagus nerve in the neck is intimately associated with the carotid sheath and is immediately deep to the internal jugular vein. The vagus nerve may be injured during the dissection and division of the lower portion of the internal jugular vein. Identification of the vagus nerve before division of the internal jugular is mandatory.
The hypoglossal nerve in the neck travels under the internal jugular vein, passes over the internal and external carotid arteries, and continues inferior to the posterior belly of the digastric muscle to enter the tongue musculature. Identifying this nerve is important to avoid injury.
-
Brachial plexus and phrenic nerve
The phrenic nerve lies above the anterior scalene muscle and deep to the transverse cervical artery. The brachial plexus exits lower in the neck and then passes between the anterior and middle scalene muscles.
Identify the anterior and middle scalene muscles before clamping the lymphatic structures. Avoid dissection in the supraclavicular area before phrenic and brachial plexus visualization.
-
Thoracic duct: The thoracic duct, located in the lower left neck, arises posterior to the internal jugular vein and anterior to the phrenic and transverse cervical artery. The anatomy is variable, and the duct has multiple interdigitated channels.
For more information about the relevant anatomy, see Neck Anatomy.and Practical Guide to Neck Dissection. [11]
Contraindications
Since the development of newer surgical procedures minimizing surgical morbidity, the contraindications to a neck dissection have been controversial. However, patients who have too great a surgical risk because of cardiopulmonary disease and whose condition cannot be optimized preoperatively should not be considered for this operation.
Patients in whom preoperative imaging suggests deep infiltration of the tumor in the prevertebral space, scalene muscles, levator scapula muscle, phrenic nerve, and brachial plexus should not be considered suitable candidates. Despite a poor prognosis due to the advanced disease, the resulting morbidity, if not possible mortality, poses no advantage to the patient.
The treatment of patients undergoing carotid vessel management remains controversial. From a radiologic standpoint, carotid artery encasement is defined as disease having surrounded the common carotid or internal carotid artery over more than 270° of encirclement. Many have advocated the preoperative evaluation of these patients with a balloon occlusion test.
Patients who can tolerate the occlusion of the ipsilateral carotid artery without any evidence of neurologic dysfunction may be candidates in whom the carotid segment may be safely resected. The help of a vascular surgeon or a neurosurgeon in these cases may be advisable for reconstruction of the resected segment.
Because patients with significant carotid artery involvement often have preexisting atherosclerotic vascular disease, resection of the carotid artery results in significant morbidity, if not mortality, for most of these individuals.
Contraindications for radical neck dissection also include the following:
-
Primary tumor that cannot be controlled
-
N0 neck
-
Distant metastatic disease
-
Fixed neck mass in the deep cervical fascia and/or skull base involvement
-
Circumferential or near circumferential involvement and invasion of the carotid vessels if the patient cannot tolerate a balloon occlusion test
Extensive metastatic involvement of the platysma and cervical skin is not uncommon and is not a contraindication for a radical neck dissection. However, it requires a wide resection of the area affected with local and regional flap reconstruction of the sacrificed tissue.
-
The skin incision is made through the platysma, and the flap is elevated in the subplatysmal plane. In the superior lateral aspect of the flap, leaving the greater auricular nerve and the external jugular vein on the sternocleidomastoid muscle is important. The posterior flap is elevated toward the trapezius muscle.
-
The sternocleidomastoid muscle is exposed and incised above the clavicle with Bovie electrocautery.
-
The anterior and posterior belly of the omohyoid is identified. Note that the omohyoid crosses the internal jugular vein laterally.
-
The internal jugular vein is identified in the lower aspect of the neck, and a 2-0 silk suture is then passed around the vein and tied.
-
2-0 silk sutures and suture ligatures are placed as shown.
-
The supraclavicular fatty tissue is opened using blunt dissection with identification of the phrenic nerve. The phrenic nerve appears as a white cord down the midline of the anterior scalenus muscle. The internal jugular vein has been ligated and transected. The carotid artery is seen on the top of the image. The transverse cervical artery is seen at the bottom of the image.
-
The submental fatty tissue, the submandibular nodes, and the submandibular gland have been removed and displaced inferiorly together with the specimen.
-
The internal jugular vein is identified superiorly, medial to the posterior belly of the digastric muscle. The ligation of the internal jugular vein at this point is performed with a 2-0 silk suture and a distal suture ligature.
-
Final aspect of the surgical wound after removal of the operative specimen.
-
Axial contrast-enhanced neck CT showing an extensive mass of the left side of the neck.
-
Patient in supine position and head turned to the right side. Radical Left Neck Dissection completed: The classical radical neck dissection encompasses the lymphatic nodes in levels I-V. View of the surgical wound after removal of the operative monoblock specimen. S = Superior. I = Inferior. M = Medial. L = Lateral. (1) Anterior and posterior bellies of the digastric muscle. (2) Carotid artery and vagus nerve. (3) Anterior cervical nerve root. (4) Phrenic nerve. (5) Brachial plexus. (6) Internal jugular vein, inferior aspect, cut and ligated. (7) Anterior scalene muscle. (8) External jugular vein, inferior aspect, cut and ligated. (9) Hypoglossal nerve. (10) Sternocleidomastoid muscle, superior aspect, cut.
-
Selective Radical Neck Dissection 2-5 Part 1. Video courtesy of Dr. Nader Sadeghi.
-
Selective Radical Neck Dissection 2-5 Part 2. Video courtesy of Dr. Nader Sadeghi.
-
Selective Radical Neck Dissection 2-5 Part 3. Video courtesy of Dr. Nader Sadeghi.
-
Selective Radical Neck Dissection 2-5 Part 4. Video courtesy of Dr. Nader Sadeghi.
-
Selective Radical Neck Dissection 2-5 Part 5. Video courtesy of Dr. Nader Sadeghi.










