Definition and Efficacy
Cardiac markers are used for the diagnosis and risk stratification of patients with chest pain and suspected acute coronary syndrome (ACS) and for management and prognosis in patients with acute heart failure, pulmonary embolism, and other disease states. Cardiac markers can be classified into those that signify myocardial necrosis (creatine kinase-MB [CK-MB] fraction, myoglobin and cardiac troponins), those that indicate myocardial ischemia (ischemia modified albumin), those that suggest myocardial stress (natriuretic peptides), and those markers of inflammation and prognosis (C-reactive protein [CRP], soluble CD40 ligand [sCD40L], and homocysteine). [1]
The cardiac troponins, in particular, have become the cardiac markers of choice for patients with ACS, eclipsing CK-MB and myoglobin in terms of clinical value. Indeed, cardiac troponin is central to the definition of acute myocardial infarction (MI) in the consensus guidelines from the European Society of Cardiology (ESC) and the American College of Cardiology (ACC): These guidelines recommend that cardiac biomarkers should be measured at presentation in patients with suspected MI, and that the only biomarker that is recommended to be used for the diagnosis of acute MI at this time is cardiac troponin due to its superior sensitivity and accuracy. [2, 3, 4, 5, 6]
For example, patients with elevated troponin levels but negative CK-MB values who were formerly diagnosed with unstable angina or minor myocardial injury are now reclassified as non–ST-segment elevation MI (NSTEMI), even in the absence of diagnostic electrocardiographic (ECG) changes.
Similarly, only one elevated troponin level above the established cutoff is required to establish the diagnosis of acute MI, according to the ACC guidelines for NSTEMI. [3, 7, 8]
These changes were instituted following the introduction of increasingly sensitive and precise troponin assays. Up to 80% of patients with acute MI will have an elevated troponin level within 2-3 hours of emergency department (ED) arrival, versus 6-9 hours or more with CK-MB and other cardiac markers. [6]
Accordingly, most have advocated relying solely on troponin and discontinuing the use of CK-MB and other markers. [9, 10, 11, 12, 13] Nevertheless, CK-MB and other markers continue to be used in some hospitals to rule out MI and to monitor for additional cardiac muscle injury over time.
Note that cardiac markers are not necessary for the diagnosis of patients who present with ischemic chest pain and diagnostic ECGs with ST-segment elevation. These patients may be candidates for thrombolytic therapy or primary angioplasty. Treatment should not be delayed to wait for cardiac marker results, especially because the sensitivity is low in the first 6 hours after symptom onset. ACC/American Heart Association (AHA) guidelines recommend immediate reperfusion therapy for qualifying patients with ST-segment elevation MI (STEMI), without waiting for cardiac marker results. [14, 15]
Go to Myocardial Infarction and Complications of Myocardial Infarction for more complete information on these topics.
Markers of Myocardial Necrosis and Ischemia
Cardiac troponins (cTn)
The troponins are regulatory proteins found in skeletal and cardiac muscle. Three subunits have been identified: troponin I (TnI), troponin T (TnT), and troponin C (TnC). The genes that encode for the skeletal and cardiac isoforms of TnC are identical; thus, no structural difference exists between them. However, the skeletal and cardiac subforms for TnI and troponin TnT are distinct, and immunoassays have been designed to differentiate between them.
Two different reference ranges are used in troponin assays. The upper percentile reference limit gives the upper limit of what can be expected in a normal, healthy, adult population, whereas the coefficient of variation (CV) is the percentage variation in assay results that can be expected when the same sample is repeatedly analyzed.
Fourth universal definition of acute myocardial infarction (MI)
Recognizing that cardiac troponin measurements may be elevated in disease states not primarily related to myocardial ischemia, a fourth universal definition of acute MI was developed by the American College of Cardiology (ACC), European Society of Cardiology (ESC), American Hospital Association (AHA), and World Health Federation (WHF) in 2018.
First, myocardial injury is defined as elevated cardiac troponin values with at least one value above the 99th percentile upper reference limit. Myocardial injury is considered acute if there is a rise and/or fall of cardiac troponin values. The term "myocardial infarction" is to be used when there is acute myocardial injury with clinical evidence of acute myocardial ischemia and with detection of a rise and/or fall of cardiac troponin values within at least one value above the 99th percentile upper reference limit and at least one of the following features [16] :
-
Symptoms of myocardial ischemia
-
New ischemic electrocardiographic (ECG) changes
-
Development of pathological Q waves, imaging evidence of new loss of viable myocardium, or new regional wall motion abnormality in a pattern consistent with ischemia
-
Identification of a coronary thrombus by angiography or autopsy
The definition was updated to manage the fact that nonischemic myocardial injury, as occurs in association with heart failure, arrhythmia, myocarditis, renal failure, pulmonary embolism, and percutaneous or surgical coronary procedures, also result in elevated cardiac markers as cardiac troponins become the standard. [17]
Troponin assays: sensitivity, specificity, and precision
The sensitivity, specificity, and precision of the different commercially available troponin assays vary considerably. These differences are related to a lack of standardization, the use of different monoclonal antibodies, the presence of modified TnI and TnT in the serum, and variations in antibody cross-reactivity to the myriad detectable forms of TnI that result from its degradation.
At present, the 99th upper reference limit of cardiac troponin is still the best-established criterion for the diagnosis of acute MI. [18, 19] Only one manufacturer produces the TnT assay, and its 99th percentile cutoffs and the 10% CV are well established. However, up to 20-fold variation has occurred in results obtained with the multitude of commercial TnI assays currently available, each with their own 99th percentile upper reference limits and 10% CV levels.
In the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) IV study, a relatively insensitive point-of-care TnI assay was used to screen patients for study eligibility. In a subsequent study, the blood samples were reanalyzed using the 99th percentile cutoff of a far more sensitive central laboratory TnT assay. [20] The more sensitive 99th percentile cutoff of this TnT assay identified an additional 96 (28%) of 337 patients with a positive TnT result but negative point-of-care TnI; these patients had higher rates of death or MI at 30 days. [20]
In a similar reanalysis of the TACTICS-TIMI 18 trial, three different TnI cutoffs were compared on 1821 patients to evaluate the 30-day risk of death or MI: the 99th percentile, 10% CV, and the World Health Organization (WHO) acute MI cutoffs. [21, 22] (The WHO cutoffs define acute MI using creatine kinase-MB [CK-MB] and report troponin levels as either a higher “acute MI level” or a lower “intermediate level” that is correlated with “leak” or “minor myocardial injury.”)
Using the 10% CV cutoff identified, an additional 12% more cases were identified relative to the WHO acute MI cutoff. The 99th percentile cutoff identified an additional 10% of cases relative to the 10% CV cutoff, as well as a 22% increase in the number of cases over the WHO acute MI cutoff. Nevertheless, the odds ratios for the adverse cardiac event rates of death or MI at 30 days were similar for all three cutoffs, suggesting that the lower cutoffs detected more patients with cardiovascular risk without sacrificing specificity. [21, 22]
The American Association for Clinical Chemistry Academy (AACC) (formerly the National Academy of Clinical Biochemistry [NACB]) working with the ACC/ESC guidelines has recommended adoption of the 99th percentile upper reference limit as the recommended cutoff for a positive troponin result. [19, 23, 24] Ideally, the precision of the assay at this cutoff level should be measured by a CV that is less than 10%.
However, most TnI assays are imprecise at the 99th percentile reference limit. [25] Some have therefore recommended that the cutoff level be raised to the slightly higher 10% CV level instead of the 99th percentile reference limit to ensure adequate assay precision.
In addition, studies have shown that populations within the 99th percentile reference limit include patients with low troponin levels who nevertheless have an elevated cardiac risk, and that the true 99th percentile cutoff for a healthy patient population is actually a factor of 10-50 lower. Accordingly, these investigations suggest that higher sensitivity or ultrasensitive troponin assays are necessary. [22] A high-sensitivity cardiac troponin (hs-cTn) can quantify lower cardiac troponin concentrations by up to 10 fold lower than conventional assays while also detecting measurable cardiac troponin values in over 50% of healthy subjects. These tests are very precise in that their CV is below 10% at the 99th percentile upper reference limit. Therefore, the advantage of ultrasensitive troponins is based on the premise that lower cutoff levels achieve higher sensitivity that will allow earlier diagnosis, often within 90 minutes of presentation. High-sensitivity troponins confer additional clinical benefits in that they help detect myocardial injury due to acute ischemia earlier, sometimes before a larger infarct occurs. [26]
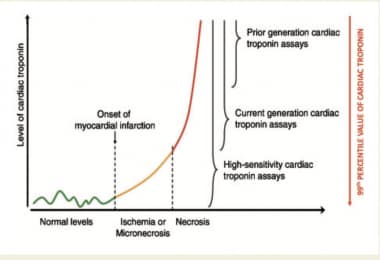 Cardiac Markers. This graph describes the improved sensitivity of the cardiac troponin assay. Whereas conventional cardiac troponin assays could detect the red region in patients with myocardial necrosis, the newer high-sensitivity assays can detect people with myocardial injury, early onset myocardial infarction, and baseline cardiac troponin levels in 50% of healthy patients. Courtesy of Oxford University Press [Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. 2017 Dec 1;113(14):1708-18. Online at https://academic.oup.com/cardiovascres/article/113/14/1708/4158239.]
Cardiac Markers. This graph describes the improved sensitivity of the cardiac troponin assay. Whereas conventional cardiac troponin assays could detect the red region in patients with myocardial necrosis, the newer high-sensitivity assays can detect people with myocardial injury, early onset myocardial infarction, and baseline cardiac troponin levels in 50% of healthy patients. Courtesy of Oxford University Press [Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. 2017 Dec 1;113(14):1708-18. Online at https://academic.oup.com/cardiovascres/article/113/14/1708/4158239.]
Additionally, the introduction of hs-cTn assays into practice necessitates serial measurements to differentiate a number of previously mentioned disease states that cause myocardial injury. [27]
Of note, the 99th percentile upper reference limit has been shown to differ between men and women when measured with high-sensitivity assays. [19, 23, 24, 28]
To optimize use of the assay in the emergency department (ED), it is important to be familiar with the particular troponin assay available in the individual laboratory and to know whether the cutoff is set at the 10% CV level or at the 99th percentile upper reference limit.
Point-of-care assays
The AACC recommendations specify that cardiac markers be available in hospitals on an immediate basis 24 hours per day, 7 days per week, with a turnaround time of 1 hour or less. [23, 29] Point-of-care (POC) devices that provide rapid results should be considered in hospitals whose laboratories cannot meet these guidelines.
POC assays for CK-MB, myoglobin, and the cardiac troponins TnI and TnT are available. Only qualitative TnT assays are available as POC tests, but both quantitative and qualitative POC TnI assays are currently marketed.
In a multicenter trial, the time to positivity was significantly faster for the POC device than for the local laboratory (2.5 h vs 3.4 h). [30]
In another multicenter study, which evaluated the i-STAT POC TnI assay in comparison with the central laboratory in 2000 patients with suspected acute coronary syndrome (ACS), POC testing reduced the length of stay by approximately 25 minutes for patients who were discharged from the ED. [31, 32] POC high-sensitivity assays are available as well. The increasing sensitivity of POC assays coupled with the benefit of rapid turnaround time make them attractive clinical tools in the ED.
Prognostic value of troponin
In addition to its use in the diagnosis of MI, an elevated troponin level can identify patients at high risk for major adverse cardiac events (MACE). [33, 34] Specifically, data from a meta-analysis indicated that an elevated troponin level in patients without ST-segment elevation is associated with a nearly four-fold increase in the cardiac mortality rate. [35] In patients without ST-segment elevation who were being considered for thrombolytic therapy, initial TnI levels on admission correlated with mortality at 6 weeks, but CK-MB levels were not predictive of adverse cardiac events and had no prognostic value. [33]
Other studies revealed that an elevated troponin level at baseline was an independent predictor of mortality, even in patients with chest pain and acute MI with ST-segment elevation (STEMI) who were eligible for reperfusion therapy. [36, 37]
Data from the ARTEMIS study, comprising 1137 diabetic patients with stable coronary artery disease (CAD) and 649 normoglycemic patients, found that high levels (≥14 ng/L) of hs-TnT was an independent strong predictor of cardiac death or hospitalization for heart failure in patients with diabetes and CAD (as were B-type natriuretic peptide, hs-C-reactive protein [hs-CRP], and soluble suppressor of tumorigenicity-2 [sST2] in a multivariate analysis). [38] In the nondiabetic group, only hs-CRP and sST2 were predictive for these outcomes.
Data from the Acute Decompensated Heart Failure National Registry (ADHERE) involving information from 23,696 patients hospitalized with acute heart failure showed that increased levels of troponin and creatinine were the strongest predictors of in-hospital worsening heart failure. [39]
The TIMI IIIB, GUSTO IIa, GUSTO IV ACS, and FRISC trials all demonstrated a direct correlation between the level of TnI or TnT and the mortality and adverse cardiac event rate in ACS. [33, 36, 40, 41, 42]
Cardiac troponin levels have also been associated with higher mortality in patients with pulmonary embolism, presumably due to their significance as markers of right ventricular strain, particularly in normotensive patients. [27]
More recently, elevated high-sensitivity troponin T levels in patients hospitalized with coronavirus disease 2019 (COVID-19) may indicate a higher risk for in-hospital mortality. [43, 44]
CK-MB
Prior to the introduction of cardiac troponins, the biochemical marker of choice for the diagnosis of acute MI was the CK-MB isoenzyme. The criterion most commonly used for the diagnosis of acute MI was two serial CK-MB elevations above the diagnostic cutoff level or a single result more than twice the upper limit of normal. Although CK-MB is more concentrated in the myocardium, it also exists in skeletal muscle and false-positive elevations occur in a number of clinical settings, including trauma, heavy exertion, and myopathy.
CK-MB first appears 4-6 hours after symptom onset, peaks at 24 hours, and returns to normal in 48-72 hours. Its value in the early and late (>72 h) diagnosis of acute MI is limited. However, its release kinetics can assist in diagnosing reinfarction if levels rise after initially declining following acute MI.
In the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) registry, a review of almost 30,000 patients revealed that discordant troponin and CK-MB results occurred in 28% of patients. [45] However, patients who were troponin negative but CK-MB positive had in-hospital mortality rates that were not significantly increased from patients who were negative for both biomarkers.
Similarly, in a report of more than 10,000 patients with ACS from the multicenter Global Registry of Acute Coronary Events (GRACE) registry, in-hospital mortality was highest when both troponin and CK-MB were positive, intermediate in troponin-positive/CK-MB-negative patients, and lowest in patients in whom both markers were negative and in those who were troponin negative/CK-MB positive. [46] Thus, an isolated CK-MB elevation has limited prognostic value in patients with a non-ST elevation ACS.
Despite updates to guidelines promoting cardiac troponin as the only cardiac biomarker necessary to diagnose MI, biomarker protocols vary across the United States. A 2017 survey of 824 US hospitals undergoing Chest Pain Center accreditation by the Society of Cardiovascular Patient Care revealed that only 24% use cardiac troponin alone, 49% use the 99th percentile of the upper reference limit for their cut point, and most use both cardiac troponins and CK-MB in serial measurements to rule out MI. [47]
CK-MB/CK relative index
The relative index calculated by the ratio of CK-MB (mass) to total CK can assist in differentiating false-positive elevations of CK-MB arising from skeletal muscle. A ratio of less than 3 is consistent with a skeletal muscle source, whereas ratios greater than 5 are indicative of a cardiac source. Ratios between 3 and 5 represent a gray zone. No definitive diagnosis can be established without serial determinations to detect a rise.
The CK-MB/CK relative index was introduced to improve the specificity of CK-MB elevation for MI. However, sensitivity for acute MI falls when concurrent cardiac injury and skeletal muscle injury is present. In an ED-based study to evaluate the CK-MB relative index compared with the absolute CK-MB, specificity was increased, but there was a loss of sensitivity. [48]
The CK-MB/CK relative index is useful if patients have only an MI or only skeletal muscle injury, but not if they have both. Therefore, in the combined setting of acute MI and skeletal muscle injury (rhabdomyolysis, heavy exercise, polymyositis), the fall in sensitivity is significant.
Note that the diagnosis of acute MI must not be based on an elevated relative index alone, because the relative index may be elevated in clinical settings when either the total CK or the CK-MB is within normal limits. The relative index is only clinically useful when both the total CK and the CK-MB levels are increased.
CK-MB isoforms
The CK-MB isoenzyme exists as two isoforms: CK-MB1 and CK-MB2. Laboratory determination of CK-MB actually represents the simple sum of both isoforms. CK-MB2 is the tissue form, and it is initially released from the myocardium after MI; CK-MB2 is converted peripherally in serum to the CK-MB1 isoform rapidly after symptom onset.
Normally, the tissue CK-MB1 isoform predominates; thus, the CK-MB2/CK-MB1 ratio is typically less than 1. A result is positive if the CK-MB2 is elevated and the ratio is greater than 1.7.
CK-MB2 can be detected in serum within 2-4 hours after infarction onset and peaks at 6-9 hours, making it an early marker for acute MI. Two large studies evaluating its use revealed a sensitivity of 92% at 6 hours after symptom onset, compared with 66% for CK-MB and 79% for myoglobin. [49, 50] The major disadvantage of this assay is that it is relatively labor intensive for the laboratory.
Myoglobin
Myoglobin is a heme protein found in skeletal and cardiac muscle that has attracted considerable interest as an early marker of MI. Its low molecular weight accounts for its early release profile: Myoglobin typically rises 2-4 hours after onset of infarction, peaks at 6-12 hours, and returns to normal within 24-36 hours.
Rapid myoglobin assays are available, but overall, they have a lack of cardiospecificity. Serial sampling every 1-2 hours can increase the sensitivity and specificity; a rise of 25-40% over 1-2 hours is strongly suggestive of acute MI. However, in most studies, myoglobin only achieved 90% sensitivity for acute MI, so the negative predictive value of myoglobin is not high enough to exclude the diagnosis of acute MI.
The original studies that evaluated myoglobin used the WHO definition of acute MI that was based on a CK-MB standard. With the adoption of a troponin standard for acute MI in the ACC/ESC definition, the sensitivity of myoglobin for acute MI is substantially reduced. This significantly diminishes its utility, and a number of studies have indicated that contemporary cardiac troponin assays render the use of myoglobin measurements unnecessary. [10, 12]
Ischemia modified albumin (IMA)
IMA is a novel marker of ischemia that is produced when circulating serum albumin contacts ischemic heart tissues. IMA can be measured by the albumin cobalt binding assay that is based on this marker's inability to bind to cobalt. [51] A rapid assay with a 30-minute laboratory turnaround time has been developed and marketed as the first commercially available US Food and Drug Administration (FDA)–approved marker of myocardial ischemia.
Based on investigations of myocardial ischemia induced by balloon inflation during percutaneous coronary intervention, IMA levels rise within minutes of transient ischemia, peak within 6 hours, and can remain elevated for as long as 12 hours.
Studies on the use of IMA in patients with chest pain in the ED found sensitivities that ranged from 71% to 98% and specificities of 45-65%, with a negative predictive value of 90-97% for ACS. [52]
A multimarker approach in one study, using a combination of ECG findings, TnT levels, and IMA levels, achieved a sensitivity of 95% for ACS, [53] whereas a second study calculated that the combination of IMA, myoglobin, CK-MB, and TnI increased the sensitivity to 97% for detecting myocardial ischemia. [54]
However, IMA levels are also elevated in patients with cirrhosis, certain infections, and advanced cancer, which reduces the specificity of the assay. Further validation and outcome studies are required to evaluate the use of IMA in the ED diagnosis of ACS when the ECG and cardiac troponins levels are nondiagnostic.
Acute Coronary Syndrome Testing Strategy
In patients with definite or possible acute coronary syndrome (ACS), in addition to clinical tools such as risk stratification scores (eg, the HEART score [history, electrocardiogram (ECG), age, risk factors, initial troponin]), [55] the serial evaluation of cardiac markers is essential to diagnosing acute myocardial infarction (MI) as well as for risk stratification for major adverse cardiac events (MACE). Most contemporary clinical practice guidelines use the cardiac troponin (cTn), whether conventional or high sensitivity (hs), exclusively for these purposes. The main clinical outcome of interest after initial emergency department (ED) evaluation of patients with suspected ACS is 30-day MACE. MACE includes MI, death, target lesion revascularization, or death.
In 2018, The American College of Emergency Physicians (ACEP) updated its level C recommendations regarding testing strategies for ruling out non-ST-elevation MI (NSTEMI) in lower risk patients to include the use of the HEART score and conventional or high-sensitivity cardiac troponins (hs-cTn) as follows:
-
In adult patients with suspected acute non–ST-elevation ACS, conventional troponin testing at 0 and 3 hours among low-risk patients with ACS (defined by HEART score 0 to 3) can predict an acceptable low rate of 30-day MACE.
-
A single high-sensitivity troponin result below the level of detection on arrival to the ED, or negative serial high-sensitivity troponin result at 0 and 2 hours is predictive of a low rate of MACE.
-
In adult patients with suspected acute non–ST-elevation ACS who are determined to be low risk based on validated accelerated diagnostic pathways that include a nonischemic ECG result and negative serial high-sensitivity troponin testing results both at presentation and at 2 hours can predict a low rate of 30-day MACE, allowing for an accelerated discharge pathway from the ED.
A 2021 prospective, multicenter study comprising 1452 patients suggests that a HEART score without the troponin measurement (ie, HEAR score) of less than 2 is a safe and reliable workup strategy to exclude NSTEMI, with a very low risk of MACE at 45 days. [56] Furthermore, a two-step HEART strategy combining the HEAR and HEART scores is potentially safe and could significantly lower the need for measuring troponin levels in very low-risk patients (HEAR score < 2; HEART score < 4) who present to the ED with chest pain.
The American Heart Association and American College of Cardiology (AHA/ACC) 2014 guideline for the management of ACS/NSTEMI recommends that cardiac troponin (I or T) be measured at presentation and 3-6 hours after symptom onset in all patients in which there is clinical suspicion. [4] Additional troponin measurements are indicated beyond 6 hours in patients with an initial normal serial troponin value and ischemic ECG findings and/or intermediate/high-risk clinical features. Timing of presentation and time of onset of symptoms should be considered when interpreting the troponin values. Finally, the guidelines state that creatine kinase (CK)-MB and myoglobin are not useful for the diagnosis of ACS. All of the above recommendations are cited with level A evidence. [4]
Few studies on the "time to positivity" have been performed, but serial samples that become positive in the 12- to 24-hour window are considered unlikely, unless the patient has ongoing symptoms of ischemia after admission. Although acute MI can therefore be ruled out in patients with negative serial marker results through the 6- to 9-hour period after presentation regardless of clinical suspicion, MACE is another consideration for higher risk patients.
Troponins in Therapeutic Management of ACS
Clinical trials have demonstrated the benefits of using cardiac markers as an indicator for specific therapeutic interventions in acute coronary syndrome (ACS). However, this use remains investigational; currently, no validated therapeutic algorithms are based on an isolated positive marker result in the absence of other clinical or electrographic (ECG) findings.
Subgroup analysis of trials with low–molecular-weight heparin (LMWH) showed a decreased cardiac event rate in patients with a positive result for troponin T (TnT) and who were treated with an LMWH. [57, 58]
Similarly, in the Platelet Receptor inhibition for Ischaemic Syndrome Management (PRISM) trial, patients with an elevated troponin I (TnI) who were treated with the glycoprotein (GP) IIb/IIIa inhibitor tirofiban (Aggrastat) demonstrated a significant decrease in cardiac events compared with patients without an elevated TnI level. [59] No significant difference in outcomes was seen in patients without TnI elevations who were treated with tirofiban when compared with placebo.
In the Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) trial, patients who were treated with the GP IIb/IIIa inhibitor eptifibatide (Integrilin) within 6 hours of symptom onset obtained the greatest benefit, and subgroup analysis showed that patients with an elevated troponin level also had better responses to therapy than did those whose troponin result was negative. [60]
Finally, in the Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy–Thrombolysis in Myocardial Infarction (TACTICS-TIMI) 18 trial, patients with elevations in TnI or TnT had a significant reduction in death, MI, or rehospitalization for ACS within 6 months after being treated with early invasive therapy consisting of aspirin, heparin, tirofiban, and catheterization/revascularization within 4-48 hours. [61, 62] Subset analysis noted that an elevation of CK-MB did not benefit the early invasive group when compared with the conservative management group. However, early invasive therapy did benefit the subgroup of patients with elevated troponin levels but normal CK-MB levels. [63]
These studies suggest that a positive troponin result alone is an independent predictor of high risk for adverse cardiac events, and that therapy with LMWHs and/or GP IIb/IIIa inhibitors appears to confer the most benefit on patients with elevated cardiac troponins levels.
Troponins in Chronic Renal Failure
Patients with chronic renal failure (CRF) who are on hemodialysis are at increased risk of coronary artery disease and acute coronary syndrome (ACS), and cardiovascular disease accounts for about 50% of deaths in these patients. Early studies revealed a high prevalence of elevated cardiac troponin (cTn) levels in patients with CRF, especially troponin T (TnT). However, the clinical significance of an elevated TnT result is unclear.
Biochemical studies have demonstrated that the troponin elevation originates from the myocardium and is not related to the myopathy associated with renal failure. Yet, patients with CRF frequently have chronic congestive heart failure (CHF) and hypertension, which may independently elevate the troponin level. In addition, data suggest that elevated troponin levels in asymptomatic patients may reflect subclinical microinfarctions that are clinically distinct from ACS.
Large prospective studies have confirmed the association between TnT elevation and cardiac mortality in patients with CRF. The GUSTO IV ACS trial showed that patients with renal insufficiency and an elevated TnT had the highest overall risk of the composite endpoint of death or acute myocardial infarction (MI), [64] and two other prospective studies reported that an elevated TnT—but not troponin I (TnI)—increased the risk of long-term mortality. [65, 66] Whether elevated TnT increases cardiac risk in the short term (ie, 30 d) is unclear, but patients without short-term risk may not require hospitalization and potentially could be managed on an outpatient basis.
It has been suggested that chronically elevated troponin levels represent chronic structural cardiovascular disease, such as prior MI, chronic CHF, or hypertension in the setting of CRF. If true, these patients are at higher cardiac risk compared with the normal, healthy patient population, and troponin remains a useful marker in the setting of CRF. [67, 68]
Note that dialysis does not affect TnT or TnI levels; predialysis and postdialysis levels are essentially unchanged. Creatine kinase (CK)-MB, however, is dialyzable, and levels are decreased postdialysis. Therefore, a single elevated TnT level in patients with CRF and possible ACS is nondiagnostic for acute MI in the absence of other findings. Serial determinations are usually required, with a focus on a rise in the troponin level.
Ascertaining whether an elevated troponin in patients with CRF represents true acute MI or a false-positive result can be difficult. In patients with cardiac risk factors who are deemed clinically to be at moderate-high risk for ACS, the prudent approach would be to observe and perform serial cardiac markers over 6-9 hours. In low-risk asymptomatic patients and in the absence of any other findings indicative of ACS, the elevated troponin result is more likely to be false positive for acute MI.
Troponins in Nonischemic Heart Disease
A number of studies have demonstrated that troponin T (TnT) can be used for risk stratification of patients with heart failure without ischemia. Specifically, elevated cardiac troponins (cTn) are associated with decreased left ventricular ejection fraction and poor prognosis in patients with heart failure, and they are related to the severity of heart failure. [69]
Isolated studies have shown evidence of myocardial infarction and elevated troponin I (TnI) levels in patients with subarachnoid hemorrhage. [70] Vasoactive peptides released during acute subarachnoid hemorrhage induce deep T-wave inversions on electrocardiography that indicate myocardial injury. As mentioned previously, TnT has been shown to be an independent predictor of outcome in patients with pulmonary embolism; right ventricular strain or infarction from acute pulmonary hypertension causes the elevated troponin level.
Elevated troponin levels have also been documented in other nonischemic cardiac disease states, such as tachyarrhythmias, hypertension, myocarditis, and myocardial contusion.
Markers of Hemodynamic Stress: Natriuretic Peptides
Natriuretic peptides (NP) are neurohormones involved in maintaining homeostasis in the cardiovascular system with respect to the regulation of sodium and water excretion and blood pressure. There are three NPs: atrial NP (ANP), brain-type NP (BNP), and C type NP (CNP). In clinical practice, BNP and N-terminal pro BNP (NT-proBNP) are used primarily to guide the management of heart failure. They are also used to prognosticate in other cardiovascular diseases.
BNP is produced and secreted primarily by the ventricular myocardium in response to wall stress. Mechanical stretch is sensed by cardiac myocytes in response to increases in volume or pressure in the ventricles, which triggers the production of the prohormone proBNP. ProBNP is cleaved into BNP and NT-proBNP, the inert molecule. Both can be assayed, and they are comparable in terms of their clinical utility for the management of heart failure and for prognostic value in other cardiovascular diseases. The half-life of BNP is 20 minutes, shorter than the 120 minutes half-life of NT-proBNP. Both significantly rise in concentration in heart failure. They can be useful markers of heart failure in that lower values lower the pretest probability of a diagnosis of heart failure.
Data from guidelines and studies
Based on level A evidence from guidelines for the management of heart failure by the American College of Cardiology, American Heart Association, and the Heart Failure Society of America (ACC/AHA/HFSA), early measurement of BNP levels is recommended to support medical decision making in the diagnosis of acute heart failure, especially if there is clinical uncertainty, and to assess disease severity in chronic heart failure. [71, 72] Although the authors noted that serial BNP or NT-proBNP measurements have not been shown to improve meaningful outcomes (eg, reduction in hospitalizations, mortality from heart failure), a growing body of evidence supports the use of a single NP measurement on admission and a predischarge measure to guide prognosis. Several studies have revealed that when levels of NPs are higher prior to discharge than they were at admission for heart failure, patients are at risk of worse outcomes such as readmission and death. [71, 72]
Additionally, a growing body of literature suggests the use of BNP and/or NT-proBNP as a screening diagnostic tool for asymptomatic patients at risk of developing heart failure may be useful; however, more information is needed to determine the value proposition of this intervention. [72, 73]
Multiple studies have demonstrated that BNP may also be a useful prognostic indicator in acute coronary syndrome (ACS). Several investigations by the Thrombolysis in Myocardial Infarction (TIMI) Study Group showed that the BNP level predicted cardiac mortality and other adverse cardiac events across the entire spectrum of ACS. The mortality rate nearly doubled when both troponin I (TnI) and BNP levels were elevated.
In the Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy (TACTICS)-TIMI 18 trial, an elevated BNP level was associated with tighter culprit stenosis, higher corrected TIMI frame count, and left anterior descending artery involvement. [61] These data suggest that increased BNP levels may correlate with greater severity of myocardial ischemia and could partially explain the association between increased BNP levels and adverse outcomes.
Data from the Oral Glycoprotein IIb/IIIa Inhibition with Orbofiban in Patients with Unstable Coronary Syndromes (OPUS)-TIMI 16 and TACTICS-TIMI 18 demonstrated that baseline elevations of TnI, C-reactive protein (CRP), and BNP levels in patients with non-ST-elevated MI (NSTEMI) were independent predictors of the composite endpoint of death, MI, or heart failure. [74] The Proteomic Testing (PROMPT)-TIMI 35 trial demonstrated that transient myocardial ischemia during exercise testing was associated with an immediate rise in BNP levels. [75] In addition, the severity of ischemia was directly proportional to the elevation in BNP.
Heart failure
The presence of acute heart failure in patients with ACS is a well-known predictor of adverse cardiac events and higher risk. Therefore, it is not surprising that an elevated BNP level, as a marker of heart failure, is also predictive of adverse cardiac events in patients with ACS. Furthermore, the use of BNP and cardiac troponins on admission to the hospital is recommended by the ACC to help with prognosis in acutely decompensated heart failure. [72] Both natriuretic peptides and cardiac troponins, therefore, are clinically useful for the management of both ACS and heart failure in hospitalized patients.
Limitations
There are limitations to the use of NPs, mostly related to their lack of specificity. Elevated baseline serum levels have been found due to noncardiac patient factors, such as increasing age, female sex, pulmonary disease, sepsis, renal dysfunction, and the use of a relatively newer class of pharmacotherapy for heart failure known as angiotensin receptor-neprilysin inhibitors (ARNIs). Neprilysin is an enzyme involved in the degradation of NPs in the circulation. ARNIs inhibit neprilysin, resulting in elevated levels of NPs, which then may interfere with the prognostic utility of BNP. [76]
However, note that obese patients may have lower circulating BNP, not NT-proBNP. And NP levels may not be elevated in those patients who develop flash pulmonary edema, as the symptoms and signs may develop before the rise in levels occurs.
Markers of Cardiac Inflammation
C-reactive protein (CRP)
CRP, a nonspecific marker of inflammation, is considered to be directly involved in coronary plaque atherogenesis. Extensive studies beginning in the early 1990s showed that an elevated CRP level independently predicted adverse cardiac events at the primary and secondary prevention levels.
Data indicate that CRP is a useful prognostic indicator in patients with acute coronary syndrome (ACS), as elevated CRP levels are independent predictors of cardiac death, acute myocardial infarction (MI), and congestive heart failure (CHF). In one study, CRP was elevated in patients with unstable angina but not in those with angina caused by vasospasm; therefore, this marker may be more specifically associated with coronary artery inflammation related to atherosclerosis rather than injured myocardium. [77] In combination with troponin I (TnI) and brain-type natriuretic peptide (BNP), CRP may be a useful adjunct, but its nonspecific nature limits its use as a diagnostic cardiac marker for ACS in the emergency department (ED).
Myeloperoxidase
Myeloperoxidase (MPO) is a proinflammatory enzyme that generates reactant oxidant species and has been linked to prothrombotic oxidized lipid production, plaque instability, lipid-laden soft plaque creation, and vasoconstriction from nitrous oxide depletion. Early studies showed significantly increased MPO levels in patients with angiographically documented coronary artery disease [78] ; these findings spurred further investigation into MPO as a novel cardiac marker.
In 604 sequential patients presenting to an ED with chest pain, elevated MPO levels independently predicted an increased risk for major adverse cardiac events, including myocardial infarction (MI), reinfarction, need for revascularization, or death at 30 days and at 6 months. [79] Among the patients who presented to the ED with chest pain but who were ultimately ruled out for MI, an elevated MPO level at presentation predicted subsequent major adverse cardiovascular outcomes. In a subgroup of patients with negative baseline troponin T (TnT), MPO levels were significantly elevated at baseline, even within 2 hours after symptom onset. [79]
MPO may be a useful early marker in the ED based on its ability to detect plaque vulnerability that precedes ACS. However, further validation studies on MPO in the general ED chest pain population are needed to determine its sensitivity and specificity, as well as its negative and positive predictive values. [80, 81]
Interleukins (ILs)
IL-6 and IL-18 are produced by many different cell types, including macrophages that reside in atherosclerotic plaques and, once released, cause a proinflammatory response. High IL-6 levels in coronary artery disease, symptomatic or asymptomatic, has been associated with a higher risk of death. Unfortunately, like many other inflammatory markers, it is elevated in several disease states, which greatly limits its specificity. [82] High serum levels of IL-18 are associated with an increased risk of developing cardiovascular disease in the general population, increased mortality in heart failure patients, and development of CHF and acute MI (AMI) in patients with ACS. [83]
Whole blood choline
Choline is produced and released when membrane phospholipids are cleaved by phospholipase D, an enzyme that is activated in ACS. Whole blood and plasma choline levels may predict major adverse cardiac events (MACE) in hospitalized patients suspected of having ACS but who have negative cardiac troponins. [84]
Questions & Answers
Overview
What is the efficacy of cardiac markers?
Where are troponin cardiac markers found and what reference ranges are used in troponin assays?
How is creatine kinase-MB (CK-MB) used as a cardiac marker?
How is myoglobin used as a cardiac marker?
How are ischemia modified albumin (IMA) cardiac markers characterized and what do they indicate?
What is the role of troponin cardiac markers in the diagnosis of myocardial infarction (MI)?
How does the sensitivity, specificity, and precision of cardiac troponin assays vary?
How is the creatine kinase-MB (CK-MB) relative index used as a cardiac marker?
How are creatine kinase-MB (CK-MB) isoforms used as cardiac markers?
What is the indication for cardiac marker point-of-care (POC) assays?
What is the prognostic value of cardiac troponin?
How are troponins used as cardiac markers in chronic renal failure (CRF)?
Which troponins are used as cardiac markers in nonischemic heart disease?
What is the role of natriuretic peptides in the cardiovascular system?
How is choline used as a cardiac marker?
How are C-reactive protein (CRP) cardiac markers characterized and what do they indicate?
How are myeloperoxidase (MPO) cardiac markers characterized and what do they indicate?
How are Interleukin (IL) cardiac markers characterized and what do they indicate?
-
Cardiac Markers. Use of cardiac markers in the emergency department. Studies on troponins in acute coronary syndrome. AMI = acute myocardial infarction.
-
Cardiac Markers. Use of cardiac markers in the emergency department. Troponin I (TnI) levels and cardiac mortality in acute coronary syndrome.
-
Cardiac Markers. Use of cardiac markers in the emergency department. Cardiac event rates in the Platelet Receptor Inhibition for Ischemic Syndrome (PRISM) Study based on troponin I (TnI) results. + ve = positive.
-
Cardiac Markers. Use of cardiac markers in the emergency department. Effect of time to treatment in patients with acute coronary syndrome (ACS) who are treated with the GIIb/IIIa inhibitor eptifibatide. MI = myocardial infarction; Rx = treatment.
-
Cardiac Markers. This graph describes the improved sensitivity of the cardiac troponin assay. Whereas conventional cardiac troponin assays could detect the red region in patients with myocardial necrosis, the newer high-sensitivity assays can detect people with myocardial injury, early onset myocardial infarction, and baseline cardiac troponin levels in 50% of healthy patients. Courtesy of Oxford University Press [Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. 2017 Dec 1;113(14):1708-18. Online at https://academic.oup.com/cardiovascres/article/113/14/1708/4158239.]
Tables
What would you like to print?
- Definition and Efficacy
- Markers of Myocardial Necrosis and Ischemia
- Acute Coronary Syndrome Testing Strategy
- Troponins in Therapeutic Management of ACS
- Troponins in Chronic Renal Failure
- Troponins in Nonischemic Heart Disease
- Markers of Hemodynamic Stress: Natriuretic Peptides
- Markers of Cardiac Inflammation
- Questions & Answers
- Show All
- Media Gallery
- References